Left superior vena cava with associated venous variations
Vaishaly Bharambe*, Vasanti Arole and P. Vatsalaswamy
Dr. D. Y. Patil Medical College, Pimpri, Pune, Maharashtra, India
- *Corresponding Author:
- Dr. Vaishaly Bharambe
D-9 State Bank Nagar, Panchvati, Pashan Road, Pune 411008, Maharashtra, India
Tel: +91 (982) 2910845
E-mail: vaishalybharambe@yahoo.co.in
Date of Received: November 29th, 2011
Date of Accepted: April 13th, 2012
Published Online: January 13th, 2013
© Int J Anat Var (IJAV). 2013; 6: 9–12.
[ft_below_content] =>Keywords
superior vena cava, accessory hemiazygos, brachiocephalic vein
Introduction
Precise anatomical knowledge of the great vessels of the neck and thorax and their variations is essential for safe anesthesia, intensive care practice, placement of central venous catheters, pacemaker implantation using the transvenous approach, etc.
Occurrence of persistent left superior vena cava (SVC) with absent right SVC are venous system variations that could impede such procedures. Persistent left SVC in 80% of the cases is found to co-exist with the right SVC [1]. Its association with absence of right SVC is unusual.
In the present case we report multiple venous variations occurring simultaneously in the same cadaver.
Case Report
During routine dissection of the thoracic region, in an 45-year-old formalin fixed male cadaver:
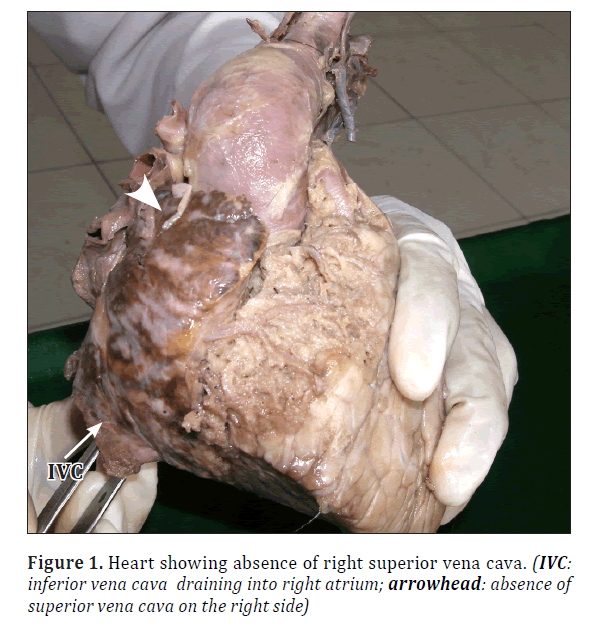
• The SVC was absent on the right side (Figure 1).
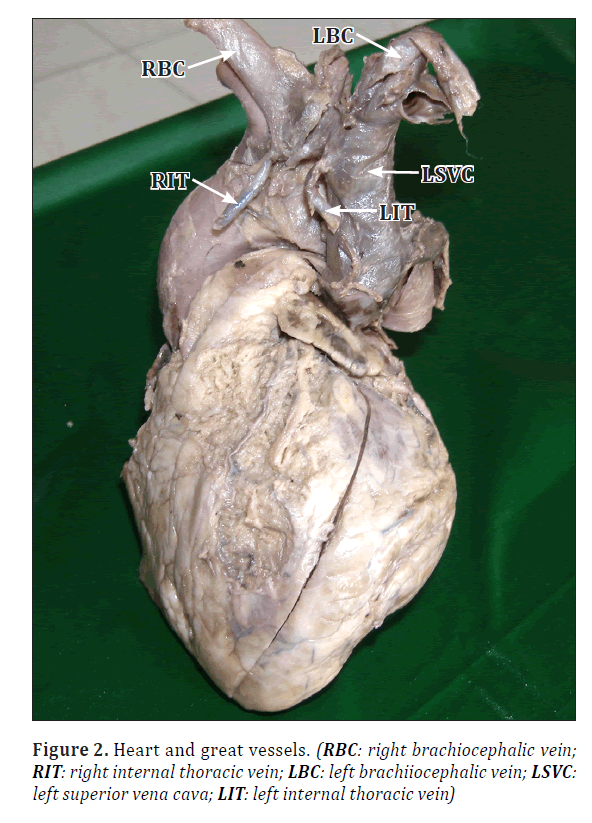
• just posterior to the manubrium sterni to join the left brachiocephalic vein behind the 1st costo-sternal joint to form the left SVC (Figure 2).
