HONEY AND DATE SYRUP AS NATURAL ALTERNATIVES TO FORMALIN IN THE DEMONSTRATION OF CONNECTIVE TISSUE
2 Department of Medical Laboratory, Pathology Unit, HopeXchange Medical Centre, Kumasi, Ghana
3 Department of Medical Laboratory Science, University of Energy and Natural Resources, Sunyani, Ghana
4 Department of Theoretical and Applied Biology, Kwame Nkrumah, University of Science and Technology, Kumasi, Ghana
5 Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Received: 07-Jan-2023, Manuscript No. pulhhr-23-6025; Editor assigned: 10-Jan-2023, Pre QC No. pulhhr-23-6025 (PQ); Accepted Date: Jan 30, 2023; Reviewed: 11-Jan-2023 QC No. pulhhr-23-6025 (Q); Revised: 13-Jan-2023, Manuscript No. pulhhr-23-6025 (R); Published: 06-Feb-2023, DOI: 10.37532/pulhhr.23.7 (1).1-9.
Citation: Bright OA, Albert OS , Michael AK , et al. Honey and date syrup as natural alternatives to formalin in the demonstration of connective tissue. J Histol Histopathol Res Vol 7 No 1 2023; 7(1):1-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background:In anatomical science, formaldehyde (10% buffered formalin) is still accustomed as the gold standard fixative however, as detailed by the Environmental Protection Agency (EPA) and the International Agency for Cancer Research (IARC) the use of formalin causes health hazards due to its carcinogenicity. Hence, we considered to substitute formalin with natural Honey and Date syrup to achieve a formalin free laboratory for preservation of the biological specimens. And this is because honey and date syrup have been used for millennia as preservatives and antibacterial agents. Research has demonstrated that honey and date syrup have acidic and dehydrating characteristics.
Aim: The impact of these abilities on tissue fixation was investigated in this study to assess the potential of honey and date syrup as natural alternatives to formalin as fixatives. Methods: Dates and honey were obtained from the local market in Navrongo in the Upper East region of Ghana and Kumasi in the Ashanti region of Ghana respectively for the study. Varying concentrations of 45%, 65% and 85% of honey and date syrup were prepared after the glucose, fructose concentration and pH of honey and date syrup were determined. Albino rats were anesthetized by using chloroform vapor to produce unconsciousness. In the state of sleep, each albino rat was placed in a horizontal position, and deep incisions were made and heart, kidney, liver and muscles were carefully harvested and washed in 10% formalin, Honey and Date syrup respectively. The tissues were fixed in the honey and date syrup concentrations with 10% formalin as control for 12 hours and prolonged observation made for 10 working days. The fixed tissues were observed for tissue integrity after staining and scored by a panel.
Result: The findings of the study indicate the higher concentrations of 65% to 85% honey and date syrup are suitable for long term gross preservation of tissues and organs as their efficacy does not vary significantly (P>0.05) from 10% formalin. Lower concentrations of 45% give excellent tissue staining characteristics.
Conclusion: Honey and date syrup at low concentrations (45% and 65%) can serve as alternatives help to decrease and abolish the use of formalin in the near future.
Key Words
Formalin; Fixation; Honey; Date syrup; Tissue fixative; Occupational hazard
Introduction
Any intrinsic Preservation of the anatomical specimen in its natural state is a high goal for all histological and cytological examinations. This procedure comprises a series of chemical reactions that prevent cell and tissue degradation, as well as autolysis and putrefaction. In diagnostic pathology, the fundamental and overarching purpose of tissue fixative is to preserve cells and tissue components [1]. Fixation alters the tissue on a molecular level, retaining structure but also hardening it, making sectioning and staining easier, according to Srii. Over the last century, there have been no huge advancements in the field of tissue fixation. Formaldehyde (10% buffered formalin) is the gold standard fixative used in anatomical pathology laboratories for regular diagnostic pathology and immunohistochemistry. It is widespread, easy to utilize, and can be used in a variety of tissues. It is also cheap, widely acknowledged worldwide and takes less time to get ready. According to Srii [2], the Environmental Protection Agency (EPA) and the International Agency for Cancer Research (IARC) have recognized formalin as a potential human carcinogen (IARC). Inhalation of formaldehyde by repeated or extended inhalation of the mucous membrane of the eyes, nose, or upper respiratory system in an occupational setting poses a health risk. There have been attempts to generate non-toxic, safer substitutions by substituting less harmful chemicals for formaldehyde. There are no aldehyde components in non-formalin fixatives; therefore, there is no risk of toxicity. Because of their favourable characteristics, natural alternatives now have more potential. According to the study, the antibacterial qualities of honey and date syrup are acidic and dehydrating. In addition to its injury cure and antimicrobial capabilities, research has highlighted the antiautolysis and tissue strengthening properties of honey and the autopreserving nature of date syrup [3]. Meat was kept with honey for several days in ancient Rome [2]. Piatek-Koziej, expounded on the effects of natural sweetener solutions on the size, weight, and form of cardiac tissue samples over time, up to a period of one year of observation. These solutions included 10% ethanol base honey solutions and 10% water base honey solutions. In the current study, we used honey and date syrup as fixation elements for preserving specimens of tissue. We explored their cellular properties using different concentrations of honey and date syrup and stains. This study made use of naturally accessible components such as honey and date syrup as alternatives to formalin, to address the toxicity of formalin [4].
Materials and Methods
Ethics statement
The study protocol was reviewed and approved by the Committee for Human Research and Ethics (CHRE) of School of Science, Department of Basic and Applied Biology, University of Energy and Natural Resources (UENR) Sunyani, Ghana (CHRE/ AP/045/22).
Study area
The research was carried out in the Department of Pathology, HopeXchange medical Centre, Kumasi on Latitude 6.6608089°N and Longitude 1.6561068°W with Digital Address: AK-W362-2858 and the department of Medical Laboratory Technology, Garden City University College, Kenyasi, on Latitude 6.7392°N and Longitude 1.5652°W with Digital Address: AK-391-4815 to investigate the efficacy and potency of some concentrations of honey and date syrup solution in the fixation of some connective tissues.
Study design
Experimental study and purposive sampling from February to March 2022. Connective tissues (heart, kidney, muscle, and liver) were fixed in honey (45%, 65%, 85%), date syrup (45%, 65%, 85%) and neutral buffered formalin (10%), respectively and dehydrated in an alcoholic environment subsequent to decolorization and deparaffinization using xylene. Deparaffinized sections of the date and honey fixed tissues were stained with hematoxylin and eosin. The formalin-fixed tissue was de-alcoholarised using standard xylene processing subsequent to deparaffinization with xylene and staining with hematoxylin and eosin would be done.
Procurement of honey and date
The forest honey (traditionally obtained honey from forests) was obtained from Navrongo in the Upper East region of Ghana. The quality of the honey was verified by dropping it on a surface and examining its flatness and spreading, as well as burning it following the light match test according to Abubakar [5]. The dates were obtain-ed from Alabar (a traditional market), in Kumasi in the Ashanti region of Ghana. Forest and old date fruit (date fruit collected in the traditional manner and left to ripen) were purchased.
Laboratory procedure
Extraction of date syrupe
The process of extraction entailed combining pitted unpitted dates with an equal amount of water in an open pot and heated to boiling, and then sifting. The remaining date on the filter was boiled for a secondary extraction to obtain more juice. The juice was concentrated with further heating and evaporation to obtain the date syrup. Date syrup is similar to date juice in terms of characteristics, but it is more concentrated. One of the most popular date derivatives is date syrup, often known as "rub" or "dibs," which is created by boiling and evaporating date juice.
Honey and date syrup pH
The pH of the date syrup and honey was determined using a pH meter. Orion Star A211 instruments with a pH range of -2 to 20, and a precision of 0.002). The manufacturer's instructions were followed as SOP using 10 g of date syrup and honey, respectively
Glucose content
This was accomplished using the A.Kruss optronic GmbH Refractometer, which is based on the light refraction through liquid principle. The glucose concentration was determined in D (refractive index scale) or g/dl after the absorbance was measured at 589 nm within 4 seconds. The result was recorded for honey and date syrup.
Experimental specimens and organs
Fresh tissues of kidney, lung, heart, liver, and muscle were obtained from five laboratory rats, which were purchased from the College of Science, Department of Biochemistry and Biotechnology, Kwame Nkrumah University of Science and Technology, Kumasi, and kept in a well-structured metal cage at the department of medical laboratory technology, Garden City University College, Kenyasi-Kumasi. The animals were well fed with animal feed before the day of animal sacrifice.
Animal sacrifice
The laboratory rats were anesthetized by using chloroform vapor. Longitudinal abdominal incision was made and fresh organs were carefully harvested.
Tissue preparation
The tissues for this study were obtained from the organs harvested from the laboratory rats and included heart, muscle, kidney and liver tissues. Only soft tissues measuring 0.5 cm×1 cm or greater and having a thickness ranging from 1.5 mm to 3 mm were labelled and taken for further processing. A total of 30 fresh soft tissue samples were obtained for the study. Each tissue specimen was cut into five equal sections (30×5=150 total bits). The tissue samples were sorted into three groups, each having 50 tissue samples: Group A, Group B, and Group C. Tissues in Group A were fixed in different concentrations of honey (45%, 65% and 85% respectively), Group B tissues were fixed in date syrup, and Group C fixed in formalin.
Preparation of fixatives (working solution)
Group A: Varied concentration of honey (45%, 65% and 85%) with pH: 6.09- varied concentrations of honey 45ml, 65ml and 85ml was mixed with 55ml,35ml and 15ml of distilled water respectively. Group B: Varied concentration of date syrup (45%, 65% and 85%) with pH: 4.1-varied concentrations of date syrup 45 ml, 65 ml and 85 ml was mixed with 55 ml, 35 ml and 15 ml of distilled water respectively
Group C: 10% neutral buffered formalin (7.0-7.4)-10 ml NBF mixed with 90 ml of distilled waterand the tissues were immediately washed in 10% of honey, 10% date syrup and 10% formalin respectively. Washed tissues were immersed immediately in the varied concentrations of honey and date syrup (45%, 65% 85% and 10%) neutral buffered formalin following removal from albino rat.
