Left dominant coronary artery with intramyocardial segment of its circumflex branch
Received: 02-Mar-2023, Manuscript No. ijav-23-6247; Editor assigned: 04-Mar-2023, Pre QC No. ijav-23-6247 (PQ); Accepted Date: Mar 23, 2023; Reviewed: 18-Mar-2023 QC No. ijav-23-6247; Revised: 23-Mar-2023, Manuscript No. ijav-23-6247 (R); Published: 30-Mar-2023, DOI: 10.37532/1308-4038.16(3).249
Citation: Tessema CB. Left Dominant Coronary Artery with Intramyocardial Segment of Its Circumflex Branch. Int J Anat Var. 2023;16(3):271-273.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
During the dissection of the heart of an 86-year-old male cadaver a relatively larger left coronary artery was incidentally detected. This artery divided into anterior interventricular and circumflex branches as usual, and the circumflex branch further divided into SA-nodal branch, left atrial branches, left marginal branch and left ventricular branches. As the circumflex branch coursed through the posterior aspect of the coronary sulcus it became covered by myocardial bridge and tunneled through the myocardium which it exited near the crus. Then it gave branch to the AV-nodal branch, right atrial branch, left and right ventricular branches, and the posterior interventricular artery. The smaller right coronary artery supplied only the sternocostal aspects of the right atrium and ventricle. Since such variant intramyocardial course may be associated with significant acute coronary syndrome or sudden death, every clinician should be aware of myocardial bridges particularly in condition refractory to medical and surgical treatment.
Keywords
Coronary artery; Circumflex artery; Intramyocardial segment; Myocardial bridge
INTRODUCTION
The distribution pattern of coronary arteries, depending on which one of them gives branch to the posterior interventricular artery supplying the posterior interventricular septum, can be classified into right dominance, left dominance or co-dominance (balanced) pattern [1]. As documented by previous studies, right coronary dominance is a common feature followed by left dominance and co-dominance. Right coronary dominance is positively correlated with triple-vessel disease than left dominance and co-dominance patterns and may serve as an independent risk factor for triple-vessel coronary artery disease [1]. On the other hand, the left coronary dominance pattern, which is higher in males than in females, is associated with a high prevalence of stenosis [2]. Though the different dominance types have associations with coronary vascular diseases, the left coronary artery dominance pattern puts a larger area of the myocardium at risk and therefore, carries a higher risk of death and reinfarction than the others [3]. This was consistent with what was previously noted by Veltman et al. 2015 [4].
Myocardial bridging is a congenital variant involving a segment of coronary artery coursing through myocardial wall, in different depth and length, being potentially affected by systolic compression [5]. Despite the wide spread notion that myocardial bridging almost exclusively affects the middle portion of the anterior interventricular (left anterior descending) artery and is rare in the other coronary artery branches, there are well documented evidences indicating that it can affect all major epicardial coronary arteries and any of their branches. Muehrcke DD et al. 2021 [6] reported a case of circumflex (left circumflex) coronary artery completely covered by myocardium in a 52-year-old patient with severe two-vessel coronary artery disease encountered during CABG. Moreover, myocardial bridge involving the left main coronary artery, circumflex artery, and anterior interventricular artery [7]) and severe bridging involving the anterior interventricular, the circumflex and posterior interventricular arteries were also reported [8]. In the majority of the cases myocardial bridging is asymptomatic (clinically silent), but there is evidence that it can be the cause of refractory anginal pain not responding to therapy and/or sudden death. It can also be a cause of technical difficulty during coronary bypass surgery [9]. Broad range of existing data show that myocardial bridging can result in clinical conditions with several possible manifestations like angina pectoris, myocardial infarction, arrhythmias, depressed left ventricular function, left bundle branch block, myocardial stunning, apical ballooning syndrome, early death after cardiac transplantation, and sudden death [10]. It was also implicated that myocardial bridging is protective against accumulation of atherosclerotic plaque in the bridged segment but promotes plaque formation and coronary stenosis proximal to the bridged segment, however, Jiang L et al. 2018 [11] in their study concluded that myocardial bridging might act as a potential protective element against severe obstructive atherosclerosis even in the whole coronary system.
