Growth Of Microalgae Chlorella Vulgaris At The Photobioreactor For Biodiesel Production
2 Department of Biosciences, Middle East Technical University, Üniversiteler mah. Eskişehir yolu No 1. 06800, Çankaya Ankara, Turkey, Email: haoktem@metu.edu.tr
3 Middle East Technical University, NANObiz Teknokent Galyum Blok No 14-18. 06800 Çankaya, Ankara, Turkey, Email: haoktem@metu.edu.tr
Received: 03-Feb-2018 Accepted Date: Feb 20, 2018; Published: 01-Mar-2018
Citation: Erturk H, Sudagidan M, Yurdakul M et al. Growth Of Microalgae Chlorella Vulgari̇s At The Photobioreactor For Biodiesel Production. J Microbiol Biotechnol Rep. 2018;2(1): 29-31.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Microalga is known to have higher lipid contents and biodiesel efficiency than most plant oil sources e.g. palm oil. We studied the growth of Chlorella vulgaris at the photobioreactor in our laboratory. We aimed to use this photobioreactor of laboratory scale as feed stream to an open pond larger scale bioreactor for future work. Photobioreactor had three compartments which had separate controls for light and air circulation. Temperature was kept at 22°C-26°C. The circulation rate was 180 L/h. The light intensity was set at 16 hours on and 8 h off. The nutrient powder was dissolved in sterile water and the pH of the solution was 6.5-6.7. Inoculation of culture was performed aseptically. The algae culture was an original strain of Chlorella vulgaris. This specific culture was proposed for use as bioenergy due to high lipid content. Culture growth was maintained aseptically and samples were taken from the reactor periodically for microbial analysis. The continuous growth was achieved at the bioreactor without contamination for more than 9 months. Slurry was centrifuged, dried and algae biomass was obtained. Extraction of lipids of the dried algae was performed by Bligh and Dyer method Extracted lipid was subject to transesterification reaction for production of fatty acid methylesters (FAMES).The lipid contents of sample was analysed by GC-FID. The results for the lipid contents were: Palmitic acid: 28%, Linoleic acid: 26%, Heptadecanoic acid: 12%, Oleic acid: 10%, Palmitoleic acid: 3%, stearic acid: 5% and the rest is arachidic acid, myristic acid etc.
Introduction
Microalgae are widely used organisms in biotechnological areas and their use has been increased in recent years. Microalgae are very important as they
i. Adaptable to variable growing conditions
ii. Non pathogenic and
iii. They have high growth rates.
Therefore, it is very important to conduct research and development studies with algae cultures as they are considered as clean, sustainable energy sources. Most microalgae are known to have high lipid and protein contents. Lipid extraction from microalgae C. Vulgaris has been conducted by some researchers [1-3].
The main objectives of the research
First objective was to grow C. vulgaris at the photobioreactor in the laboratory. This special type of strain was suggested to be used as biodiesel and biofertiliser. It was an aim to grow the microalgae at the controlled photobioreactor conditions and extract the lipids. Second objective was to apply the transesterification reaction for the lipids to obtain fatty acid methy esters (FAMEs). The obtained biodiesel was to be analysed for lipid content and quality. The use of microalgae cultures for R&D purposes has been studied in our University laboratory in Konya with regard to the above objectives [4].
Materials and Methods
The algae strain was an original strain of C. vulgaris obtained from the USA. Biotechnology company. This strain was isolated from spring waters and was supposed to have high lipid and protein contents. The specific medium for growth of C. vulgaris was provided by the supplier company from the USA.
Photobioreactor had three compartments which had separate controls for light and air circulation. Temperature was kept at 22°C-26°C. The circulation rate was 180 L/h. The light intensity was set at 16 h on and 8 h off.
Before inoculation of the bioreactor with the algae culture, sterilization of inside of the bioreactor was performed by circulating disinfectant hipochlorite solution and then rinsing with distilled water. The growth medium for C. Vulgaris was supplied by the supplier company in powder form. The powder was sterilised by UV light under the sterile hood and then dissolved in autoclaved distilled water as recommended. The innoculation of the bioreactor was done aseptically. Each compartment of the bioreactor received 17 L of sterile medium before culture inoculation. A total of 51 L of the medium was prepared and used for the experiment. Sample was taken from the outlet of the reactor periodically for microbial analysis of the water to check for any contamination. No contamination was detected from the medium samples.
Experimental
Liquid algae samples were taken from the bioreactor for preliminary experiments of lipid analysis. Slurry was dried and algae biomass was obtained.
Extraction of lipids was done according to Bligh and Dyer procedure [5] suggested to be suitable for C. vulgaris.
Lipid extraction from microalgae method.
Extraction process was performed by Bligh and Dyer method. First, 100 mL microalgal suspension was mixed with methanol and chloroform in 2:1 v/v ratio and mixed thoroughly in a vortex. Then, chloroform and water were added to the mixture to a final ratio of 1:1:0.9 v/v for methanol: chloroform: water, respectively. The final ratio of pellet to methanol, chloroform and water mixture should be 1:40 w/v. The mixture was shaken for 10 min in a separating funnel and incubated until two distinct phases were visible. The lower phase containing the micro algal lipids was separated and evaporated by rotary evaporator. Finally liquid content was quantified gravimetrically.
Then obtained lipid was used for transesterification reaction for biodiesel production. Reaction was carried out at 60°C for 3 h with 1:2 methanol: lipid ratio (w/w) in presence of KOH catalyst. After reaction was ended, product was cooled immediately and centrifuged. Obtained biodiesel was analyzed with GC.
Analysis
GC-FID method
GC-FID Shimadzu model. Column type: RT-2560 type; ID: 0.25 mm, Temperature: 250 C (max) Detector FID; Temparature 250°C.
Results
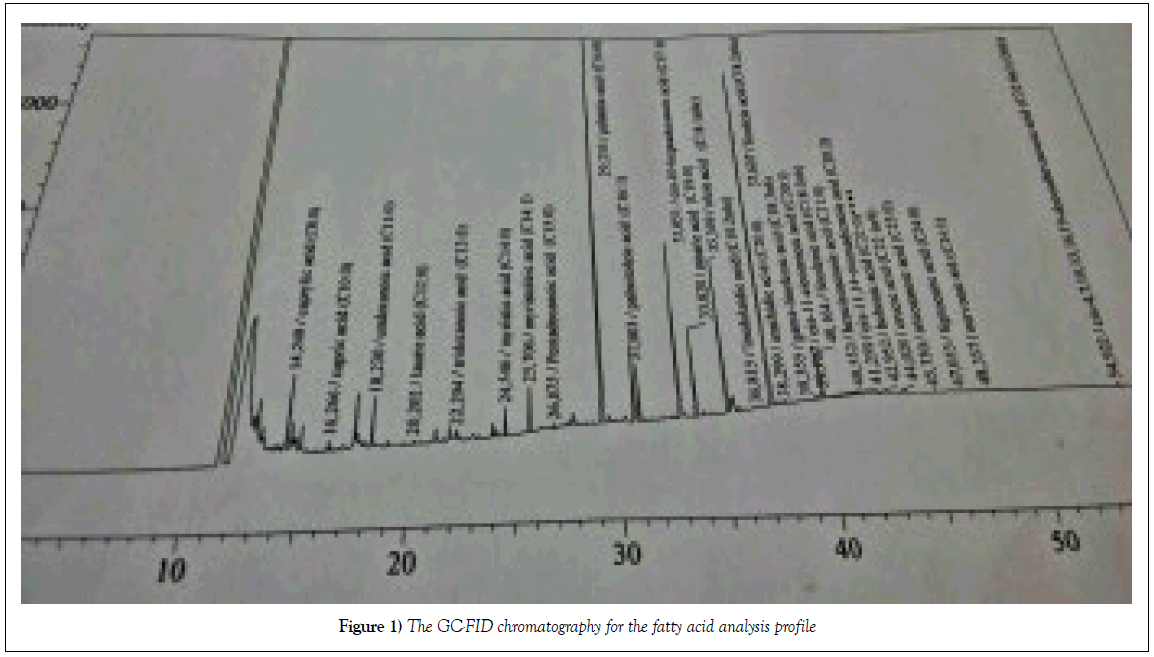
The lipid contents of sample was analysed by GC–FID at the lab.
➢ The results showed the lipid contents as:Palmitic acid: 28%, Linoleic acid: 26%, Heptadecanoic acid: 12%, Oleic acid: 10%, Palmitoleic acid: 3%, stearic acid: 5% and the rest is arachidic acid, myristic acid etc (Figure 1).
Figure 1: The GC-FID chromatography for the fatty acid analysis profile
Discussion
Results are as expected from literature although some variations for the lipid compositions existed. The ratio of lipids obtained are known to vary according to bioreactor type [2].
Future studies should be done to improve the yields [6] obtained for biodiesel, of FAMES (fatty acid methyl esters).
➢ Samples were taken from the bioreactor and observed under Leica fluorescence microscope. The microscope image of C. Vulgaris (Figure 2) showed pictures of green circular cells surrounded by white walls. The growth of C. Vulgaris at the photobioreactor are shown in Figure 3.
REFERENCES
- Castellanos SC. Batch and continuous studies of C. vulgaris in photo bioreactors. M.S. Thesis, The University of Western Ontario, Canada 2013.
- Frumento D, Casazza AA, Al Arni S, et al. Cultvation of C. vulgaris in tubular photo bioreactors. Biochemical Engineering Journal. 2013;81:120-25.
- Nautiyal P, Subramanian K A, Dastidar MG. Production and characterization of biodiesel from algae. Fuel Processing Technology. 2014;120:79-88.
- Erturk H, Oktem H A. Long term maintenance and use of microalgae cultures for R&D purposes. Biotechnology Congress 2015, abstract book p71, Konya, Turkey.
- Onay M, Sonmez C, Oktem HA, et al. Evaluation of various extraction techniques for efficient lipid recovery from thermo resistant microalgae. AJAC. 2016;7:141-50.
- Nagarajan S, Chou S K, Cao S et al. An updated comprehensive techno economic analysis of microalgae biodiesel. Bioresource Technology. 2013;145:150-6.
Keywords
C. Vulgaris; Lipids; FAMES; Photobioreactor; Biodiesel