• Right and left internal thoracic veins were draining into the right brachiocephalic vein and the left SVC, respectively (Figure 2).
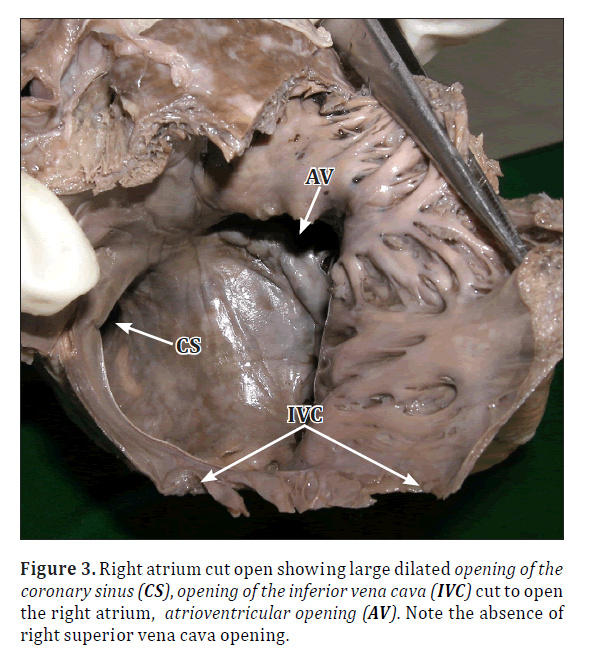
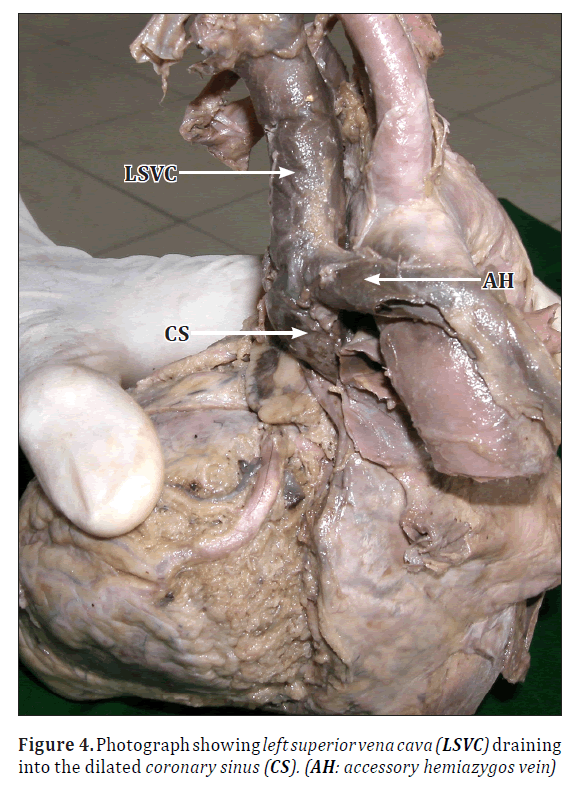
• The left SVC was draining into the right atrium via a dilated coronary sinus (Figures 3, 4).
• The azygos vein drained into the accessory hemiazygos vein, which was arching above the root of left lung and draining into the left SVC (Figure 4).
Dissection of the thoracic and abdominal regions revealed no evidence of transposition of viscera.