METHOD
During the dissection of the coronary vessels in the heart of an 86-year-old male cadaver who died of cardiovascular accident secondary to hypertension, a smaller right coronary artery and relatively larger left coronary artery with unusual branching and distribution patterns were detected. The arteries were further dissected by carefully peeling off the epicardium and cleaning of the subepicardial fat. The main coronary arteries and their branches were painted red for the purpose of clear visualization and photographs were taken for illustrations.
CASE REPORT
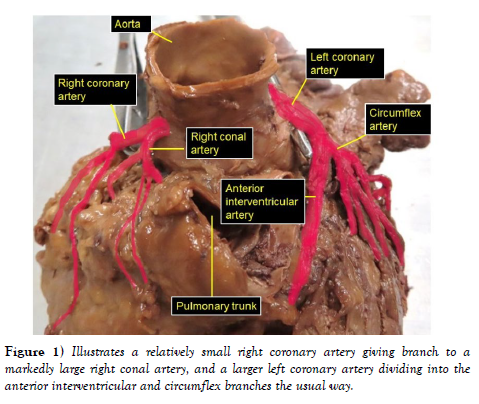
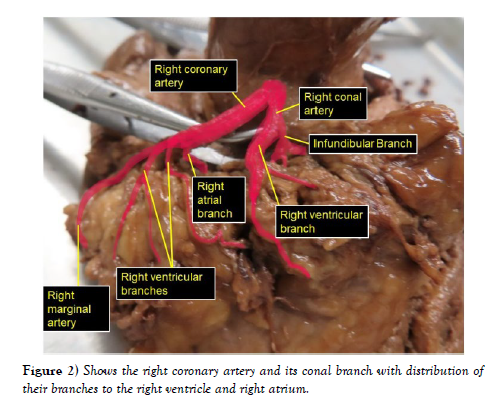
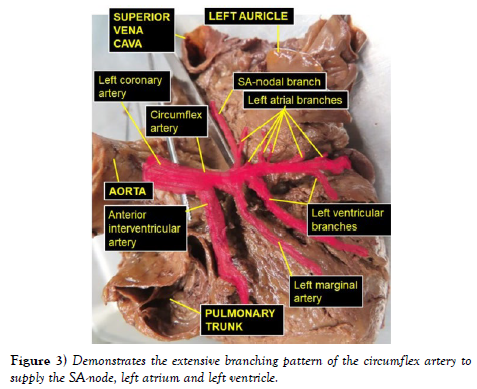
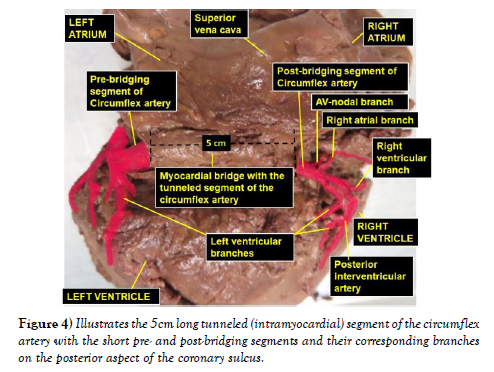
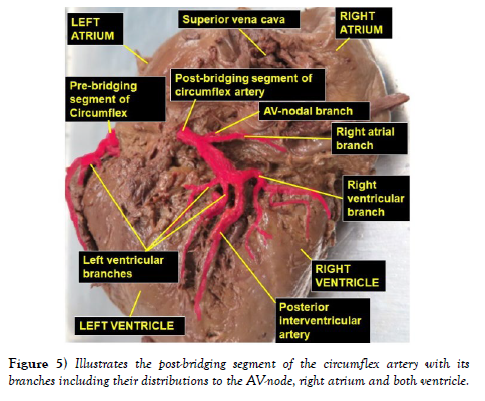
The dissection and cleaning of the vessels revealed a smaller right coronary artery originating from the right aortic sinus and a larger left coronary artery arising from left aortic sinus (Figure 1). The right coronary artery gave branch to an unusually large right conal artery that distributed to the conus arteriosus (infundibulum) and the upper left portion of the right ventricle. Then continued through the right part of the coronary sulcus (atrioventricular groove), gave multiple short right atrial branches in coronary sulcus and ventricular branches that descended on the sternocostal surface of the right ventricle and terminated as the right marginal artery along the right margin of the heart (Figure 2). The larger left coronary artery arose from the left aortic sinus as usual and ran to the left between the pulmonary trunk and the left auricle, where it divided into the anterior interventricular and circumflex arteries (Figure 3). The circumflex artery then coursed through the left part of the coronary sulcus. Before turning around the left margin of the heart, it gave branch to the SA-nodal branch, multiple left atrial branches, left marginal branch and left ventricular branches (Figure 3). The SA-nodal branch ascended on the medial surface of the left atrium dorsal to the ascending aorta to enter the right atrium at the root of the superior vena cava (Figure 3). After the circumflex artery turned around the left margin of the heart it almost entirely became intramyocardial (tunneled) except its short pre- and post-bridging segments. Its pre-bridging segment gave branches to left side of posterior left ventricle and dived deep into the myocardium for about 5 cm (intramyocardial segment) (Figure 4). Close to the crus (junctional area between the coronary sulcus and the posterior interventricular sulcus), it exited the myocardium as post-bridging segment and ramified into AVnodal branch, right atrial branch, right and left ventricular branches and the posterior interventricular artery (Figures 4-5).
