Histological analyses in seasonal variability of pathological impact on Mytilus galloprovincialis (Lamarck, 1819) of Moroccan North Atlantic Coast
Received: 02-Jan-2018 Accepted Date: May 30, 2018; Published: 20-Jun-2018
Citation: Sanaa B. Histological analyses in seasonal variability of pathological impact on Mytilus galloprovincialis (Lamarck, 1819) of Moroccan North Atlantic Coast. J Histol Histopathol Res 2018;2(1): 17-21.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
We histopathologically investigated the seasonal variability in health status of a natural population of mussels (Mytilus galloprovincialis) in the Atlantic coast region of Morocco. We hypothesized those external conditions and the health status of hosts synergistically disturbed M. galloprovincialis and determined infections of endoparasites due to the stress upon the host. In Moulay Bousselham, an ecologically important habitat for the indigenous shellfish, varieties of endoparasites were identified from samples collected between March 2009 and March 2011. Pathological status of M. galloprovincialis was examined and interpreted based on statistical relationships between major parasites and environmental variables and temporal changes in physiological conditions of M. galloprovincialis. Ciliophora, Nematopsis, and Rickettsia-like organisms were observed throughout the study period. Their prevalences were found to be statistically related to temperature and salinity, and to the host’s own physical condition. Bacteria colonies were often observed in gill tissues, while some hosts exhibited severe infections in their digestive and pericardial glands. Hemocytic infiltration was not found in association with bacterial infections. Lesser prevalent parasites found in our study included Proctoeces maculatus, gill ciliate Ancistrum mytili, Pseudoklossia-like coccidian, and Mytilicola intestinalis. While these infections did not total more than 7% in annual prevalence, they may, based on their known effects, pose a significant threat to M. galloprovincialis. In our study, many cases of hemocytic infiltration were found in gonad, digestive gland, and gills; not always were they found in association with pathogens (such as Ciliophora or Nematopsis).
Keywords
Bivalve (Mytilus galloprovincialis); Mytilicola, granulocytomas; Hemocytic infiltration; Seasonal impact; Merja zerga
Research of pathological risk has become increasingly more important as the global aquacultural sector of shellfish has grown [1]. Parasitic infections have been suggested as one of the major causes of mass mortality in both natural and cultured stocks of shellfish [2-7]. Considering the recent expansion of aquacultures in the North Atlantic Coast region of Africa, scientific investigations of parasitic impacts to shellfish should be enhanced with the new needs. No sufficient pathological studies concerning bivalves in Morocco have been done [8-10]. Within Moroccan waters, Steinhausia sp. was the focus of a study in which its geographical distribution, seasonal variation and parasitic association with hemocytic infiltration responses were investigated [10].
Regarding Mytilus galloprovincialis in the Mediterranean Sea, other known pathogens include: microsporidia; the turbellarian, Urastoma cyprinae; the copepods, Mytilicola intestinalis and Mytilicola orientalis [11]; and Steinhausia mytilovum [12,13]. Villalba suggested the presence of Marteilia refringens (a major pathogen found in the oyster Ostrea edulis) to be a major cause of mortality in M. galloprovincialis. M. refringens was also found in a cultured population of mussels in Morocco, along with the other pathogens, although requisite status monitoring and impact studies have yet to be conducted [9].
The pathogenic resistance of mussels (including M. galloprovincialis) has been discussed in relation to their environment, and sensitivity to local thermal conditions was indicated [9,14].
Our study aimed to pathologically assess the health status in natural populations of mussel Mytilus galloprovincialis in the Atlantic Coast region of Morocco. Occurrences of endoparasitic species in M. galloprovincialis were monitored and histopathologically investigated in Moulay Bousselham, Morocco (GPS 34°53’ N; 6°17’ W). This area is characterized by the wide range of seasonal variability in especially water temperature, nearly 10°C, was observed in the past study [10]. The past observation of the seasonal variability in the physical conditions and reproductive status of M. galloprovincialis brought us the hypothesis that there is relationship between the environmental variability and pathological status of the M. galloprovincialis [10]. Based on data collected between March 2009 and March 2011, water temperature was shown to vary according to season, with the lowest temperature being recorded in November 2010 (14°C) and the highest in the summer months of July and August (23°C) [10]. Throughout survey years, the condition index followed a seasonal cyclic pattern, going from a minimum in April to a maximum in June. During the summer spawning period, from July to August, the condition index decreased; it also decreased from autumn to winter [10].