Discussion
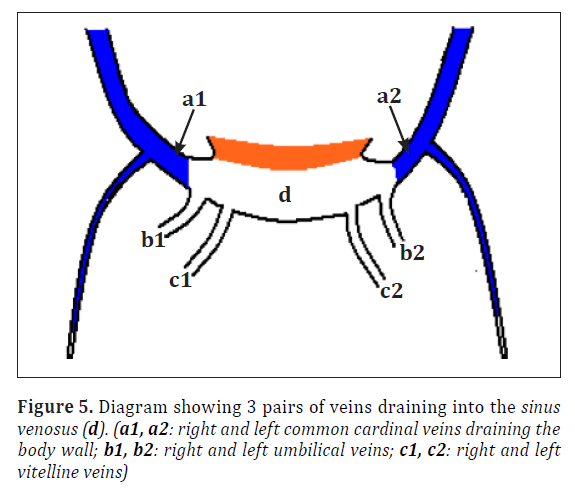
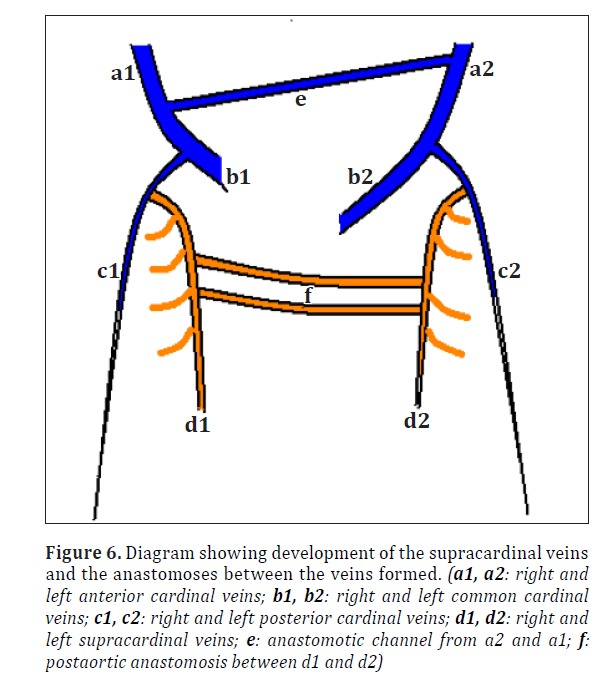
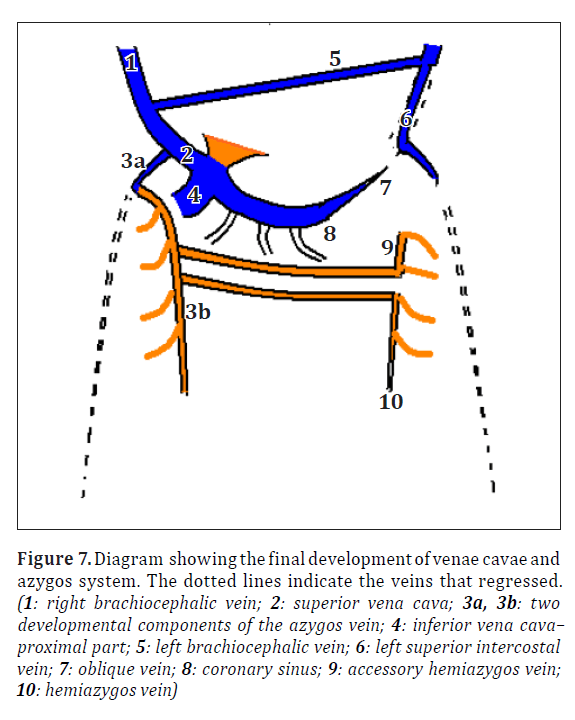
To understand the present variations the normal development of the related venous system needs to be reviewed. In the 5th week of intrauterine life 3 pairs of major veins can be distinguished draining into the two horns of sinus venosus [2] (Figure 5). Formation of supracardinal veins and anastomoses between the right and left side veins is shown in Figure 6. Final development with shunting of blood from left to right is shown in Figure 7.