Figure 1: Illustrates a relatively small right coronary artery giving branch to a markedly large right conal artery, and a larger left coronary artery dividing into the anterior interventricular and circumflex branches the usual way.
Figure 2: Shows the right coronary artery and its conal branch with distribution of their branches to the right ventricle and right atrium.
DISCUSSION
Even though different studies showed a wide range of variability in the percentages of coronary artery dominance in the general population, all studies agree that right dominance (82% – 89%) is the most common pattern followed by left dominance (5% – 12%) as second common and codominance (3% - 7%) as a least common pattern [1]. The study of Aricatt et al. 2023 [2] on four thousand angiograms revealed similar percentages of dominance patterns within the above ranges, where right dominance was found to be 85.5%, left dominance 9.7% and codominance in 4.8% of the cases. These two authors also emphasized on the clinical relevance of the dominance patterns and according to the first author, right dominance pattern is closely correlated with triple-vessel disease than left dominance or codominance patterns. Whereas, the second author described a high prevalence of stenosis in the left dominance pattern, which is a common feature in males, followed by the right dominance and codominance patterns. Though, such clinical correlations exist in the different dominance patterns, the left dominance pattern that supplies a large area of the myocardium confers a high risk of death and reinfarction than others. This increased risk begins soon after an acute event and continues, and even increases, throughout follow-up [3]. This is in agreement with a previous study done by Veltman et al 2015 [4] on 1131 patients regarding the risk carried by the left dominance pattern, but Veltman et al concluded that, after surviving the first 30-days post-ST segment elevation myocardial infarction, coronary vessel dominance has no influence on long term outcome.
As clearly depicted by Galiuto L. 2015 [5], similar to the wide range of variability in the dominance patterns, the incidence of myocardial bridging is also variable depending on the diagnostic method used including various imaging techniques. Many investigations done in this area did conclude that myocardial bridging almost exclusively affects the middle portion of the anterior interventricular artery, while other coronary vessels are rarely affected. However, there is a tangible evidence that myocardial bridging can affect all major coronary arteries and their corresponding branches as a group or individually including the complete covering of circumflex artery by myocardium [6], myocardial bridging involving the left main coronary artery, circumflex artery and anterior interventricular artery in the same heart [7], and myocardial bridging in all major epicardial vessels like anterior interventricular, circumflex and posterior interventricular arteries in a single heart [8].