We investigated the pathological status and the relationships between dominant parasites and environmental variables. Interpretations were made based on the temporal changes in physiological conditions of Mytilus galloprovincialis within the selected habitat.
Materials and Methods
Moulay Bousselham is characterized by rocky bands extending approximately 500m to 1km from the coastline north of the Marja Zerga lagoon. Cliffs fall parallel to the bank and sandy beaches feature rocky boundaries at the lagoon level. The area is influenced by seasonal upwelling and marked by a biodiversity based on primary production [15]. Mytilus galloprovincialis and Perna perna coexist as natural stock in this area.
Mytilus galloprovincialis samples, with a mean shell length of 45.45mm, were collected each month from March 2009 to March 2011 to a total of 660 individuals (n=30 mol-1). Also during this period, a thermosalinometer was used to measure water temperature and salinity at a sampling depth of 3 cm. While samples in January 2010 and January 2011 weren’t taken due to environmental conditions.
Sampled M. galloprovincialis were individually examined in the laboratory immediately after transportation from the field. Calipers were used to measure mussel size (shell length) and a scientific scale determined, to the nearest 0.001g, the measurements of weight; total wet weight (TW), wet meat weight (WMW), and shell weight (SW). The condition index (CI) of individual M. galloprovincialis was calculated according to the formula of Aguirre in 1979 [16]:
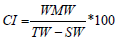
Small size 2.75 to 20.11. The organs from each M. galloprovincialis sample were anatomically examined and fixed in Davidson’s fluid more than 24 hours; they included: gill, mantle, gonad and gonoducts, digestive gland tubules, stomach/intestine, and connective tissue. The specimens were then dehydrated in a graded ethanol series before being embedded in paraffin. Two parallel oblique-longitudinal sections (2-μm thick) were made from the mantles of each specimen; hematoxylin and eosin were then used to stain the resulting sections prior to examination. These histological sections were examined under an optical microscope to identify the presence of parasites and pathological alterations.
To determine the monthly pathological state, including parasitic infections or diseases, we used the prevalence calculated as:
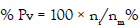
Pv was the monthly prevalence in percentage, ni indicated the number of infested individuals, and nm was the number of mussels analysed in month [17-30]. Hemocytic infiltration, being a major biological response to parasitism was also taken into account by the analyses and its presence was recorded.
In this paper we were not looking for disseminated neoplasia (contagious cancer/leukemia).
The obtained observations of parasites and indices were quantitatively analyzed. The time series for the prevalence of dominant parasites was investigated, and changes in pathological state were compared with it throughout survey years.
Seasonal dependency of parasitic prevalence was tested using the Kruskal Wallis Test. To evaluate ecological (i.e., environmental and biological) association between presence or absence of parasites and on-site enviromental conditions, salinity and temperature, and biological conditions as Condition index IC, generalized liner model was applied. Wald statistic were evaluated to determine significant environmental variables and biological conditions associated to presence or absence of the dominant parasites at α = 0.05 level. We also used the GLM to examine the dependency of hemocytic infiltrations upon dominant causal parasites. R version 2.15.2 (R Core Team) was applied for all explanatory analyses and statistical tests in this study.
Results
Salinity and temperature
Nano filtered Changes in the level of salinity (from local exchanges of water between the coast and the lagoon) were an indication of its impact distribution. It was variable within individual seasons and ranged from 33 to 36 during the period of study.
The water’s temperature recorded variations according to the season. For three successive years from 2009 to 2011, the lowest level of temperature was recorded in November 2010 (14°C). The highest temperature was recorded in summer (23°C) in July and August.
Parasitical observations
A variety of parasites and hemocytic alterations were found within the study area; while Martelia refringens, a notably virulent protozoan in the Mediterranean, was absent from observations.