Gross fixation of tissues
The albino rat was dissected and immediately photographed. The tissues of the albino rat were harvested and washed with 10% pure honey, 10% date syrup and 10% formalin, respectively. The washed organs from respective fixatives were then fixed in (45%, 65%, 85%) pure honey, (45%, 65%, 85%) date syrup and 10% buffered formalin. The tissues were assessed after durations of 12 hours, 24 hours, 48 hours, 3 days, 1 week and 2 weeks.
Fixation of histological tissue processing
A total of 30 fresh soft tissue samples were obtained for the study. Each tissue specimen was cut into five equal sections (30×5=150 total bits). The tissue samples were sorted into three groups, each having 50 tissue samples: Group A, Group B, and Group C. Tissues in Group A were fixed in honey of varied concentrations (45%, 65% and 85% respectively) for 14 days, Group B tissues were fixed in date syrup of varied concentrations (45%, 65% and 85% respectively) while the tissues of Group C were fixed in 10% buffered formalin for 14 days, all tissues were grossed and cut into tiny bits for tissue processing after the 14 days fixation (Tables 1 and 2).
TABLE 1.Tissue processing schedule
| Stage | Reagent | Duration |
|---|---|---|
| 1 | 70% Alcohol | 45 min |
| 2 | 80% Alcohol | 45 min |
| 3 | 90% Alcohol | 45 min |
| 4 | 100% Alcohol (absolute alcohol) | 1 hr |
| 5 | 100% Alcohol (absolute alcohol) | 1 hr |
| 6 | 100% Alcohol (absolute alcohol) | 1 hr |
| 7 | xylene | 1.30 hr |
| 8 | xylene | 1.30 hr |
| 9 | Infiltration and impregnation (paraffin wax) | 45 min |
| 10 | Infiltration and impregnation (paraffin wax) | 45 min |
| 11 | Infiltration and impregnation (paraffin wax) | 1 hr |
| 12 | Infiltration and impregnation (paraffin wax) | 1.30 hr |
TABLE 2. Haematoxylin and eosin staining schedule
| Stage | Regeant | Duration |
|---|---|---|
| 1 | Deparaffinize and rehydrate sections | 30 sec |
| 2 | xylene | 4 min×3 min |
| 3 | 100% ethanol | 2 min×3 min |
| 4 | 95% ethanol | 1 min×3 min |
| 5 | 80% ethanol | 1 min×3 min |
| 6 | 70% ethanol | 1 min×3 min |
| 7 | Deionised water | 1 min×3 min |
| 8 | Harris Hematoxylin | 1 min×3 min |
| 9 | Tap water | 1 min×5 min |
| 10 | 1% Acid alcohol | 1 min×30 sec |
| 11 | Tap water | 1 min×5 min |
| 12 | 70% alcohol | 1 min×3 min |
Statistical analysis
Data was entered into Microsoft Excel 2016 and analyzed using GraphPad Prism v5 (GraphPad software, San Diego, USA). Homogeneity analysis was done to identify how equivalent various parameter are. One-way and Two-way ANOVA analysis was carried out at 95% confidence level coupled with Bonferroni Post-Hoc analysis when significant difference (P<0.05) was detected.
Results
The three fixatives used were honey, date syrup and formalin. The six criteria used to assess the fixed sections include nuclear details, cytoplasmic details, nuclear staining, cytoplasmic staining, clarity and morphology. Two independent examiners observed the obtained biopsied samples under digital microscope. Graph pad prism v.5 statistics was used to compare the inter-observer variability.
Physicochemical qualities of honey and date syrup
The honey and date syrup used in the study was good with deep amber and dark brown colors respectively. The pH of honey was recorded to be 6.09 while that of date syrup was 4.1 at temperature of 26°C. The honey used for the study had glucose content of 75.18% and fructose content of 83.99% whereas date syrup recorded glucose content of 74.64% and fructose content of 74.63% as shown in Table 1. The statistical analysis of the data indicates there is no significant difference (P>0.05) in the physicochemical properties of the honey and date syrup used for the fixation of the various tissues obtained from the sacrificed animals (Table 3).
TABLE 3 Physicochemical properties of honey and date syrup
| Parameter | Honey | Date syrup | p-value |
|---|---|---|---|
| Glucose content | 75.18%a | 74.64%a | 0.264 |
| Fructose content | 83.99%a | 74.63%a | 0.264 |
| Refractive index | 1.474a | 1.473a | 0.264 |
| Temperature | 26°Ca | 26°Ca | 0.264 |
| Color | Deep amber | Dark brown | |
| pH | 6.09a | 4.1a | 0.264 |
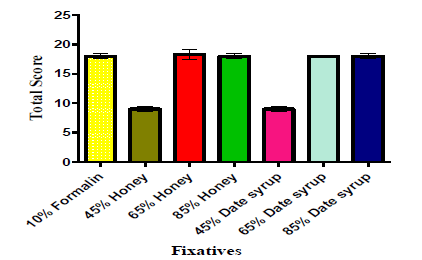
Gross fixation study
Figure 1, shows the impact of different concentrations of honey, date syrup and 10% buffered formalin on gross fixation. Tissues fixed in 45% honey and date syrup gave moderate fixation with total score of 9(50%) after 1 week and was significantly different (P<0.05) from the standard 10% formalin. Tissues fixed in 65%, and 85% honey and date syrup gave good preservation for up to 2 weeks with total score of 18 (100%) similar (P>0.05) to the formalin-fixed counterparts.