Figure 6. Diagram showing development of the supracardinal veins and the anastomoses between the veins formed. (a1, a2: right and left anterior cardinal veins; b1, b2: right and left common cardinal veins; c1, c2: right and left posterior cardinal veins; d1, d2: right and left supracardinal veins; e: anastomotic channel from a2 and a1; f: postaortic anastomosis between d1 and d2)
Figure 7. Diagram showing the final development of venae cavae and azygos system. The dotted lines indicate the veins that regressed. (1: right brachiocephalic vein; 2: superior vena cava; 3a, 3b: two developmental components of the azygos vein; 4: inferior vena cava–proximal part; 5: left brachiocephalic vein; 6: left superior intercostal vein; 7: oblique vein; 8: coronary sinus; 9: accessory hemiazygos vein; 10: hemiazygos vein)
Normally as observed in Figures 5, 6 and 7 large portions of left cardinal veins are obliterated during embryonic life due to left-to-right shunting of blood.
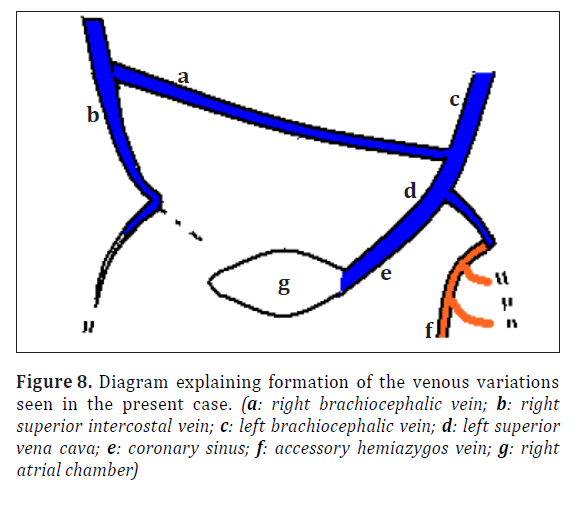
It is proposed that in the present case there could have been shunting of blood from right to left through the oblique anastomoses (Figure 8). The development following such an occurrence could have resulted in the left anterior cardinal vein distal to the oblique anastomosis forming the left brachiocephalic vein. The oblique anastomosis itself formed the right brachiocephalic vein. The persistent left anterior cardinal vein proximal to the anastomosis and the left common cardinal vein together formed the left SVC. Left horn of sinus venosus formed coronary sinus. The proximal part of the persistent left supracardinal vein along with the proximal part of the posterior cardinal vein formed the accessory hemiazygos vein draining into the left SVC.
With the regression of rest of right posterior cardinal vein, the azygos vein via the post-aortic anastomotic channels (Figure 6) drained into the accessory hemiazygos vein.
In 1954, 46 cases of persistent left SVC were reviewed, out of which, 65% cases showed double SVC, 39% showed transposition of thoracic and abdominal viscera and many cases had associated conditions like Fallot’s tetralogy, tricuspid atresia and Eisenmenger’s complex [3].
In 1998 double SVC was detected during central venous catheterization of a 3-year-old boy with no other related structural variations observed [1].
A case of persistent left SVC was discovered in 2001 after insertion of a pulmonary artery catheter [4].
In 2004 a study was carried out on 10 patients of persistent left SVC where 30% cases were found to be having associated congenital heart disease, 30% cases had absent right SVC and a dilated coronary sinus and 50% cases showed absence of left brachiocephalic vein [5].
A study carried out in 2007 on 152 cases undergoing surgery for congenital heart disease, reported 3% cases having a persistent left SVC and dilated coronary sinus, one of these cases showing absence of right SVC [6].
Finding of persistent left SVC was reported in 2007 in a 60-year-old cadaver where a narrow communicating vein connected it to the right superior vena cava. The left SVC drained via dilated coronary sinus into the right atrium [7].
A left SVC with dilated coronary sinus was detected in an accident victim with thoracic injuries in 2008 [8].
Conclusion
In the present case, a left SVC was observed with the absence of right sided SVC, presence of right brachiocephalic vein crossing to the left and joining the left brachiocephalic vein to form the left SVC, accessory hemiazygos vein draining into the left SVC, right internal thoracic vein draining into the right brachiocephalic vein and left internal thoracic vein draining into left SVC. There were no associated congenital cardiac malformations nor situs inversus.
The present case is clinically important because left SVC can impede manipulation of a cardiac venous catheter in or through the coronary sinus which can result in hypotension, angina or cardiac arrest [1]. Left SVC can also cause difficulties in placing of a pulmonary artery catheter or pacemaker in the right ventricle because of the orientation of the coronary sinus [1]. There may be higher incidences of cardiac arrhythmias in these patients [7]. The cardiac catheter may pass through the dilated coronary sinus during exploration of the right atrium thus appearing to pass right across the heart and being mistaken for presence of an atrial septal defect [3]. Venous variations often complicate mediastinal surgeries with intraoperative haemorrhage due to damage to variant large vessels having life-threatening consequences [9]. Occurrence of left SVC can be indicative of congenital heart malformations. Left SVC may not always produce symptoms, therefore going undetected [3].
Despite the clinical importance of these venous variations they often go undetected because there is no alteration in the hemodynamics of patients with agenesis of right SVC and persistent left SVC and such patients being asymptomatic. [10] In cases where there is double SVC the persistence of left SVC may go undetected as most central catheters are placed on the right [1].
These variations can be detected by a radiological picture of heart showing straight borders on both sides and a broad pedicle [3] and by angiocardiography.
An attempt has been made in this article to explain the possible embryological basis for the venous variations observed and their clinical significance. Study of such cases not only increases awareness of their clinical implications in the present scenario but also has high educational value for students of embryology.
References
- Higgs AG, Paris S, Potter F. Discovery of left sided superior vena cava during central venous catheterization. Br J Anaesth. 1998; 81: 260–261.
- Sadler TW. Langman’s Medical Embryology. 10th Ed., Philadelphia, Lippincott Williams & Wilkins. 2006; 186–189.
- Campbell M, Deuchar DC. The left-sided superior vena cava. Br Heart J. 1954; 16: 423–439.
- Masuda Y, Imaizumi H, Satoh M, Hazama K, Nakamura M, Chaki R, Asai Y. Persistent left-sided superior vena cava diagnosed after flow-directed pulmonary artery catheterization; report of a case. Masui. 2001; 50: 1109–1112. (Japanese)
- Gonzalez-Juanatey C, Testa A, Vidan J, Izquierdo R, Garcia-Castelo A, Daniel C, Armesto V. Persistent left superior vena cava draining into the coronary sinus: report of 10 cases and literature review. Clin Cardiol. 2004; 27: 515–518.
- Erdogan M, Karakas P, Uygur F, Mese B, Yamak B, Bozkir MG. Persistent left superior vena cava the anatomical and surgical importance. West Indian Med J. 2007; 56: 72–76.
- Paval J, Nayak S. A persistent left superior vena cava. Singapore Med J. 2007; 48: e90–e93.
- Goyal SK, Punnam SR, Verma G, Ruberg FL. Persistent left superior vena cava:a case report and review of literature. Cardiovasc Ultrasound. 2008; 6: 50.
- Pyrzowski J, Spodnik JH, Lewicka A, Poplawska A, Wojcik S. A case of multiple abnormalities of the azygos venous system: a praeaortic interazygos vein. Folia Morphol (Warsz). 2007; 66: 353–355.