Even though myocardial bridging, as a congenital variant, remains asymptomatic or clinically silent in the majority of the cases, it can be a cause of sudden death and various forms of cardiac conditions caused by coronary artery involvement [9], [10]). There is also a common understanding that myocardial bridging protects against the formation of atherosclerotic plaques in the bridged segment, while it promotes the formation of plaques in the segment proximal to the bridge. However, Jiang et al. [11] in their study done on 6774 patients with symptoms of angina; expanded this to the extent that myocardial bridging might provide protection against atherosclerosis not only in the bridged segment but also in the entire coronary system.
In correlation to all these, the finding of a hugely left dominance pattern like in this current case report, with distribution to all parts of the left ventricle, left atrium, posterior aspects of the right atrium and right ventricle including the SA- and AV-nodes may put the major portion of the myocardium at risk. Furthermore, the presence of myocardial bridge might impose additional risk, particularly in the parts of the myocardium supplied by branches from the post-bridging segment of the circumflex artery like AV-node, posterior right atrium and, posterior right and left ventricles.
CONCLUSION
The finding in this case report that noted left dominant coronary artery with myocardial bridging over a significant portion of the circumflex artery, can be a proof of previous descriptions that myocardial bridging can affect the major coronary vessels and any of their branches. This report has also showed the unique branching pattern of the pre-bridging and post-bridging segments of the circumflex artery that can be of diagnostic importance and may be helpful in the predictions of probable outcomes in symptomatic cases. This can also enhance the awareness of clinician in the consideration of myocardial bridges as differential diagnosis, particularly in coronary artery related conditions that do not adequately respond to medical and surgical treatment.
ACKNOWLEDGEMENT
I am thankful to the donor and his families for their invaluable donation and consent for education, research and publication. I would also like to express my gratitude to the department of biomedical sciences for the encouragement and uninterrupted support. As always, I am also grateful to Denelle Kees and John Opland for their immense assistance during the dissection of this cadaver in the gross anatomy lab.
References
- Peng L, Guo X, Gao Y, Guo Q, Zhang J, et al. Impact of right coronary dominance on triple-vessel coronary artery disease: a cross-sectional study. Medicine. 2018; 97: 32 (e11685).
- Aricatt DP, Prabhu A, Avadhani R, Subramanyam K, Ezhilan J, et al. A study of coronary artery dominance and its clinical significance. Folia Morphol. 2023; 82(1): 102-107.
- Abu-Assi E, Castineira-Busto M, Gonzalez-Salvado V, Raposeiras-Roubin S, Abumuaileq RR-Y, et al. Coronary artery dominance and long-term prognosis in patients with ST-segment elevation myocardial infarction treated with primary angioplasty. Rev Esp Cardiol. 2016; 69(1):19-27.
- Veltman CE, van der Hoeven BL, Hoogslag GE, Boden H, Kharbanda RK, et al. Influence of coronary artery dominance on short- and log-term outcomes in patients after ST-segment elevation myocardial infarction. Eur Heart J. 2015; 36:1023-1030.
- Galiuto L. How to access functional significance of myocardial bridging in athletes: a personalized medicine approach. Biomed J Sci & Tech Res. 2020; 26(2): 004-313.
- Muehrcke DD, Shimp W, Casillas M. Intraoperative angiography to find an intramyocardial coronary artery. Clin Surg. 2021; 6: 3025.
- Kumar B, Wardhan H, Nath RK, Sharma A. A rare case of myocardial bridge involving left main, left circumflex, and left anterior descending coronary arteries. J Am Coll Cardiol. 2012; 59(10):965.
- Gupta MD, Girish MP, TrehanV, Tyagi S. Myocardial bridging in all major epicardial vessels. JACC Cardiovasc Interv. 2014; 7(10):e129-31.
- Navarro A, Sladden D, Casha A, Manche A. The difficulty in identifying and grafting an intramuscular coronary artery. Malta Med J. 2019; 3(1):14-16.
- Ibarrola M. Myocardial bridge a forgotten condition: A review. Clin Med Img Lib. 2021; 7:182.
- Jiang L, Zhang M, Zhang H, Shen L, Shao L, et al. A potential protective element of myocardial bridge against severe obstructive atherosclerosis in the whole coronary system. BMC Cardiovasc Disord. 2018; 18(1):105.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref