Kidney stones: Mechanism of formation, pathogenesis and possible treatments
Received: 22-Feb-2018 Accepted Date: Nov 25, 2018; Published: 10-Dec-2018
Citation: Gupta S, Shamsher SK. Kidney stones: Mechanism of formation, pathogenesis and possible treatments. J Biomol Biochem 2018;2(1):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
With a very high recurrence rate in both males and females, kidney stones also called as urolithiasis (nephrolithiasis) are affecting a remarkably significant population around the globe. As kidney stones formation largely depends on urine’s oxalate content rather than the calcium concentration of the urine, kidney stones are predominantly made up of oxalate and also there are multiple steps involved in the pathogenesis of calcium oxalate including nucleation, crystal growth, crystal aggregation and crystal retention. Although there are several therapies such as thiazide like diuretic, allopurinol, painkillers, dietary modifications or in severe conditions shock-wave treatment, ureterenooscopic and percutaneous nephrolithotomy are being presently used for kidney stones treatment, however, the side effects that these treatments possess and reoccurrence of the disease have motivated researchers to search for better and safer options. Herbal drugs as well as several medicinal plants display antiurolithiatic activity and thus perform an important role in treatment of kidney stone disease. Studies in both humans and animal models of hyperoxaluria-associated calcium oxalate kidney stone disease indicated that oxalate-degrading microorganisms, in particular the more widely-studied Oxalobacter formigenes are both capable of and important in regulating urinary levels of oxalate. Such regulation of oxalate levels in the body have displayed prominent reductions in hyperoxaluria followed by diminished rate of recurrent stone formation. The present work discusses the mechanism of stone formation, several forms of kidney stones as well as the various therapeutic measures for treatment of urinary stones including many medicinal plants as herbal option as well as oxalate degrading enzyme.
Keywords
Urolithiasis; Hypercalciurea; Hyperoxaluria; Nephrolithiasis; Calcium oxalate; Oxalate degrading bacteria
Introduction
Urolithiasis or kidney-urinary tract stone disease has emerged as a severe health concern throughout the World [1]. With a high recurrence rate in both, males and females as well as affecting around 12% of the population globally, kidney stones or urolithiasis (nephrolithiasis) is marked by the formation of urinary calculi in the urinary tract [2]. In certain parts of the world such as the Middle East countries the hazards of urolithiasis are there is much greater [3]. Because of the inhibiting ability of oestrogen, high capacity of estrogen and greater muscular mass kidney stones are more prevalent in males rather than in females and affects all age groups from less than 1 year old to more than 70 years. Increase in incidence of nephrolithiasis has caused increased morbidity and economic burden across the world. The prime cause of nephrolithiasis is the super saturation of urine with calcium and oxalate that leads to pathological mineralization in the kidneys [4]. Calcium oxalate accounts for the maximum percentage of kidney stones (approximately 75%) while calcium hydroxyl phosphate (brushite or calcium hydroxyapatite) magnesium ammonium phosphate (struvite or triple phosphate), urate and cystine constitute around 50%, (10-20)%, 5% and (1-2)% of the kidney stones respectively [5]. Kidney or urinary stones vary widely in their size from micrometers to several centimetres in diameter and so remain unnoticed most of the times for long periods and are often discovered incidentally on radiography or ultrasound scanning or by painful manifestations [6]. The study of papillary biopsies in patients with kidney stones have demonstrated that on the basis of the type of stone and the urine chemistry, the sequence of events leading to stone formation differ greatly [7]. Hence involving a number of physicochemical events such as super-saturation, nucleation, growth, aggregation and retention within the kidneys development of kidney stone or urolithiasis is a complex process.
In the urinary tract, stones are located at different sites such as the urinary bladder, ureters and the kidneys. Staghorn (filling numerous major and minor calices) and non-staghorn are the two classes into which the kidney stones have been grouped. Although the proximal, middle or distal are the various locations that have been reported for ureteral stones, the nonstaghorn stones are mainly pelvic or calyceal in nature. Small kidney stones of less than 5 mm in diameter possess the largest possibility of being passed out easily followed by stones of 5mm to 7 mm that have about 50% possibility but stones over 7 mm donot pass out on their own and often require urological intervention. While only 10% of the stones in the kidneys have to be removed surgically, 90% of them move out of the urinary tract and as the stone moves down the urinary tract, renal colic or flank pain begins to grow [8]. Although elimination of the kidney stones can be accomplished through various methods such as shock wave lithotripsy, percutaneous nephrostolithotomy, ureteroscopy and open or laproscopic interventions however these therapies exhibit severe side effects are also highly expensive and painful. In this regard several medicinal plants demonstrated effective results in treatment of kidney stones disease.
Mechanism of Kidney Stone Formation
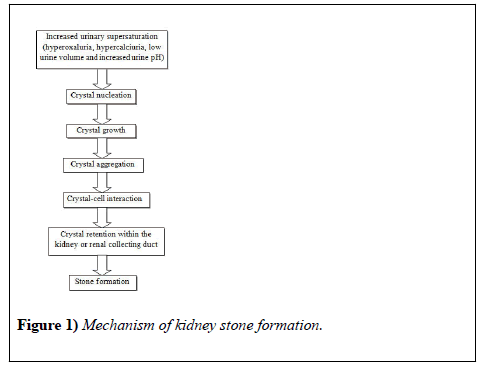
The uneven proportion of inhibitors and promoters are responsible to the complex process of stone formation in kidneys or urolithiasis [9]. Supersaturation of the urine with calcium and oxalate salts followed by crystalline particle formation is the major reason behind kidney stone formation with supersaturation being the driving force [5]. The mechanism involved in the process of stone formation (Figure 1) include nucleation of crystals fractions, growth or gathering of these crystals to a size so that they can interact with some intra-renal structure(s), confinement of these crystals inside the kidney or renal collecting system succeded by further aggregation and/ or secondary nucleation ultimately forming the clinical stone [10]. Calcium oxalate (CaOx) forms the major proportion of the kidney stones that is around 80% [11], while calcium phosphate (CaP) forms a small percentage (15%) of these stones [12]. The crystals formed either in renal tubular fluid or in the renal interstitial fluid that is supersaturated with respect to these constituents, which in turn might be a consequence of reduced urine volume, an alteration in urine pH, mechanisms causing increased secretion of stone forming constituents such as hypercalciuria, hyperuricosuria, hypocitraturia, hyperoxaluria or a combination of these factors [13]. The urine and presumably, the tubular fluid of stone formers are often more highly supersaturated than that of normal healthy adults, which favors nucleation and growth of crystals [10]. Long-term accretion of additional elements, crystalline as well as organic matrix produces the clinical stone. As already explained extreme excretion of calcium or oxalate either alone or in combination as well as low volume of urine leads to an enhanced calcium oxalate supersaturation. The reciprocal actions between genetic susceptibility and environmental factors in different proportions promote both hypercalciuria and hyperoxaluria. A few genetic disorder which a rare autosomal recessive disorder, dietary routine including greater intake of oxalate and low calcium intake, potential abnormalities of anion transporters found in both gut and kidney, enhanced absorption of oxalate in the intestines other than mal absorptive diseases (enteric hyperoxaluria), changes in the normal flora of the gut thereby decreasing degradation of oxalate in the colon may cause one form of hyperoxaluria called primary hyperoxaluria types 1 and 2 [14]. Some rare genetic disorders such as adenine phosphoribosyltransferase (APRT) deficiency, cystinuria, and Dent disease, familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) and primary hyperoxaluria (PH) may lead to severe or chronic kidney stone diseases in children [15]. The above mentioned processes in humans are influenced by several factors known as promoters and inhibitors that either support or prevent the process of stone formation thus affecting a person’s ability to promote or prevent stone formation. Stone formation is facilitated by promoters while inhibitors prevent it (Table 1). Among the promoters of calcium oxalate stones are high oxalate, sodium, calcium, urate, low urione pH and low urine volume while the inhibitors include organic substances such as nephrocalcin and urinary prothrombin fragment-1, osteopontin and various inorganic substances such as citrate, magnesium [5].
| Components | Pathophysiological mechanism | Reference |
|---|---|---|
| Promoters: | ||
| Uric acid or urate | Has an Antagonistic effect on substances in the urine. Promotes heterogeneous nucleation by uric acid or monosodium urate. Binding of calcium oxalate to cells is increased. |
[24] [25] |
| Urine pH | Highly acidic pH enhances calcium oxalate crystallization. Formation of uric acid crystals is enhanced. Promotes secondary nucleation of calcium oxalate by formation of calcium phosphate precipitates. |
[10] |
| Urine volume | Promotes crystallization | [2] |
| Hypercalciuria | Increased intestinal calcium absorption which may be 1, 25-dihydroxyvitamin D [1,25(OH)2D] dependent or independent. Decreased renal calcium reabsorption. Increases movement of calcium from bones. Enhances urine supersaturation and hence crystallization of calcium. |
[26] [13] |
| Hyperoxaluria | Because of an innate error in metabolism oxalate overproduction occurs. Increased dietary intake and bioavailability Increased intestinal oxalate absorption. Urinary supersaturation and formation of calcium oxalate crystals. |
[27] [13] |
| Inhibitors: | ||
| Alkaline pH | Inhibits cystine and uric acid stone formation | [10] |
| Citrate | Forms complexes with calcium and therefore lower calcium oxalate supersaturation, inhibits aggregation of preformed crystals and attachment of crystals to urinary epithelium. | [28] |
| Pyrophosphate | Formation of both calcium oxalate and calcium phosphate crystals is inhibited. | [29] |
| Phytate | Inhibits calcium crystallization both in the intra-papillary tissue and urine. | [30] |
| Magnesium | Inhibits of growth (and presumably nucleation) of crystals as well as aggregation. While for the attachment of calcium oxalate crystals its supra-physiologic concentrations are required. | [31] |
| Glycoproteins | Inhibits spontaneous nucleation from metastable solutions as well as the growth of preformed crystals. | [10] |
Table 1: Promoters and Inhibiters of stone formation
Major Types of Kidney Stones
There are several locations for the tones along the urinary tract such as the kidneys, ureters and urinary bladder. Kidney stones are categorized as either staghorn (filling numerous major and minor calices) or nonstaghorn. Non-staghorn stones are described as calyceal or pelvic in location, while ureteral stones are defined as proximal, middle or distal. Small stones of the kidney, i.e., less than 5 mm in diameter have a greater possibility of passing out, while this possibility reduces as the size of the stones increases with those of 5 mm to 7 mm having only 50% chance, and the ones over 7 mm cannot pass out and always require urological intervention [7]. With differences in their pathogenesis and composition kidney stones have been categorized into several types.
Calcium stones: Calcium in combination with oxalate, phosphate and also with uric acid constitutes the major proportion of kidney stones [16]. Oxalate being a naturally occurring substance is found in various food sources such as some fruits, vegetables, nuts, and chocolate which exhibit high oxalate levels. It is also produced metabolically by the liver. Normal oxalate level in a healthy adult is around 20-40 mg/d [17]. A number of dietary components such as elevated vitamin D doses, oxalate rich diet, various metabolic disorders and intestinal bypass surgery lead to increased concentrations of oxalate and calcium in the urine. Calcium oxalate and calcium phosphate stones are white, grey or black colored therefore demonstrating radio-opaque appearance, being roughly of 1 cm in diameter and in the radiographs these stones appear as dense and sharply circumscribed structures [18]. Medical conditions such as renal tubular acidosis and hyperparathyroidism demonstrate an association with calcium phosphate stones [19].
Struvite stones: A second type comprising about 10-15% of kidney stones are triple phosphate or struvite stones which are formed in presence of bacterial infection and is a crystalline substance made up of magnesium ammonium phosphate. The development of struvite stones is favored by the bacterial enzyme urease that splits the bacteria into ammonia and carbon dioxide thereby making the urine alkaline. Mostly found in humans with certain metabolic diseases including gout, idiopathic hypercalciuria and hyperparathyroidism, the struvite stones are large, glared and laminated [2].
Uric acid stones: Yellow-orange, round and smooth stones made up of uric acid constitute around 5-10% of the kidney stones which in the radiographs appear nearly transparent unless they have been mixed with calcium crystals or struvite [18]. These stones are usually square, diamond or rod shaped, pleomorphic crystals which are polarizable [12]. People with abnormalities such as gout syndrome, obesity or those taking a diet rich in proteins, purines especially ones eating meat and fish possess uric acid kidney stones [20].
Protease-related stones: This type of stone is usually observed in HIVpositive patients using protease inhibitor indinavir sulphate drug. The use of this drug may lead to the formation of such stones in 4-12% of the patients under treatment [21].
Cystiene stones: These stones are caused due to hereditary disorders which cause the kidneys to secrete excessive amino acids (cystinuria) and are rare. With a moderate radio-opaque and rounded appearance these stones are marked with shiny crystallites and are greenish-yellow in color [22].
Silica stones: Medicines as well as herbal products including zonisamide, sulfa medications, indinavir, guaifene sin, laxatives (when abused), acetazolamide, ciprofloxacin, triamterene, ephedrine, loop diuretics, topiramate, and products containing silica promote the formation of such stones which are also known as drug-induced stones [23].
Therapeutic Options and Methods of Prohibition of Kidney Stones
Spontaneous passage of the urinary stones is the only objective of conventional management, the most persistent option for treatment of renal colic [6]. Kidney stones can be prevented and treated by drinking plenty of water, eating vegetarian diet and a diet heavy on herbs [2]. The dreadful nature of kidney stones urges for improved treatment options although dietary restrictions with reduced calcium and oxalate intake and greater fluid intake has been one of the most usual method for the inhibition of kidney stones. Several investigations reveal that kidney stones can be prevented by intake of diet rich in vegetables and fruits. Green tea, pomegranate, oregano, parsley, common madder, raspberry, yellow-fruit nightshade, khela, black cumin etc. are the some of the dietary plants that have been observed to be significantly effective in preventing the formation of kidney stones and treating hyperoxaluria [32]. Narcotic analgesic, thiazide like diuretic, allopurinol and potassium citrate are the main drugs which are prescribed as treatment strategy for preventing kidney stones [33]. For patients in pain treatments such as shock-wave therapy, endoscopic management such as both ureterenoscopic and percutaneous nephrolithotomy offer efficient ways to treat stones and provide rapid relief [2], while open surgery or laproscopic treatment is usually performed in combination with the treatment of other diseases e.g., renal pelvic stenosis [6]. Treatment of kidney stones with diuretics and analgesics although is a common approach but their excessive consumption leads to intense side effects while shock waves cause traumatic side effects as well as may lead to infections and formation of residue stone fragments and hence have inspired researchers to move towards natural and safer remedies like oxalate degrading enzymes and herbal medications [18]. Management of the initial processes of stone formation such as the crystallization events is the ideal method for treatment and prevention of urolithiasis. Medicinal plants such as Amaranthus spinosus, Bambusa nutans, Abutilon indicum, Phyllanthus emblica, Cinnamomum bejolghota and Amaranthus viridis play important part in the prevention of kidney stones and exhibit good antiurolithiatic property and therefore due to their clinically manifested antimutagenic, immunomodulatory as well as adaptogenic effects, herbal drugs have generated great curiosity among the people [34].
Oxalate-Degrading Bacteria as new Therapeutic Tools for Preventing Kidney Stones
The discovery of oxalate-degrading bacteria in the human gastrointestinal tract has opened the way to explore several investigations regarding their potential role in reducing the urinary excretion of oxalic acid [35]. By selectively enhancing the natural oxalate degrading microflora of the gut, i.e., Bifidobacterium sp., Bacillus sp., Oxalobacter formigenes and Porphyromonas gingivalis, humans as well as animals can counter hyperoxaluria and oxalate rich diets as has been affirmed by several researches [36]. An oxalate degrading bacterial enzyme namely oxalate decarboxylase from Bacillus subtilis proved to be a possible therapeutic option for treating calcium oxalate stone disease in human. Oxalate decarboxylase breaks down oxalate thereby producing formate and carbon dioxide. Oral therapy of a cross-linked formulation of oxalate decarboxylase has proved to be an effective therapeutic option for hyperoxaluria as it strongly reduced the oxalate content in urine in hyperoxaluria model of mice [37]. Previously, the heterologous expression of this enzyme in Lactobacillus plantarum (L. plantarum) was developed and utilized as a potential probiotic for depletion of intestinal dietary oxalate [38]. For herbivores, transgenic plants expressing fungal oxalate decarboxylase may significantly reduce nutritional stress due to diminished oxalate content in the feed [39,40].
Conclusion
Attributed to a number of factors such as nutritional disorders, genetic disorders and physiological disorders renal urolithiasis or kidney stones is a common pathological condition. The process of stone formation encompasses a number of steps such as crystal nucleation, aggregation, binding to the kidney, more aggregation and secondary nucleation. Although kidney stone formation can be prevented by controlling the dietary intake of oxalate, adequate nutrient intake, also suitable medication and various therapies are available for treating renal stones but re-occurrence of kidney stones as well as the side effects posed by these treatments has become a great issue that has attracted the people to use better and safer medications such as herbal drugs and medicinal plants which play vital roles in kidney stone diseases treatment. Treatment with oxalate-degrading bacteria or purified oxalate-degrading enzymes (isolated from bacterial and fungal sources) could be promising new therapeutic options for patients with hyperoxaluria. Novel and practical techniques can be developed in the near future that can easily analyze the physicochemical process involved in stone formation that will surely be a boon for this field. The above document thereby contributes towards a better understanding of the basis of calcium and non-calcium kidney stones formation, both molecular as well as genetic basis. It will prove to be helpful in giving greater insight in the development of better treatment of kidney stone. Based on the elemental pathophysiological mechanisms of nephrolithiasis, targeted therapy using microbial oxalate degrading enzymes for prevention and treatment of nuclei formation or salt crystallization may prove to be highly successful with or without using herbal formulations.
Conflicts of Interest
Further, the authors have no conflict of interest among themselves at their place of work or with the institution
Acknowledgement
The authors are thankful to the Department of Biotechnology, Himachal Pradesh University, Shimla for providing resources to the authors.
REFERENCES
- Allison MJ, Sidhu H, et al. Materials and methods for treating or preventing oxalate-related disease. United States patent 2004.
- Aray P, Pandey S, Verma V, et al. Kidney stone formation and use of medicinal plants as anti-urolithiatic agents. UJPSR 2017;2(4):43-48.
- Heilberg IP, Schor N, et al. Renal stone diseases: causes, evaluation and medical treatment. Arq Bras Endocrinol Metabol. 2006;50(4):823-31.
- Chhiber N, Sharma M, Kaur T, Singla SK, et al. Mineralization in health and mechanism of kidney stone formation. Int J Pharm Sci Invent. 2014;3(10):25-31.
- Aggarwal PK, Narula S, Kakkar M, Tondon C, et al. Nephrolithiasis: Molecular Mechanism of Renal Stone Formation and the Critical Role Played by Modulators. BioMed Res Int 2013; ID: 292953, 21 pages.
- Fisang C, Anding R, Müller SC, Latz S, Laube N, et al. Urolithiasis-an Interdisciplinary Diagnostic, Therapeutic and Secondary Preventive Challenge. Dtsch Arztebl Int 2015;112:83-91.
- Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol 2010;25:831-41.
- Lingeman JE, Matlaga B, Evan AP. Surgical management of urinary lithiasis. In: Walsh PC, Retik AB, Vaughan ED,Wein AJ, eds. Campbell’s urology. Philadelphia: Saunders, 2006:1431-1507.
- Sathish R, Natarajan K, Nikhad MM et al. Effect of hygrophila spinosa t.anders on ethylene glycol induced urolithiasis in rats. AJPCR 2010;3(4):61-3.
- Ratkalkar VN, Kleinman JG, et al. Mechanisms of Stone Formation. Clin Rev Bone Miner Metab 2011;9(3-4):187-97.
- Mandel NS, Mandel GS, et al. Urinary tract stone disease in the United States veteran population. II. Geographical analysis of variations in composition. J Urol 1989;142:1516-21.
- Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 2005;67:576-91.
- Sakhaee K, Maalouf NM, Sinnott B, et al. Kidney Stones 2012: Pathogenesis, Diagnosis, and Management. J Clin Endocrinol Metab 2012; 97(6):1847-60.
- Coe FL, Evan AP, Worcester E, et al. Kidney stone disease. J Clin Invest 2005;115:2598-608.
- Edvardsson VO, Goldfarb DS, Lieske JC, Lasic LB, Anglani F, Milliner DS, Palsson R, et al. Hereditary Causes of Kidney Stones and Chronic Kidney Disease. Pediatr Nephrol 2013;28:1923-1942.
- Barbasa C, Garciaa A, Saavedraa L, Muros M, et al. Urinary analysis of nephrolithiasis markers. J Chromatogr B 2002;781:433-55.
- Han H, Segal AM, Seifter JL, Dwyer JT, et al. Nutritional Management of Kidney Stones (Nephrolithiasis). Clin Nutr Res 2015;4:137-52.
- Tiwari A, Soni V, Londhe V, Bhandarkar A, Bandawane D, Nipate S, et al. An overview on potent Indigenous herbs for urinary tract infirmity: urolithiasis. Asian J Pharm Clin Res 2012;5:7-12.
- Mirian AB, Ita PH, Schor N, et al. Phyllanthus niruri as a promising alternative treatment for nephrolithiasis. International Braz J Urol 2010;36(6):657-64.
- Hamid M, Mohammad MN, Ghanea L, et al. Evaluation of the Raphanus sativus effect on urinary pH. J of Res in Med Sc 2007;12(2):58.
- Aggarwal A, Tandon S, Singla SK, Tandon C, et al. Diminution of oxalate induced renal tubular epithelial cell injury and inhibition of calcium oxalate crystallization in vivo by aqueous extract of Tribulus terrestris. International Braz J Urol 2010;36(4):480-89.
- Combest W, Newton M, Combest A, Kosier JH, et al. Effects of herbal supplements on the kidney. Urol Nurs 2005;25(5):381-6.
- Umashankar D, Chandra R, Chawla AS, Deepak M, Singh D, Handa SS, et al. High pressure liquid chromatographic determination of bergenin and (+)- afzelechin from different parts of Paashaanbhed (Bergenia ligulata yeo). Phytochem Anal 1999;10(1):44-7.
- Farell G, Huang E, Kim SY, Horstkorte R, Lieske JC, et al. Modulation of proliferating renal epithelial cell affinity for calcium oxalate monohydrate crystals. J Am Soc Nephrol 2004;15:3052-62.
- Pak CY, Sakhaee K, Peterson RD, Poindexter JR, Frawley WH, et al. Biochemical profile of idiopathic uric acid nephrolithiasis. Kidney Int 2001;60:757-761.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW, et al. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2011;79:393-403.
- Holmes RP, Goodman HO, Assimos DG, et al. Contribution of dietary oxalate to urinary oxalate excretion. Kidney Int 2001;59:270- 76.
- Kok DJ, Papapoulos SE, Blomen LJMJ, Bijvoet OLM, et al. Modulation of calcium oxalate monohydrate crystallization kinetics in vitro. Kidney Int 1988;34:346-50.
- Grases F, Conte A, et al. Urolithiasis, inhibitors and promoters. Urol Res 1992;20:86-8.
- Grases F, Isern B, Sanchis P, Perello J, Torres JJ, Costa-Bauza A, et al. Phytate acts as an inhibitor in formation of renal calculi. Front Biosci 2007;12:2580-7.
- Lieske JC, Farell G, Deganello S, et al. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res 2004;32:117-23.
- Nirumand MC, Hajialyani M, Rahimi R, Farzaei MH, Zingue S, Nabavi SM, Bishayee A, et al. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int J Mol Sci. 2018;19:765.
- Moe OW. Kidney stones: pathophysiology and medical management. Lancet 2006;367:333-444.
- Havagiray R, Shashi A, Jain SK, Sabharwal M, et al. Herbal treatment for urinary stones. IJPSR 2010;1:24-9.
- Giardina S, Scilironi C, Michelotti A, Samuele A, Borella F, Daglia M, Marzatico F, et al. In vitro anti-inflammatory activity of selected oxalate degrading probiotic bacteria: potential applications in the prevention and treatment of hyperoxaluria. J Food Sci 2014;79:384-90.
- Miller AW, Dearing D, et al. The metabolic and ecological interactions of oxalate-degrading bacteria in the Mammalian gut. Pathogens 2013;2(4):636-652.
- Campieri C, Campieri C, Bertuzzi V, Swennen E, Matteuzzi D, Stefoni S, Pirovano F, Centi C, Ulisse S, Famularol G, et al. Reduction of oxaluria after an oral course of lactic acid bacteria at high concentration. Kidney Int 2001;60:1097-105.
- Alberta A,Tiwaria V, Paula E, Ganesana D, Ayyavub M, Kujura R, Ponnusamyc S, Shanmugamd K, Sasoe L, Selvama GS, et al. Expression of heterologous oxalate decarboxylase in HEK293 cells confers protection against oxalate induced oxidative stress as a therapeutic approach for calcium oxalate stone disease. J Enzyme Inhib Med Chem 2017; 32:426-33.
- Kesarwani M, Azam M, Natarajan K, Mehta A, Datta A, et al. Oxalate decarboxylase from Collybia velutipes. Molecular cloning and its overexpression to confer resistance to fungal infection in transgenic tobacco and tomato. J Biol Chem 2000;275:7230-8.
- Dias BBA, Cunha WB, Morais LS, Vianna GR, Rech EL, de Capdeville G, Aragão FJL, et al. Expression of an oxalate decarboxylase gene from Flammulina sp. in transgenic lettuce (Lactuca sativa) plants and resistance to Sclerotinia sclerotiorum. Plant Pathol 2006;55:187-193.