High-performance liquid chromatography for the determination of oxymatrine content in radix Sophorae tonkinensis
- *Corresponding Author:
- Yu Cai
601 Huangpu Street West, Tianhe District, Guangzhou, China 510632.
Telephone: 86-13119582329
E-mail: 1710001232@qq.com
Zhihai Huang
55 Huanxi Road, University City, Panyu District, Guangzhou, China 510006.
Telephone: 86-02-81887233
E-mail: 1-long-1@163.com
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
HPLC; Oxymatrine; radix Sophorae tonkinensis
Radix Sophorae tonkinensis is the processed lateral root of Sophorae tonkinensis Gapnep [1]. It is bitter, cold, toxic, in the lung and stomach meridian. It has been used as a Chinese medicinal herb to treat jaundice, inflammation and aches, and to clear away heat and purging pathogenic fire [2,3]. Radix Sophorae tonkinensis mainly include alkaloid, flavonoids and triterpenoid, but oxymatrine and matrine are the primary effective components [4,5]. However, oxymatrine and matrine are also the primary toxic components. The Pharmacopoea of the People’s Republic of China states that the safe and effective dosage of radix Sophorae tonkinensis is 3 g to 6 g. If the dosage exceeds 12 g, it can cause severe adverse reactions such as nausea and vomiting [6,7]. Radix Sophorae tonkinensis from different origins have different oxymatrine content. The aim of the present study was to establish a highperformance liquid chromatography (HPLC) method for the determination of oxymatrine, and to determine the oxymatrine content of different origins of radix Sophorae tonkinensis to provide an evaluation standard for controlling radix Sophorae tonkinensis quality.
Methods
Materials
The reference substance of oxymatrine was purchased from Guangzhou Institute for Drug Control (Guangzhou, China). Radix Sophorae tonkinensis was provided by The Second Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine (Guangzhou, China). Methanol and phosphoric acid were chromatographic grade, and all the other chemicals were analytical grade.
Instrumentation
All HPLC experiments were performed on a self-assembled HPLC (SHIMADZU, Japan), with the C18 chromatographic column (R&C, Guangzhou). The extraction process of radix Sophorae tonkinensis used rotary evaporators (EYELA, Shanghai), ultrasonic cleaner (Ningbo, China) and an electronic balance (ACCULAB, Beijing).
Chromatographic conditions
C18 as chromatographic column (250 mm × 4.6 mm, 5 μm), methanol/3% phosphoric acid in water (80:20) as the mobile phase, 220 nm as the ultraviolet wavelength detection, 1 mL/min as the flow rate, 30°C as the column temperature.
Oxymatrine standard preparation
7.5 mg oxymatrine standard was precisely measured and dissolved in methanol in a 50 mL volumetric flask, obtaining a 150 μg/mL oxymatrine standard solution. Subsequently, 2 mL, 4 mL, 6 mL and 8 mL of the solution was diluted with methanol to obtain 30 μg/mL, 60 μg/mL, 90 μg/mL and 120 μg/mL oxymatrine standard solutions.
Preparing radix Sophorae tonkinensis samples
Radix Sophorae tonkinensis was crushed and screened, then taking screened powder (approximately 0.5 g) gave a respective weight, denoted M. Trichloromethane-methanol-ammonia (40:10:1) was used to dispose the radix Sophorae tonkinensis samples for 30 min, and all samples were subsequently obtained from organic solvent extraction with 30 min ultrasonic treatment. All disposed samples were filtered, then 10 mL of filtrate was measured to recover solvents to dry under decompression at 38°C to obtain the residue, the residue was diluted by methanol, then transferred to a 10 mL volumetric flask. After mixing and filtering with 0.45 μm filter membrane, radix Sophorae samples were obtained. The blank groups were treated as the samples but without radix Sophorae tonkinensis.
The content of oxymatrine
Twenty microlitres of radix Sophorae tonkinensis samples were injected in the HPLC system, the peak area was recorded, mass concentration (C) was obtained and the content of Oxymatrine from radix Sophorae tonkinensis was determined. The content of Oxymatrine was calculated by 51C/1000M.
Results
Specific inspection study
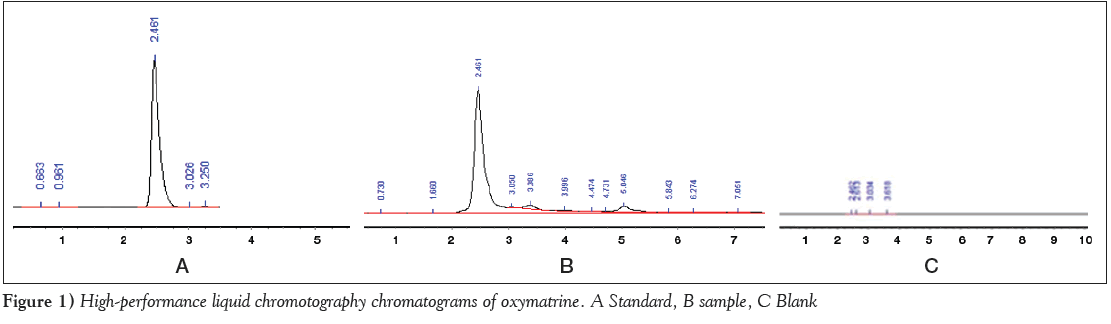
According to the described chromatographic conditions, oxymatrine standard and radix Sophorae tonkinensis samples were effective separated. The other ingredients did not interfere with the oxymatrine chromatography peak. The method had high specificity; specificity results are shown in Figure 1.
Standard curve drawing
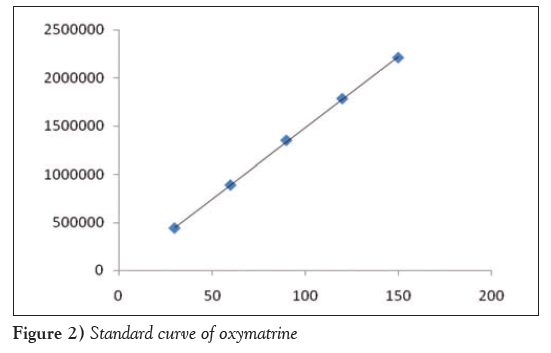
With peak area (y) as the ordinate and mass concentration (x, μg/mL) as the abscissa, the standard curves for oxymatrine were linear in the concentration range of 30 μg/mL to 150 μg/mL (y=14739x+9487.6, r=0.9998); a standard curve is shown in Figure 2.
Precision, stability, repeatability and recovery tests
Taking the same concentration of oxymatrine standard solution, continuously investigated six times, the RSD result of precision test was 0.941%; taking screened radix Sophorae tonkinensis powder (approximately 0.5 g), disposing samples as described, then investigating its peak area every 2 h, the RSD result of stability test was 1.423% in 12 h; taking screened radix Sophorae tonkinensis powder (approximately 0.5 g), disposing samples as described, then continuously investigated its peak area six times, the RSD result of repeatability test was 1.879%; taking screened radix Sophorae tonkinensis powder (approximately 0.25 g) for the six groups, disposing them as described, then adding 0.150 mg oxymatrine standard solution to the six radix Sophorae tonkinensis samples and investigating its peak area, the average recovery was 99.22%, and RSD was 1.874%. The results of recovery tests are shown in Table 1. Precision, stability, repeatability and recovery tests were well within the 5% acceptable range.
| NO* | Samples contained, mg | added, mg | Measured, mg | Recovery, % | average recovery, % | Relative SD, % |
|---|---|---|---|---|---|---|
| 1 | 5.707 | 0.150 | 5.854 | 98.00 | ||
| 2 | 5.710 | 0.150 | 5.859 | 99.33 | ||
| 3 | 5.703 | 0.150 | 5.852 | 99.33 | ||
| 4 | 5.703 | 0.150 | 5.849 | 97.33 | ||
| 5 | 5.700 | 0.150 | 5.854 | 102.67 | ||
| 6 | 5.705 | 0.150 | 5.853 | 98.67 | ||
| Total | 99.22 | 1.874 |
*All Radix Sophorae tonkinensis were from Guangxi, China, batch number: 121003; the content of oxymatrine was 22.82 mg/g
Table 1: Results of recovery tests (n=3)
Oxymatrine content of different origin radix Sophorae tonkinensis samples
Approximately 0.5 g of radix Sophorae tonkinensis powder from different origins disposed them as described, recording the peak area, the content of oxymatrine from radix Sophorae tonkinensis was obtained and is presented in Table 2.
| Origin | batch number | Oxymatrine content, mg/g |
|---|---|---|
| Heilongjiang, China | 121001 | 24.72 |
| 121002 | 24.85 | |
| 121003 | 22.82 | |
| Guilin, Guangxi, China | 1209021411 | 7.18 |
| 1209021412 | 6.98 | |
| 1209021413 | 6.99 | |
| Nanning, Guangxi, China | 120901 | 9.28 |
| 120902 | 8.43 | |
| 120903 | 8.27 |
Table 2: Results of different origin of Radix Sophorae tonkinensis sample determination (n=3)
As the results in Table 2 demonstrate, radix Sophorae tonkinensis coming from Heilongjiang origin had the highest oxymatrine content 24.85 mg/g. Radix Sophorae tonkinensis of Guilin origin had the lowest content, and the highest content was approximately four times the lowest (a considerable difference).
Discussion
Oxymatrine has a unique oxygen structure; it can lower blood pressure, resist viruses, strengthen the heart, resist arrhythmia, and treat jaundice, inflammation and aches. Existing research shows that oxymatrine has antitumour activity, mainly by inhibiting DNA synthesis in tumour cells and restraining enzyme activity to inhibit the growth of tumour cells [8]. Oxymatrine is the one of the main effective ingredients of radix Sophorae tonkinensis; studying the different oxymatrine content from different origins of radix Sophorae tonkinensis, the peak concentration was 24.85 mg/g, but the lowest concentration was 7.18 mg/g. The reasons are as follows: different geographical environment causes the different oxymatrine content, and different growth periods lead to the difference [9,10].
Choosing amino as chromatographic column, acetonitrile/isopropyl alcohol/3% phosphoric acid in water (80:5:15) as the mobile phase, chromatographic peak of oxymatrine appeared overlapping; choosing C18 as chromatographic column, methanol/2.5% phosphoric acid in water (5:95) as the mobile phase, the chromatography of oxymatrine appeared baseline drift; choosing C18 as chromatographic column, methanol/water (75:25) as the mobile phase, the chromatography of oxymatrine could not be separated. Finally, choosing C18 as chromatographic column (250 × 4.6 mm, 5 μm), methanol/3% phosphoric acid in water (80:20) as the mobile phase, 220 nm as the ultraviolet wavelength detection, 1 mL/min as the flow rate and 30°C as the column temperature, good specificity was obtained.
The present study established a HPLC methodology for determination of oxymatrine content from radix Sophorae tonkinensis. Because oxymatrine is one of the main effective ingredients and is stable, the content of oxymatrine can be a standard for controlling and evaluating the quality of radix Sophorae tonkinensis to offer theoretical basis.
Foundation Support
Science and Technology Program of Guangdong, China; Project number: 2012B031800200.
References
- WP, Wu X, Li JP, et al. Acute toxicity experiment of Radix Sophorae tonkinensis. Lishizhen Medicine and Materia Medica Research 2013;04:771-3.
- Kang ZY, Yu CP. The extraction process of Radix Sophorae tonkinensis research and discussion. Zhongyaocai 2007;1:101-2.
- China Pharmacopoeia Committee. Chinese pharmacopoeia. Chinese medical science and technology publishing 2010;1:25-6.
- H Yafei, H Jiwei, T Ling, et al. Studies on fingerprint spectrum of Radix Sophorae Tonkinensis from Guangxi. Zhongyaocai 2005;281-3.
- Ding PL, Chen DF. phenolic compounds research of Radix Sophorae tonkinensis. Zhongcaoyao 2008;2:186-8.
- Song MQ, Zhu JS, Chen JL, et al. Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World J Gastroenterol 2007;13:1788-93.
- Y Long, XT Lin, KL Zeng, et al. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int 2004;3:69-72.
- Hou Y, Cao W, Li T, et al. Oxymatrine induced HepG2 apoptosis of liver cancer possible mechanism. Academic Journal of Second Military Medical University 2008;29:634-8.
- Peng YH, Jiang CY, Lin W, et al. Matrine and oxymatrine content in different growth duration of Radix Sophorae tonkinensis. Chinese Journal of Experimental Traditional Medical Formulae 2014;8:72-5.
- Huang YF, Huang JW, Tao L, et al. Studies on fingerprint spectrum of Radix Sophorae Tonkinensis from Guangxi. Zhongyaocai 2005;281-3.
- *Corresponding Author:
- Yu Cai
601 Huangpu Street West, Tianhe District, Guangzhou, China 510632.
Telephone: 86-13119582329
E-mail: 1710001232@qq.com
Zhihai Huang
55 Huanxi Road, University City, Panyu District, Guangzhou, China 510006.
Telephone: 86-02-81887233
E-mail: 1-long-1@163.com
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
The purpose of the present study was to establish a high-performance liquid chromatography method for the determination of the oxymatrine content of radix Sophorae tonkinensis and to comparatively analyze the content of oxymatrine in radix Sophorae tonkinensis from different origins. C18 was used as a chromatographic column (250 mm × 4.6 mm, 5 μm), methanol/ 3% phosphoric acid in water (80:20) as the mobile phase, 220 nm as the ultraviolet wavelength detection, 1 mL/min as the flow rate and 30°C as the column temperature. All samples were subjected to organic solvent extraction with ultrasonic treatment before the oxymatrine content from radix Sophorae tonkinensis was measured. Oxymatrine in the range of 30 μg/mL to 150 μg/mL had a good linear relationship (r=0.9998), and the average recovery was 99.22%, suggesting that this method is accurate and has high reproducibility. The established high-performance liquid chromatography method for determining oxymatrine content from radix Sophorae tonkinensis is efficient and stable. This may be an evaluation criteria used for quality control given that different origins of radix Sophorae tonkinensis have wide-ranging differences in oxymatrine content.
-Keywords
HPLC; Oxymatrine; radix Sophorae tonkinensis
Radix Sophorae tonkinensis is the processed lateral root of Sophorae tonkinensis Gapnep [1]. It is bitter, cold, toxic, in the lung and stomach meridian. It has been used as a Chinese medicinal herb to treat jaundice, inflammation and aches, and to clear away heat and purging pathogenic fire [2,3]. Radix Sophorae tonkinensis mainly include alkaloid, flavonoids and triterpenoid, but oxymatrine and matrine are the primary effective components [4,5]. However, oxymatrine and matrine are also the primary toxic components. The Pharmacopoea of the People’s Republic of China states that the safe and effective dosage of radix Sophorae tonkinensis is 3 g to 6 g. If the dosage exceeds 12 g, it can cause severe adverse reactions such as nausea and vomiting [6,7]. Radix Sophorae tonkinensis from different origins have different oxymatrine content. The aim of the present study was to establish a highperformance liquid chromatography (HPLC) method for the determination of oxymatrine, and to determine the oxymatrine content of different origins of radix Sophorae tonkinensis to provide an evaluation standard for controlling radix Sophorae tonkinensis quality.
Methods
Materials
The reference substance of oxymatrine was purchased from Guangzhou Institute for Drug Control (Guangzhou, China). Radix Sophorae tonkinensis was provided by The Second Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine (Guangzhou, China). Methanol and phosphoric acid were chromatographic grade, and all the other chemicals were analytical grade.
Instrumentation
All HPLC experiments were performed on a self-assembled HPLC (SHIMADZU, Japan), with the C18 chromatographic column (R&C, Guangzhou). The extraction process of radix Sophorae tonkinensis used rotary evaporators (EYELA, Shanghai), ultrasonic cleaner (Ningbo, China) and an electronic balance (ACCULAB, Beijing).
Chromatographic conditions
C18 as chromatographic column (250 mm × 4.6 mm, 5 μm), methanol/3% phosphoric acid in water (80:20) as the mobile phase, 220 nm as the ultraviolet wavelength detection, 1 mL/min as the flow rate, 30°C as the column temperature.
Oxymatrine standard preparation
7.5 mg oxymatrine standard was precisely measured and dissolved in methanol in a 50 mL volumetric flask, obtaining a 150 μg/mL oxymatrine standard solution. Subsequently, 2 mL, 4 mL, 6 mL and 8 mL of the solution was diluted with methanol to obtain 30 μg/mL, 60 μg/mL, 90 μg/mL and 120 μg/mL oxymatrine standard solutions.
Preparing radix Sophorae tonkinensis samples
Radix Sophorae tonkinensis was crushed and screened, then taking screened powder (approximately 0.5 g) gave a respective weight, denoted M. Trichloromethane-methanol-ammonia (40:10:1) was used to dispose the radix Sophorae tonkinensis samples for 30 min, and all samples were subsequently obtained from organic solvent extraction with 30 min ultrasonic treatment. All disposed samples were filtered, then 10 mL of filtrate was measured to recover solvents to dry under decompression at 38°C to obtain the residue, the residue was diluted by methanol, then transferred to a 10 mL volumetric flask. After mixing and filtering with 0.45 μm filter membrane, radix Sophorae samples were obtained. The blank groups were treated as the samples but without radix Sophorae tonkinensis.
The content of oxymatrine
Twenty microlitres of radix Sophorae tonkinensis samples were injected in the HPLC system, the peak area was recorded, mass concentration (C) was obtained and the content of Oxymatrine from radix Sophorae tonkinensis was determined. The content of Oxymatrine was calculated by 51C/1000M.
Results
Specific inspection study
According to the described chromatographic conditions, oxymatrine standard and radix Sophorae tonkinensis samples were effective separated. The other ingredients did not interfere with the oxymatrine chromatography peak. The method had high specificity; specificity results are shown in Figure 1.
Standard curve drawing
With peak area (y) as the ordinate and mass concentration (x, μg/mL) as the abscissa, the standard curves for oxymatrine were linear in the concentration range of 30 μg/mL to 150 μg/mL (y=14739x+9487.6, r=0.9998); a standard curve is shown in Figure 2.
Precision, stability, repeatability and recovery tests
Taking the same concentration of oxymatrine standard solution, continuously investigated six times, the RSD result of precision test was 0.941%; taking screened radix Sophorae tonkinensis powder (approximately 0.5 g), disposing samples as described, then investigating its peak area every 2 h, the RSD result of stability test was 1.423% in 12 h; taking screened radix Sophorae tonkinensis powder (approximately 0.5 g), disposing samples as described, then continuously investigated its peak area six times, the RSD result of repeatability test was 1.879%; taking screened radix Sophorae tonkinensis powder (approximately 0.25 g) for the six groups, disposing them as described, then adding 0.150 mg oxymatrine standard solution to the six radix Sophorae tonkinensis samples and investigating its peak area, the average recovery was 99.22%, and RSD was 1.874%. The results of recovery tests are shown in Table 1. Precision, stability, repeatability and recovery tests were well within the 5% acceptable range.
| NO* | Samples contained, mg | added, mg | Measured, mg | Recovery, % | average recovery, % | Relative SD, % |
|---|---|---|---|---|---|---|
| 1 | 5.707 | 0.150 | 5.854 | 98.00 | ||
| 2 | 5.710 | 0.150 | 5.859 | 99.33 | ||
| 3 | 5.703 | 0.150 | 5.852 | 99.33 | ||
| 4 | 5.703 | 0.150 | 5.849 | 97.33 | ||
| 5 | 5.700 | 0.150 | 5.854 | 102.67 | ||
| 6 | 5.705 | 0.150 | 5.853 | 98.67 | ||
| Total | 99.22 | 1.874 |
*All Radix Sophorae tonkinensis were from Guangxi, China, batch number: 121003; the content of oxymatrine was 22.82 mg/g
Table 1: Results of recovery tests (n=3)
Oxymatrine content of different origin radix Sophorae tonkinensis samples
Approximately 0.5 g of radix Sophorae tonkinensis powder from different origins disposed them as described, recording the peak area, the content of oxymatrine from radix Sophorae tonkinensis was obtained and is presented in Table 2.
| Origin | batch number | Oxymatrine content, mg/g |
|---|---|---|
| Heilongjiang, China | 121001 | 24.72 |
| 121002 | 24.85 | |
| 121003 | 22.82 | |
| Guilin, Guangxi, China | 1209021411 | 7.18 |
| 1209021412 | 6.98 | |
| 1209021413 | 6.99 | |
| Nanning, Guangxi, China | 120901 | 9.28 |
| 120902 | 8.43 | |
| 120903 | 8.27 |
Table 2: Results of different origin of Radix Sophorae tonkinensis sample determination (n=3)
As the results in Table 2 demonstrate, radix Sophorae tonkinensis coming from Heilongjiang origin had the highest oxymatrine content 24.85 mg/g. Radix Sophorae tonkinensis of Guilin origin had the lowest content, and the highest content was approximately four times the lowest (a considerable difference).
Discussion
Oxymatrine has a unique oxygen structure; it can lower blood pressure, resist viruses, strengthen the heart, resist arrhythmia, and treat jaundice, inflammation and aches. Existing research shows that oxymatrine has antitumour activity, mainly by inhibiting DNA synthesis in tumour cells and restraining enzyme activity to inhibit the growth of tumour cells [8]. Oxymatrine is the one of the main effective ingredients of radix Sophorae tonkinensis; studying the different oxymatrine content from different origins of radix Sophorae tonkinensis, the peak concentration was 24.85 mg/g, but the lowest concentration was 7.18 mg/g. The reasons are as follows: different geographical environment causes the different oxymatrine content, and different growth periods lead to the difference [9,10].
Choosing amino as chromatographic column, acetonitrile/isopropyl alcohol/3% phosphoric acid in water (80:5:15) as the mobile phase, chromatographic peak of oxymatrine appeared overlapping; choosing C18 as chromatographic column, methanol/2.5% phosphoric acid in water (5:95) as the mobile phase, the chromatography of oxymatrine appeared baseline drift; choosing C18 as chromatographic column, methanol/water (75:25) as the mobile phase, the chromatography of oxymatrine could not be separated. Finally, choosing C18 as chromatographic column (250 × 4.6 mm, 5 μm), methanol/3% phosphoric acid in water (80:20) as the mobile phase, 220 nm as the ultraviolet wavelength detection, 1 mL/min as the flow rate and 30°C as the column temperature, good specificity was obtained.
The present study established a HPLC methodology for determination of oxymatrine content from radix Sophorae tonkinensis. Because oxymatrine is one of the main effective ingredients and is stable, the content of oxymatrine can be a standard for controlling and evaluating the quality of radix Sophorae tonkinensis to offer theoretical basis.
Foundation Support
Science and Technology Program of Guangdong, China; Project number: 2012B031800200.
References
- WP, Wu X, Li JP, et al. Acute toxicity experiment of Radix Sophorae tonkinensis. Lishizhen Medicine and Materia Medica Research 2013;04:771-3.
- Kang ZY, Yu CP. The extraction process of Radix Sophorae tonkinensis research and discussion. Zhongyaocai 2007;1:101-2.
- China Pharmacopoeia Committee. Chinese pharmacopoeia. Chinese medical science and technology publishing 2010;1:25-6.
- H Yafei, H Jiwei, T Ling, et al. Studies on fingerprint spectrum of Radix Sophorae Tonkinensis from Guangxi. Zhongyaocai 2005;281-3.
- Ding PL, Chen DF. phenolic compounds research of Radix Sophorae tonkinensis. Zhongcaoyao 2008;2:186-8.
- Song MQ, Zhu JS, Chen JL, et al. Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World J Gastroenterol 2007;13:1788-93.
- Y Long, XT Lin, KL Zeng, et al. Efficacy of intramuscular matrine in the treatment of chronic hepatitis B. Hepatobiliary Pancreat Dis Int 2004;3:69-72.
- Hou Y, Cao W, Li T, et al. Oxymatrine induced HepG2 apoptosis of liver cancer possible mechanism. Academic Journal of Second Military Medical University 2008;29:634-8.
- Peng YH, Jiang CY, Lin W, et al. Matrine and oxymatrine content in different growth duration of Radix Sophorae tonkinensis. Chinese Journal of Experimental Traditional Medical Formulae 2014;8:72-5.
- Huang YF, Huang JW, Tao L, et al. Studies on fingerprint spectrum of Radix Sophorae Tonkinensis from Guangxi. Zhongyaocai 2005;281-3.