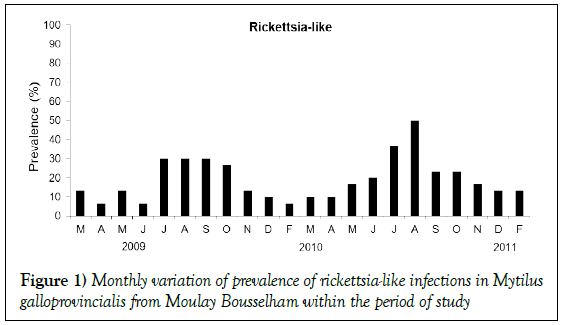
Basophilic inclusions of Rickettsia-like prokaryotes (RLPs) were detected with greater frequency in gills (18%) than digestive glands (1.81%); in rare instances, they were observed as free bacteria colonies in pericardial glands. Their mean prevalence was 19.09% (95% IC 16.16–22.30%). Prevalence in digestive glands was 1.81% (95% IC 0.94–3.15%). The highest presence of this infection was recorded during the summer of 2010 in June and August when prevalences were 37% and 50% respectively (Figure 1). Seasonal variation of their prevalence was significant (Kruskal Wallis, p<0.05). The prevalence increased steadily from spring until summer (Figure 1). No coinciding hemocytic infiltration was found.
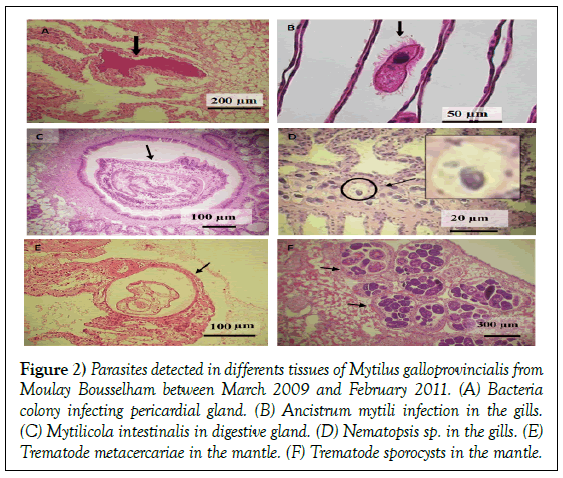
As for protozoans, Ancistrum mytili was found; however, its average prevalence was less than 2% of the total: 1.51% (95% IC 0.72 -2.76%). The total prevalence of protozoans fluctuated by 3% in summer 2009, December 2009, and March 2010; it fluctuated 10% in February and April of 2010. Local hemocytic infiltration did not tend to occur with Ancistrum mytili infections (Figures 2a and 2b).
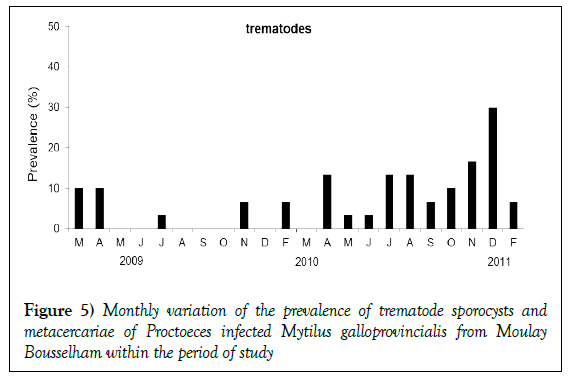
Figure 2: Parasites detected in differents tissues of Mytilus galloprovincialis from Moulay Bousselham between March 2009 and February 2011. (A) Bacteria colony infecting pericardial gland. (B) Ancistrum mytili infection in the gills. (C) Mytilicola intestinalis in digestive gland. (D) Nematopsis sp. in the gills. (E) Trematode metacercariae in the mantle. (F) Trematode sporocysts in the mantle.
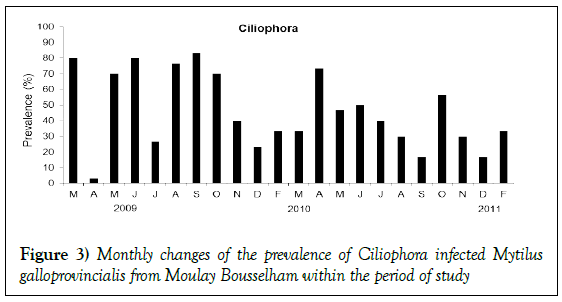
Throughout the sampling period, infections of Ciliophora were observed in the digestive epithelial cells. The mean prevalence was high: 46.06% (95% IC 42.20–49.94%). It exceeded 50% in all seasons except winter (Figure 3), though no indication of statistical dependency upon season was found (Kruskal Wallis test, p>0.05). No coinciding hemocytic infiltration was observed.
A copepod sp., Mytilicola intestinalis, was detected in stomachs from two individual mussels; the first in March 2009 and the other in December 2009 (Figure 2c). Antennae hooks were attached to the intestinal epithelial cells of the M. galloprovincialis samples, and such occurrences were regularly associated with hemocytic infiltration response. In March 2010, a copepod was observed in labial palp tissue without signs of hemocytic infiltration.
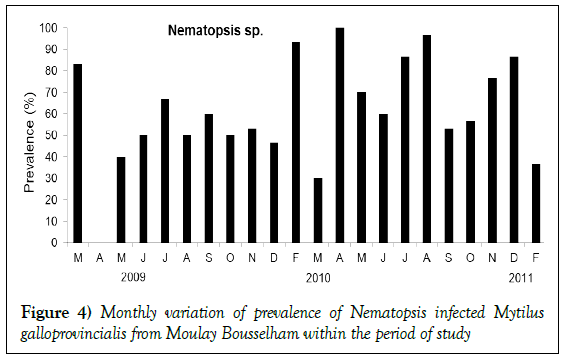
Nematopsis sp. was the most dominant parasite throughout the sampling period, with an exception in April 2009 (Figure 4). Its annual prevalence was 61.21% (95% IC 57.37–64.94%) and was observed in gills, on digestive glands, and within mantle tissues. Infections were rarely observed in the foot. Nematopsis gill infections were often associated with destruction to lamella (Figure 3d). Monthly variations in prevalence were generally high. Over 70% of the mussels observed in spring (during March 2009 and April 2010), in summer (during July and August 2010), and in winter (during February and December 2010) were infected with Nematopsis. The presence of Nematopsis was found to be independent of season (Kruskal Wallis test, p<0.05).
Sporocysts and metacercariae of trematode species Proctoeces maculatus were observed in the histological analysis. 7% (95% IC 5.14–9.18%) of the M. galloprovincialis samples were found with P. maculatus. Cases were especially frequent in December 2010 and different stages of the infections were observed. Many developed sporocysts were infecting vascular tissues of the mantle, and the metacercariae stage was observed in the foot. Two cases of heavy infections at the metacercariae stage were found to be inhibiting gametogenesis; one in March 2009 and another in February 2011. Histological immune responses were not observed in hosts infected with sporocysts.
Unidentified parasites discovered within the digestive gland lumen had an annual prevalence of 3.48% (95% IC 2.22–5.18%) and varied significantly by season (Kruskal Wallis test, p<0.05). Their prevalence was between 3% and 6% in spring and summer seasons; in autumn and winter, they were absent.
Infections of Pseudoklossia–like coccidian were observed in March and April of 2009, and May and November of 2011. In three individuals, examinations revealed macrogamonts of Pseudoklossia-like coccidian within the cells and lumen of kidney tubules. Individual tubules were observed to exhibit in excess of 6 coccidians each. No associations between Pseudoklossia spp. and hemocytic response were observed in sample hosts.
Hemocytic infiltrations
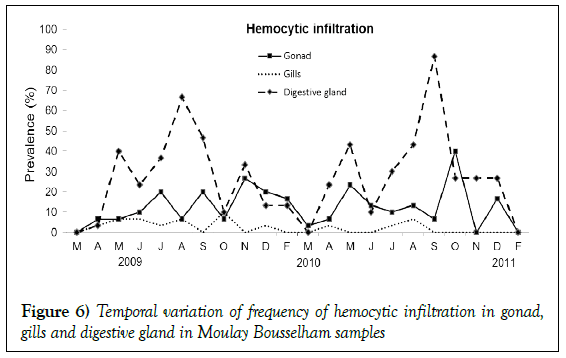
During our histological analysis, the presence of severe hemocytic infiltration in gonad, digestive gland, and gill tissue was often observed without evidence of impact from parasites (Figures 5 and 6). Cases recorded in gills were in the minority 2.42% (95% IC 1.39–3.90%). Gonads of 12.42% individuals (95% IC 11.00–15.18%) were free from potential pathogens yet exhibited hemocytic infiltration. Observations of such cases were highly variable throughout survey years. The majority of occurrences were in October 2010, and the prevalence was significantly independent of season (Kruskal wallis test p>0.05). This type of lesion was also observed in digestive gland tissues with temporal variations in prevalence. The annual prevalence of such a case was 27.42% (95% IC 24.05–30.99%) and was observed inside the tissue, surrounding intestinal and oesophagien epithelium, or in digestive diverticula. They were also observed as granulocytomas, found most often in digestive glands when the prevalence was particularly high (August 2009 and September 2010) (Kruskal wallis test p<0.005).
Association of parasites presence to abiotic and biotic parameters
The presence of RLPs had no statistical association with temperature (p>0.05), salinity (p>0.05), or CI (p>0.05) (Table 1). Ciliophora had a relationship with salinity levels (p<0.05). While Nematopsis had no relationship with temperature or salinity, significant associations were found with the mussels’ condition index (CI) (p<0.005).
Table 1: Results of logistic regression showing the relationship between the studies different ecological parameters and the presence of Rickettsia, Ciliophora, and Nematopsis; with respect to the total number of mussels at Moulay Bousselham.
| Coefficients | Estimate | Std. Error | t value | Pr (>|t|) |
|---|---|---|---|---|
| Rickettsia-like | ||||
| Intercept | -0.62849 | 0.441 | -1.425 | 0.1548 |
| Temperature | 0.01409 | 0.0079 | 1.777 | 0.0761 |
| Salinity | 0.01511 | 0.0121 | 1.249 | 0.2122 |
| Condition index | 0.0004 | 0.0007 | 0.565 | 0.572 |
| Ciliophora | ||||
| Intercept | -2.3027 | 0.544 | -4.231 | 2 E-05 |
| Temperature | 0.0227 | 0.0097 | 2.322 | 0.02 |
| Salinity | 0.0676 | 0.0149 | 4.533 | 7 E-06 |
| Condition index | -0.0006 | 0.0008 | -0.725 | 0.468 |
| Nematopsis | ||||
| Intercept | 2.6853 | 0.5361 | 5.009 | 7 E-07 |
| Temperature | -0.005 | 0.0096 | -0.521 | 0.602 |
| Salinity | 0.0006 | 0.0008 | 0.799 | 0.424 |
| Condition index | -0.0574 | 0.0147 | -3.904 | 0 |
Discussion
The cause of poly vinyl, the range is nearly optimal for the facilitation of parasitic infections in Mytilidae and other bivalves [14]. Thus, Moulay Bousselham provides an excellent model site for the detection of potential pathogens in bivalves and for the assessment of pathological risks in the surrounding area.
Cycliophora, Nematopsis, and RLPs were present throughout our study with prevalence depending on environmental dynamics. Summaries of the specific environmental conditions which facilitate their prevalence can be used to indicate potential risks of infection (Table 1). No cases of parasitism from the exceptionally virulent species M. refringens were encountered during our surveys.
RLPs were regularly observed in the gills of M. galloprovincialis sampled in past studies [3,13,16-18]. We found RLPs often in the gills of hosts, and some severe infections were also found in digestive glands. Carballal found similar cases in Cerastoderma edule samples taken from European waters [17]. Hemocytic infiltration was not found alongside these infections in our study; however, the prevalence may vary, as has been observed off the coasts of France and Spain [4]. Variability in the prevalence of hemocytic infiltration coinciding with RLPs is not easily reconciled with external factors using the data from this and past studies. A study by Figueras, for example, found RLPs most abundant in autumn, while in our study they were highest during the similar time period of late summer [19]. Seasonal changes in temperature or salinity would then be the hypothesized cause, except those particular environmental variables were not found to have a relationship with the presence of RLPs. Likewise, Minguez & Giambérini found there to be no relationship between temperature and RLPs prevalence in digestive diverticula of the kin species Dreissena polymorpha [18]. Environmental conditions in Moulay Bousselham, while not related to the presence of RLPs, may be related to the impact from RLPs.
Ciliophora-like ciliates have been observed intracellularly in digestive glands, especially inside diverticula [12,13,20,21]. Infections of such parasites have been observed in the Mediterranean Sea [13,22] and off the Atlantic coast of southern Europe [21]. Explanations for the prevalence based on physical environmental conditions have yet to be made [21,22]. It has been suggested, however, that changes in salinity and oxygen are potential drivers enhancing infections of Ciliophora-like ciliates in the North Mediterranean Sea through the influence of host conditions [23]. A statistical relationship between the prevalence of Ciliophora-like pathogens and salinity was found in the bay of M’diq, located at the coastal area of the Mediterranean Sea, and local water exchanges were suggested as the environmental driver [13]. In Moulay Bousselham, inflows to Marja Zerga change the salinity in the overall coastal area. Such oceanography may be driving the heavy prevalence of Ciliophora like ciliates. Negative impacts to the bivalve hosts from the ciliates have been noted in other studies [24]. In our study, there were no signs of the ciliates impacting host health. A threshold for tolerable environmental stress may exist in hosts which, when surpassed, allows negative impacts from the parasitism of Ciliophora-like ciliates to take effect.
The presence of Nematopsis in hosts was not found in relation with the physical environment, its significant correlation to the condition index of the mussels indicates a physiological consequence or result. A high prevalence of Nematopsis sp. was observed in our study. No apparent physical responses were found in the host tissues, consistent with Sabry [25]. We hypothesize that the presence of this parasite affected to the condition index as a consequence of a physiological response of M. galloprovincialis. Kin species Cerastoderma edule has suffered mass mortality from Nematopsis [25]. Our findings may have been the result of the negative impacts from Nematopsis in the field of the given local environment, though mortality was not able to be determined from our study.
Sabry suggested that the extensive infestation of Nematopsis may be a result of co-occurring intermediate hosts, such as coastal crustaceans, in the habitat [25-28]. In many cases, the life cycle of Nematopsis depends on specific crustacean hosts. At the M’diq aquacultural site, far from the coastline, the prevalence of Nematopsis was only 0.4% (n=750) [13].
The duration of sampling allowed us to detect other parasites, including: Proctoeces maculatus, gill ciliate Ancistrum mytili, Pseudoklossia-like coccidian, and Mytilicola intestinalis. The sum of these parasites was in the minority, at less than 7% in annual prevalence. The threat of their negative impact, however, to M. galloprovincialis is potentially serious. Depending on their state of development, trematodes can cause serious physical damage to hosts. The survey did not include observations of primordial follicles or gametes in the M. galloprovincialis to identify their sex [10]. The often-observed encapsulation of the metacercariae stage was defined as an intensive immune response of the host [29,30]. Not all observed parasites lacked detectable impacts, such as hemocytic infiltration, on their hosts; minor inflammation of lesions were seemingly the result of infections by Urastoma cyprinae and Mytilicola intestinalis. With additional stress to hosts, minor pathogens may gain the ability to cause other unexpected impacts.
Hemocytic infiltration is often observed in aquatic organisms, including mussels, which inhabit stressful environments such as estuaries [14,31]. Intensive development of hemocytic infiltration has been shown to limit the healthy growth of bivalve organs [14]. Our study found many cases of hemocytic infiltration in gonads, digestive glands, and gills; though these observations were not always associated to pathogens. Bignell suggested the possibility of organisms to have hemocytic infiltrations in the absence of parasites and that such infiltration would migrate through vesicular connective tissue to the suitable basement membrane of an organ [14]. The tissues would then be removed from the mussel via diapesis. In our study, there were no significant associations found between hemocytic lesions in organs and the dominant parasites Ciliophora or Nematopsis. Conversely, a significant association was found between digestive gland inflammation and RLPs (Table 2). The infections were more often observed in gills than digestive glands, which may be due to a specific infection process of RLPs [32-34].
Table 2: Results of logistic regression showing the relationship between the major parasites studied and the presence of hemocytic infiltration in different organs; with respect to the total number of mussels at Moulay Bousselham.
| Coefficients | Estimate | Std. Error | t value | Pr (>|t|) |
|---|---|---|---|---|
| HI in gonads | ||||
| Intercept | 0.139336 | 0.023994 | 5.807 | 9.92E-09 |
| Ciliophora | -0.0099 | 0.025933 | -0.382 | 0.703 |
| Nematopsis | -0.01191 | 0.026602 | -0.448 | 0.654 |
| Rickettsia | -0.01503 | 0.032946 | -0.456 | 0.648 |
| HI in gills | ||||
| Intercept | 0.008957 | 0.011161 | 0.803 | 0.4225 |
| Ciliophora | 0.021766 | 0.012063 | 1.804 | 0.0716 |
| Nematopsis | 0.006334 | 0.012374 | 0.512 | 0.6089 |
| Rickettsia | 0.007654 | 0.015325 | 0.499 | 0.6176 |
| HI in digestive gland | ||||
| Intercept | 2.6853 | 0.5361 | 5.009 | 7 E-07 |
| Ciliophora | -0.005 | 0.0096 | -0.521 | 0.602 |
| Nematopsis | 0.0006 | 0.0008 | 0.799 | 0.424 |
| Rickettsia | -0.0574 | 0.0147 | -3.904 | 0 |
Parasitic infections have not caused observable mortality within the site. The only pathogenic risks found to the tissue health of Mytilus galloprovincialis were from the low prevalence of trematode sporocysts, which inhibited the gametogenesis process; and Nematopsis, which had an effect on the condition index of the hosts. We do, however, recommend further histological study and continuous ecological monitoring throughout the year, with special attention to the recorded reproductive cycles and local environmental dynamics.
Conclusion
In conclusion, we found a variety of parasitical infections in the waters of Moulay Bousselham though they did not show evidence of mortal impacts to the population. Highly prevalent infections were not found in association with major physical damages; however, nuisance pathogens were found in smaller numbers. Physical damages and other impacts to individuals, and the greater population, may not only be caused by pathogens but also from the suggested environmental and ecological drivers of hemocytic infiltration and their interactions.
Pathological mortality, not having caused observable mortality, could not be examined in our study of the population of Mytilus galloprovincialis in the North Atlantic Coast of Morocco. In addition to recording temperature and salinity, we suggest ecological monitoring of a number other in-situ environmental factors which may influence parasitic prevalence and stress levels in Mytilus galloprovincialis, as well as continued histological analyses. Biological conditions of hosts are also recommended targets of study and include individual size, metabolic changes, reproductive cycles, and trophic conditions.
Acknowledgements
The completion of this work owes a lot to the Institut National de Recherches Halieutique, Morocco. That each finds here the expression of my profound gratitude.
REFERENCES
- Subasinghe RP, Bondad-Reantaso MG. Aquaculture development, health and wealth. Food and Agriculture Organization of the united nation 2001.
- Sindermann CJ. Principal diseases of marine fish and shellfish. Academic Press, New York 1970;369.
- Bower SM, Figueras AJ. Infectious diseases of mussels, especially pertaining to mussel transplantation. World Aquaculture 1989;20:89-93.
- Figueras AJ, Villalba A. Patología de Moluscos Bivalvos. Monografías Acuicultura. CAICYT 1988.
- Bower SM, Mcgladdery SE. Synopsis of infectious diseases and parasites of commercially exploited shellfish. Ann. Rev. Fish Dis 1994;4:1-199.
- Gosling P. Bivalves molluscs biology, Ecology and culture. Fishing News Books 2003:370-401.
- Powell EN, Ashton-Alcox KA. Is overwinter mortality commonplace in Delaware Bay oyster populations? The ambiguity of dredge efficiency. Journal of Shellfish Research 2013;32:639-45.
- Gam M, De montaudouin X. Do trematode parasites affect cockle (Cerastoderma edule) secondary production and elimination?. Journal of the Marine Biological Association of the United Kingdom 2009;89:1395–1402.
- Bhaby S, Belhsen OK. Seasonal dynamics of parasites on Mediterranean mussels (Mytilus galloprovincialis) and ecological determinants of the infections in Southern Alboran area, Morocco. International Journal of Parasitology Research 2013;5:116-21.
- Bhaby S. Mytilus galloprovincialis: Reproductive cycle of fields mussels close to a lagoon (North Atlantic, Moulay Bousselham, Morocco). J Mar Biol Oceanogr 2015:4.
- Kovacic I, Pavicic-Hamer D. Mytilus galloprovincialis (Lamarck, 1819) as host of Mytilicola orientalis (Mori, 1935) in the northern Adriatic Sea: presence and effect // Aquaculture International 2017;25:211-21.
- Villalba, A, Mourelle SG. Symbionts and diseases of farmed mussels Mytilus galloprovincialis throughout the culture process in the Rías of Galicia (NW Spain). Diseases of Aquatic Organisms 1997;31:127-39.
- Bignell JP, Stentiford GD. Histopathology of mussels (Mytilus sp.) from the Tamar estuary, UK. Marine Environmental Research 2011;72:25-32.
- Cherfaoui N, Doghmi H. Résiliences, système portuaire du Maroc de la naissance à 2060, ciences de l’ingenieur. 227-79.
- Rayyan A, Chintiroglou C. Steinhausia mytilovum in cultured mussels Mytilus galloprovincialis in the Thermaikos Gulf (northern Aegean Sea, Greece). Diseases of Aquatic Organisms 2003;57:271-3.
- Carballal MJ, Iglesias D. Parasites and pathologic conditions of the cockle Cerastoderma edule populations of the coast of Galicia (NW Spain). J Invertebr Pathol 2001;78:87–97.
- Minguez L, Giambérini L. Seasonal dynamics of zebra mussel parasites. Aquat. Biol 2012;15:145-51.
- Figueras AJ, Jardon CF. Diseases and parasites of mussels (Mytilus edulis, Linneaus, 1758) from two sites on the east coast of the United States. Journal of Shellfish Research 1991;10:89–94.
- Bower SM. Diseases and parasites in mussels. In: E. Gosling (ed.) The Mussel Mytilus: Ecology, Physiology, Genetics and culture. Elsevier Press, Amsterdam 1992: 543-63.
- Robledo JAF, Santarém MM. Parasite loads of rafted blue mussels (Mytilus galloprovincialis) in Spain with special reference to the copepod, Mytilicola intestinalis Aquaculture 1994;127:287-302.
- Bower SM, McGladdery E. Synopsis of infectious diseases and parasites of commercially exploited shellfish 2003.
- Criado-Aldeanueva F, Javier Soto-Navarro F. Seasonal and interannual variability of surface heat and freshwater fluxes in the Mediterranean Sea: budgets and exchange through the Strait of Gibraltar. Int. J. Climatol 2010;32: 286-302.
- Spiers ZB, Bearham D. Intracellular ciliated protozoal infection in silverlip pearl oysters, Pinctada maxima (Jameson, 1901). Journal of Invertebrate Pathology 2008; 99:247-53.
- Sabry RC, Da Silva PM. Pathological study of oysters Crassostrea gigas from culture and C. rhizophorae from natural stock of Santa Catarina Island, SC, Brazil. Aquaculture 2011;320.
- Azevedo C, Cachola R. Fine structure of the Apicomplexa oocyst of Nematopsis sp. of two marine bivalve mollusks. Diseases of Aquatic Organisms 1992;14:69-73.
- Lauckner G. Diseases of Mollusca: Bivalvia. In: Kinne O (Eds) Diseases of marine animals, Vol II. Introduction, Bivalvia to Scaphopoda. Biologische Anstalt Helgoland, Hamburg 1983;2:477–9.
- McGladdery SE, Bower SM. Diseases and parasites of scallops. In: Shumway S.E, Parsons G.J (Eds.), Scallops: Biology, Ecology and, Aquaculture 2006;595-650.
- Claire F, Hermida M. Parasites and Symbionts from Mytilus galloprovincialis (Lamark, 1819) (Bivalves: Mytilidae) of the Aveiro Estuary Portugal. Journal of Parasitology 2010;96:200-5.
- Auffert M. Bivalve hemocyte morphology. Am Fish Soc Spec Publ 1988;18:169-77:
- Bouck L, Thistle D. Responses of two morphologically similar species of benthic copepod (Harpacticoida, Dioosaccidae) to an erosion rate that occurs during winter storms. Vie Milieu 2006;56:9-14.
- Boehs G, Villalba A. Parasites of three commercially exploited bivalve mollusc species of the estuarine region of the Cachoeira River (Ilhéus, Bahia, Brazil). Journal of Invertebrate Pathology 2010;103:43-7.
- McGladdery JR, Arthur P. Aquaculture in the Third Millennium. Technical Proceedings of the Conference on Aquaculture in the Third Millennium, Bangkok, Thailand, 2000:20-5.
- Spiers Z. The identification and distribution of an intracellular ciliate in pearl oysters, Pinctada maxima (Jameson 1901). PhD thesis, Murdoch University 2008.
- Zizek S, Gombac M. Occurrence and effects of the bivalve inhabiting hydroid Eugymnanthea inquilina in cultured Mediterranean mussels (Mytilus galloprovincialis) in Solvenia. Slov Vet Res 2012;49:149-54.