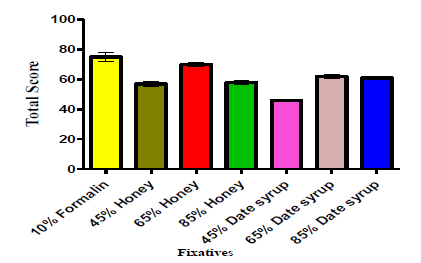
Microscopic study
Figure 2 shows the staining properties of tissues fixed in different concentrations of honey and date syrup. The 65% and 85% concentrations of both honey and date syrup gave good intense and clear nuclear and cytoplasmic staining with good preservation of tissue morphology with total scores of 70 (97.2%), 62 (86.1%) and 58 (80.6%), 61 (84.7) respectively. The 45% gave moderate intense and clear nuclear and cytoplasmic staining with moderate preservation of tissue morphology with total scores of 57 (79.2%) and 46 (63.9%) respectively. The differences in staining properties were not statistically significant (p=0.210) when compared with the formalinfixed counterparts for 65% and 85% date and honey fixatives. However, there were statistically significant differences (p=0.0026) when date syrup at 45% were compared with 10% formalin for clarity and morphology.
Photomicrographs
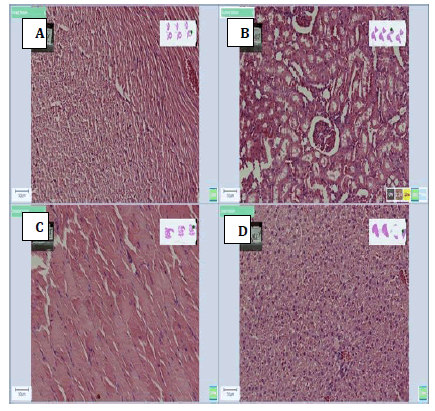
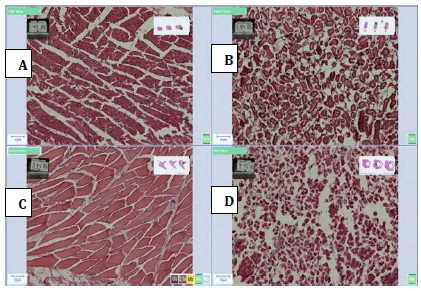
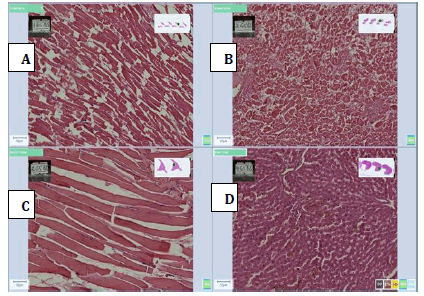
The photomicrographs are shown in Figures 3-9 (A-D). Figure 3 shows sections of heart, kidney, muscle and liver tissue fixed with 10% formalin.
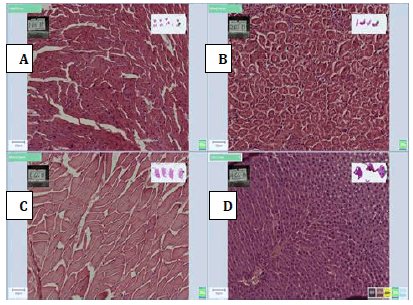
Figure 4: Photomicrographs of 45% Honey, (A) Heart, (B) Kidney, (C) Muscle and (D) Liver tissue sections stained with H and E method, (X 20) showing moderate cellular morphology with proper nuclear and cytoplasmic details with liver tissue and showing poor cellular morphology, nuclearand cytoplasmic details for heart, kidney and heart tissue but the good nuclear and cytoplasmic staining.
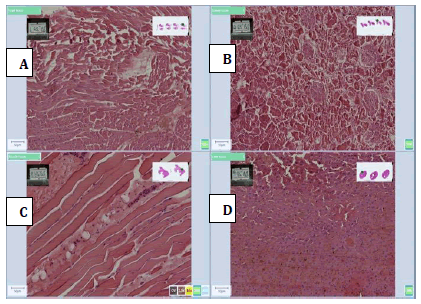
Figure 7: Photomicrographs of 45% Date syrup, (A) Heart, (B) Kidney, (C) Muscle and (D)Liver tissue sections stained with H and E method, (X 20) showing good cellular morphology with moderate nuclear and cytoplasmic details as well as good nuclear and cytoplasmic staining with A and C whilst poor cellular morphology with poor nuclear and cytoplasmic details as well as nuclear and cytoplasmic staining with B and D.
Figure 8: Photomicrographs of 65% Date syrup, (A) Heart, (B) Kidney, (C) Muscle and (D) Liver tissue sections stained with H and E method, (X 20) showing good cellular morphology with moderate nuclear and cytoplasmic details as well as good nuclear and cytoplasmic staining with A, C and D whilst poor cellular morphology with poor nuclear and cytoplasmic details as well as nuclear staining with B but then good cytoplasmic staining.
Figure 9: Photomicrographs of 85% Date syrup, (A) Heart, (B) Kidney, (C) Muscle and (D)Liver tissue sections stained with H and E method, (X 20) showing good cellular morphology with good nuclear and cytoplasmic details as well as good nuclear and cytoplasmic staining with C and D whilst poor cellular morphology with poor nuclear and cytoplasmic details as well as nuclear staining with B but then good cytoplasmic staining. Also, good nuclear and cytoplasmic staining for A
Discussion
Formalin’s usage as a fixative and embalming fluid cannot be disregarded because there have been no safer and better alternatives in our environment [6]. The primary benefit results from its nearly universal use for more than a century and all the acquired scientific information about it [7]. Additionally, formalin is inexpensive, easy to store, enables for long-term preservation, and retains lipids. Since no obvious "all-purpose" substitute has yet been discovered, it has come to be considered as the fixative that comes the closest to being ideal.[8,9]. Formalin, on the other hand, has two well-known drawbacks. To begin with, formalin is extremely toxic. Formaldehyde is classified as a human carcinogen by the IARC. International Agency for Research on Cancer (IARC) and has been linked to nasopharyngeal cancer. For the carcinogenesis of inhaled formaldehyde in respiratory nasal epithelium, Lu et al. found substantial data that can support a genotoxic and cytotoxic mechanism of action [10].
Here is a list of the different health risks that formalin poses, formalin's detrimental impact on the organ systems and health risks: Skin & mucous membrane ;Irritation of the mucous membranes of the mouth and upper respiratory tract, allergic contact dermatitis, Respiratory system; Sneezing, coughing, laryngospasm, pulmonary edema, temporary reversible decrease in lung function, degenerative diseases, inflammatory and hyperplastic changes of the nasal mucosa, asthma, chronic rhinitis, loss of olfactory functioning, Eye; Irritation, lacrimation, conjunctivitis, Gastrointestinal tract; Irritation, nausea, vomiting, diarrhoea, loss of appetite, burns and ulceration, abdominal pain, gastrointestinal hemorrhage, pharyngeal congestion, chronic pharyngitis ,Cardiovascular system; Tachypnoea, nodal tachycardia, Central nervous system; Dizziness, depression, headaches, sleep disorders, memory loss, convulsions and coma, Haematopoietic system; High serum Alanine-Amino Transferase (ALT), Lower RBC, WBC, platelet and hemoglobin counts, Renal system; Renal failure, Reproductive system; Menstrual disorder, dysmenorrhea and spontaneous abortion [4]. Fixation however maintains tissue proteins so that the tissues can be preserved in a lifelike manner after being removed from the body [11].
In order to enable accurate staining and microscopy, a fixative must adequately retain the components of the tissues. For more than a thousand years, people have used honey as a medication because of its healing and antibacterial characteristics [12]. Honey's antibacterial and antioxidative properties, have been identified to inhibit the growth of a wide variety of bacteria, fungi, protozoa, and viruses, are primarily due to its component’s hydrogen peroxide, low water content, inhibine formed by enzymatic reaction, and phytochemical factors [13]. Honey is essentially concentrated aqueous solution of inverted sugars, but it also contains a very complex mixture of other saccharides, proteins, enzymes, amino acids, organic acids, polyphenols, and carotenoid like substances, maillard reaction products, vitamins and minerals [14]. Carbohydrates constitute about 95% to 97% of the dry weight of honey [15]. Fructose and glucose are the most predominant sugars present and are responsible for most of the physical and nutritional characteristics of honey [16]. Smaller quantities of other sugars are also present such as disaccharides, trisaccharides and oligosaccharides that are formed during the ripening and storage effects of bee enzymes and the acids of honey [17]. Honey's color, aroma, flavor, density, and other physical and chemical characteristics are inherited from the plants from which it is made, while other factors, such as weather and processing, can also affect its composition and characteristics [18]. Honey has long been believed to have antimicrobial qualities and the ability to preserve compounds without having any negative impact on its consumers. A human corpse was steeped in honey to produce the fabled therapeutic compound known as "mellified man," also known as "human mummy confection." Honey was utilized in ancient Rome to extend the shelf life of meat. Because of its high acidity, hydrogen peroxide action, and low water activity, honey has antibacterial characteristics. Honey is an effective approach to turn a human corpse into a mummy due to its high acidity, hygroscopicity, and antibacterial impact [19]. Additionally, honey makes tissue rigid, making its activity comparable to that of other fixatives that work by protein cross-linking [20].
Contrarily, date syrup contains tannins, which are utilized medically as a deterrent and astringent in gastrointestinal problems [21].
According to cultivar, stage of ripening, and water content, the date, which is extremely sweet, makes up between 50% and 88% of the overall weight. Date flesh is mostly composed of sugars about two thirds and water about a fifth. The remaining date weight is made up of a variety of other substances, such as protein, fat, crude fibre, minerals, pectin, ash, crude fibre, polyphenolic various vitamins (particularly vitamin B), tannins, and more. Dates can be processed into variety of goods, including date paste, date syrup, alcohol, animal feed, date powder, several kinds of bread, marmalade, and sweet candies. Date products like DS are utilized in the culinary industry as a substitute to sugar and in the making of drinks and alcohol.
Numerous research examining the composition of DS have found strong antioxidant capacity, which may allude to the scientific basis of date fruit and DS's historic therapeutic use [22]. DS is a rich source of phenolic compounds, which are known to be potent radical scavengers. Due to their ability to oxidize, many phenolic chemicals, including polyphenols and flavonoids, have antibacterial properties. This may explain why date fruit and DS are used medicinally as antimicrobial agents [23].
In applied food microbiology, food science and technology, and cell immunology, the idea of antioxidants as potent scavengers of Reactive Oxygen Species (ROS) has recently attracted a lot of interest. Depending on the environment, interactions, structural changes, and exposure to microbes, polyphenols can either have prooxidant activity or antioxidant activity (radical scavenging and metal chelating activity). In systems that use redox active metals like iron and copper, polyphenols can function as prooxidants. As soon as the ligand for the polyphenol complex binds to Fe3+, the complex is able to reduce the iron to Fe2+ and is oxidized to a semiquinone, which can then be reduced to a quinone by reducing further Fe3+ . Fe2+ is produced when Fe3+ is reduced, and it then participates in the Fenton reaction and results in ROS generation
The Oxygen (O2) produced by bacterial aerobic respiration is necessary for the creation of cellular energy. Hydrogen Peroxide (H2O2) and the Hydroxyl Radical (OH) are two ROS produced when microorganisms respire Oxygen (O2) insufficiently. By producing the anti-ROS enzymes catalase and superoxide dismutase, aerobic respiring bacteria protect themselves from the oxidative stress brought on by the build-up of ROS, such as that brought on by exposure to polyphenols. Catalase then changes (H2O2) into water and oxygen after superoxide dismutase decreases OH to (H2O2).
The complex process of ripening dates involves the breakdown of chlorophyll, the production of carotenoids, the breakdown of the cell wall, and the conversion of starch to sugars [24].
Dates have been found to contain 19 isomeric forms and thirteen flavonoid glycosides of luteolin, quercetin, and apigenin, according to Hong et al. Flavonoids and phenols are the antioxidants that work the best. Due to their ability to chelate metals and act as radical scavengers, phenols are regarded as potent lipid peroxidation inhibitors. An aqueous extract of dates, according to Vayalil, suppresses superoxide and hydroxyl radicals, lipid peroxidation, and protein oxidation through free radical scavenging.
There is a substantial correlation between antioxidant, phenolic, and flavonoid chemicals, according to recent studies on the antioxidant activity and quantities of phenolic and flavonoid compounds in dates. Comparable findings were obtained when tissues were fixed in honey and date syrup at low concentrations in a 1:10 ratio at room temperature with a pH maintained between 4.0 and 7.0. When honey and date syrup are diluted, the glucose oxidase enzyme causes the glucose to be converted to gluconic acid and hydrogen peroxide. One potential mechanism for honey's fixing properties is the conversion of its sugars to gluconic acid. Gluconic acid is an organic acid that is easily biodegradable, non-volatile, non-corrosive, less irritating, slightly acidic, and non-odorous.
Due to its ability to preserve food from spoiling and its antibacterial qualities, glutonic acid is a common preservative in the food and pharmaceutical industries [25].
It was suggested that the phytochemical components in DS may be involved in redox processes, which are mediated by the formation of (H2O2) which result in the prevention of bacterial growth and support the traditional therapeutic use of DS. Another theory is that glucose and fructose are broken down at low pH levels in honey and date syrup to generate aldehydes, which then cross-link with amino acids in the tissue to cause fixation of the tissues. This reaction is similar to that of formaldehyde [26]. This mechanism and the HepesGlutamic Acid Buffer-Mediated Organic Solvent Protection Effect (HOPE) technology are fairly comparable. Then, similar to formaldehyde, these aldehydes crosslink with tissue amino acids, enabling fixation to take place [26].
Sabarinath used a 10% water-based honey solution as a fixative for tiny pathomorphological samples taken from the human oral cavity in their double-blind pilot investigation [27]. They came to the conclusion that this readily available, non-toxic solution could be employed as a nuclear fixative and was an excellent substitute for formalin. Similar outcomes were attained by Patil (2 who discovered that an aqueous solution of 30% jaggery and 20% honey effectively fixed goat buccal mucosa [27]. These tissues, which were submerged in the fluid for six months, displayed vitality when processed histologically using specific stains like hematoxylin and eosin [11]. A 10% water-based honey fixative was also recommended by Ozkan as a risk-free substitute for formaldehyde in the histopathological processing of relatively short segments from various types of human tissue (endometrium, breast, placenta, uterus, omentum, stomach and lung) [28]. The findings show that DS contains secondary metabolites that are linked to bioactive behaviour, in contrast to other research on date fruit and DS [29-31]. The concentration of redox active phenolic compounds and H2O2 was correlated with the degree to which DS and DS polyphenols inhibited bacterial growth. These findings corroborate the claim that the structural interaction between these bioactive chemicals, rather than an osmotic impact of sugars alone, is what causes the growth inhibition. This raises the notion that the redox active phenolic component found in DS, date fruit, and other antioxidant-rich fruits are key players in the degrading effects on microorganisms. In our study, satisfactory results were obtained with little to no statistically significant differences observed with tissue fixed in honey and date syrup, in comparison with the formalin fixed tissue and, the staining duration.
Since there is no current literature on the use of date syrup as a substitute for formalin, our endeavour is the first of its kind. Honey contains various artefacts, may including viable spores such as clostridia which may result in false positive reactions. In the present study, the honey used did not result in any such artefacts. Due to its potential toxicity, formaldehyde usage is all but prohibited in developed nations. In addition, formalin has been identified as a possible human carcinogen by two reputable organizations, the U.S. EPA and the International Agency for Research on Cancer. When combined with the recognized health risks, the use of formaldehyde should end because aspiration devices are not commonly utilized and there may be no safe way to dispose of toxic waste in developing nations. Given the aforementioned information, it is now time to consider a natural alternative like bee honey, which is readily available, simple to prepare, safe for the environment, and non-toxic. Its action is similar to that of formalin and produces results that are comparable, helping to eliminate formalin in the near future [32]. Having a small sample size and using biopsy specimens with tissues as a sampling method are shortcomings of this study. It would be better to conduct extensive research to compare the effectiveness of honey with the commonly used formalin and eventually phase out the usage of formalin. This would require a larger sample size and specimens with different tissues from the spleen and lungs. This study currently promotes using honey as a formalin alternative while highlighting its beneficial qualities.
At first, we made an effort to standardize the dilution of honey and date syrup by employing several concentrations (45%, 65%, and 85%). Higher concentrations were found to cause tissue shrinkage and loss of tissue architecture and to be ideal for large-scale and longterm tissue fixation, whereas lower concentrations could not penetrate tissues as quickly, had less preservative potential, and were perfect for both short-term fixation and small tissue samples. The best outcomes were obtained with a mixture of 65% honey and 85% date syrup. Studies done in the past on honey have demonstrated that tissues can be fixed by 10%, 20%, 50%, 70%, 90%, and 100% at both low and high concentrations [15]. Therefore, for our investigation, we took into consideration 45%, 65%, 85%, Honey and date syrup. The characteristics of the potential fixation mechanism by Honey and Date Syrup are comparable [13, 33, 34]. The pH must be acidic for this. The pH of the two drugs ranged from 4.1 to 6.09 at the doses we utilized, supporting this idea;
Conclusion
All the two natural substances: honey and date syrup gave promising results. But date syrup exceeded our expectations, even being at par with proven honey. There are several advantages of using honey and date syrup for tissue fixation: they are non- hazardous, compatible with routine processing, staining and do not require additional equipment. The natural substitutes can be used where formalin may not be available on time of biopsy and also in large scale, as in screening camps. All these natural substances are liable to develop molds over time; hence it is advisable to use thymol crystals as an antimicrobial agent. In addition, Date fixed specimen showed brownish discoloration. Nevertheless, there was no interference with subsequent staining. Some of the problems faced with usage of these natural fixatives during tissue handling and their remedy are enlisted in here; Immiscibility of natural fixatives with distilled water; Constant stirring of solution, Stickiness of natural fixatives to the tissue surface and difficulty in rinsing it off; Thorough washing under running tap water. It is heartening to know that some of the commonly available, day to day substances give pleasant surprises! Fixation of tissue by honey and date syrup is an innovative attempt. Natural fixative is the arena which requires further exploration and large-scale implementation.
Considering the novel qualities of honey and date syrup, it could be concluded that higher concentrations of 65% to 85% honey are suitable for long term gross preservation of tissues and organs. Lower concentrations of 45% give excellent tissue staining characteristics. Honey and date syrup at low concentrations (45% and 65%) can serve as alternatives help to decrease and abolish the use of formalin in the near future.
Acknowledgement
This work was supported by Mr. Chris Yaw Asare and Dr. Larbi for providing me with laboratory and materials for tissue harvesting.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Funding Statement
There was no specific funding for this work. Laboratory support was provided by the HopeXchange medical Centre.
References
- Taleb H, Maddocks SE, Morris RK, et al. The antibacterial activity of date syrup polyphenols against S. aureus and E. coli. Front microbiol. 2016 26;7:198.
- Srii R, Peter CD, Haragannavar VC, et al. Bee honey as a safer alternative for routine formalin fixation. Kathmandu Univ Med J. 2017; 60(4):308-12.
- Lalwani V, Surekha R, Vanishree M, et al. Honey as an alternative fixative for oral tissue: An evaluation of processed and unprocessed honey. J Oral Maxillofac Pathol. 2015;19(3):342.
- PiÄ?tek-Koziej K, HoÅ?da J, et al. Fixative properties of honey solutions as a formaldehyde substitute in cardiac tissue preservation. Folia Med. Crac. 2019;59(1).
- Abubakar U, Ishaq A, Okechi OO, et al. Efficacy of Various Concentrations of Honey Solution in the Fixation of Some Selected Tissues in Wistar Rats. Adv Res. (2020);21(7):34–40
- Onyije FM, Avwioro OG. Excruciating effect of formaldehyde exposure to students in gross anatomy dissection laboratory. int j occup environ med. 2012;3(2):92-95.
- Rosai J. Why microscopy will remain a cornerstone of surgical pathology. lab investig. 2007;87(5):403-8.
- Buesa RJ. Histology without formalin?. Ann Diagn Pathol. 2008;12(6):387-96.
- Hopwood D. Fixatives and fixation: a review. Histochem J. 1969;1(4):323-60.
- Lu K, Collins LB, Ru H, et al. Distribution of DNA adducts caused by inhaled formaldehyde is consistent with induction of nasal carcinoma but not leukemia. Toxicol sci. 2010;116(2):441-51.
- Patil S, Rao RS, Ganavi BS, et al. Natural sweeteners as fixatives in histopathology: A longitudinal study. J nat sci biol med. 2015;6(1):67.
- Geiling N. The Science behind honey's Eternal Shelf Life. 2013.
- Al-Maaini R, Bryant P. The effectiveness of honey as a substitute for formalin in the histological fixation of tissue. J Histotechnol. 2006;29(3):173-6.
- Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J agric food chem. 2002;50(21):5870-7.
- Alvarez-Saurez JM, Tulipani S, Romandini S, et al. Contribution of honey in nutrition and human health: a review. Mediterr. J. Nutr. Metab. 2009;1-9.
[Crossref]
- Sato T, Miyata, G. The Nutraceutical Benefit, Part 111: Honey. Nutr. pharm, 2000;16(6):468-69 [Goggle scholar][Crossref]
- Ball DW. The chemical composition of honey. J. chem. educ. 2007;84(10).
- Yuksel P, Saribas S, Bagdatli Y. Comparison of the VersaTrek and BACTEC MGIT 960 systems for the contamination rate, time of detection and recovery of mycobacteria from clinical specimens. Afr J Microbiol Res. 2011;5(9).
- Srii R, Marla V. Bee honey as a locum for routine formalin fixative. Int J Sci Res. 2016;5:498-500.
- Ramachandran S, Fontanille P, Pandey A, et al. Gluconic acid: properties, applications and microbial production. Food Technol. Biotechnol. 2006 ;44(2).
- Naskar S, Islam A, Mazumder UK, et al. In vitro and in vivo antioxidant potential of hydromethanolic extract of Phoenix dactylifera fruits. J Sci Res. 2010;2(1):144-157.
- Zhang L, Tang X, Rothman N, et al. Occupational exposure to formaldehyde, hematotoxicity, and leukemia-specific chromosome changes in cultured myeloid progenitor cells. Cancer Epidemiol Biomark Prev. 2010;19(1):80-8.
- Daglia M. Polyphenols as antimicrobial agents. Curr opin biotechnol.2012;23(2):174-81.
- Eltayeb EA, Al-hasni AS, Farooq SA. Changes in soluble sugar content during the development of fruits in some varieties of Omani date palm (Phoenix dactylifera). J Biol Sci. 1999.
- Gunter M, Bryant P. Immunocytochemical evaluation of ductal carcinoma in breast after preservation in honey. J Histotechnol.. 2009;32(2):54-9.
- Patil S, Premalatha BR, Rao RS, et al. Revelation in the field of tissue preservation–A preliminary study on natural formalin substitutes. J int oral health. 2013;5(1):31.
- Sabarinath B, Sivapathasundharam B, Sathyakumar M. Fixative properties of honey in comparison with formalin. J Histotechnol. 2014;37(1):21-5.
- Özkan N, Å?alva E, ÇakalaÄ?aoÄ?lu F, et al. Honey as a substitute for formalin?. Biotech. Histochem. 2012;87(2):148-53.
- Al-Farsi M, Alasalvar C, Al-Abid M, et al. Compositional and functional characteristics of dates, syrups, and their by-products. Food chem. 2007;104(3):943-7.
- Dhaouadi K, Raboudi F, Estevan C, et al. Cell viability effects and antioxidant and antimicrobial activities of Tunisian date syrup (Rub El Tamer) polyphenolic extracts. J agric food chem. 2011; 59(1):402-6.
- Abbès F, Kchaou W, Blecker C, et al. Effect of processing conditions on phenolic compounds and antioxidant properties of date syrup. Industrial crops and products. Ind Crops Prod. 2013;44:634-42.
- U.S. Environmental Protection Agency, Office of Air and Radiation. Report to Congress on Indoor Air Quality, Volume II Chapter 4: Assessment and Control of Indoor Air Pollution. 1989;12–27[Google scholar][ Crossref]
- White JW, Doner LW. Honey composition and properties. Beekeep U S Agric Handb. 1980;82-91.
- Henriques A, Burton NF, Cooper RA. Antibacterial activity of selected Portuguese honeys. J Apic Res. 2005;44(3):119-23.