- Palinkas A, Nagy E, Forster T, Morvai Z, Nagy E, Varga A. A case of absent right and persistent left superior vena cava. Cardiovasc Ultrasound. 2006; 4: 6.
Vaishaly Bharambe*, Vasanti Arole and P. Vatsalaswamy
Dr. D. Y. Patil Medical College, Pimpri, Pune, Maharashtra, India
- *Corresponding Author:
- Dr. Vaishaly Bharambe
D-9 State Bank Nagar, Panchvati, Pashan Road, Pune 411008, Maharashtra, India
Tel: +91 (982) 2910845
E-mail: vaishalybharambe@yahoo.co.in
Date of Received: November 29th, 2011
Date of Accepted: April 13th, 2012
Published Online: January 13th, 2013
© Int J Anat Var (IJAV). 2013; 6: 9–12.
Abstract
During routine dissection of the thoracic region of a 45-year-old male cadaver, the superior vena cava was found to be absent on the right side. The right brachiocephalic vein was passing from right to left to join the left brachiocephalic vein to form the left superior vena cava. The azygos vein was draining into the accessory hemiazygos vein which was draining into the left superior vena cava. There was a large dilated coronary sinus.
-Keywords
superior vena cava, accessory hemiazygos, brachiocephalic vein
Introduction
Precise anatomical knowledge of the great vessels of the neck and thorax and their variations is essential for safe anesthesia, intensive care practice, placement of central venous catheters, pacemaker implantation using the transvenous approach, etc.
Occurrence of persistent left superior vena cava (SVC) with absent right SVC are venous system variations that could impede such procedures. Persistent left SVC in 80% of the cases is found to co-exist with the right SVC [1]. Its association with absence of right SVC is unusual.
In the present case we report multiple venous variations occurring simultaneously in the same cadaver.
Case Report
During routine dissection of the thoracic region, in an 45-year-old formalin fixed male cadaver:
• The SVC was absent on the right side (Figure 1).
• just posterior to the manubrium sterni to join the left brachiocephalic vein behind the 1st costo-sternal joint to form the left SVC (Figure 2).
• Right and left internal thoracic veins were draining into the right brachiocephalic vein and the left SVC, respectively (Figure 2).
• The left SVC was draining into the right atrium via a dilated coronary sinus (Figures 3, 4).
• The azygos vein drained into the accessory hemiazygos vein, which was arching above the root of left lung and draining into the left SVC (Figure 4).
Dissection of the thoracic and abdominal regions revealed no evidence of transposition of viscera.
Discussion
To understand the present variations the normal development of the related venous system needs to be reviewed. In the 5th week of intrauterine life 3 pairs of major veins can be distinguished draining into the two horns of sinus venosus [2] (Figure 5). Formation of supracardinal veins and anastomoses between the right and left side veins is shown in Figure 6. Final development with shunting of blood from left to right is shown in Figure 7.
Figure 6. Diagram showing development of the supracardinal veins and the anastomoses between the veins formed. (a1, a2: right and left anterior cardinal veins; b1, b2: right and left common cardinal veins; c1, c2: right and left posterior cardinal veins; d1, d2: right and left supracardinal veins; e: anastomotic channel from a2 and a1; f: postaortic anastomosis between d1 and d2)
Figure 7. Diagram showing the final development of venae cavae and azygos system. The dotted lines indicate the veins that regressed. (1: right brachiocephalic vein; 2: superior vena cava; 3a, 3b: two developmental components of the azygos vein; 4: inferior vena cava–proximal part; 5: left brachiocephalic vein; 6: left superior intercostal vein; 7: oblique vein; 8: coronary sinus; 9: accessory hemiazygos vein; 10: hemiazygos vein)
Normally as observed in Figures 5, 6 and 7 large portions of left cardinal veins are obliterated during embryonic life due to left-to-right shunting of blood.
It is proposed that in the present case there could have been shunting of blood from right to left through the oblique anastomoses (Figure 8). The development following such an occurrence could have resulted in the left anterior cardinal vein distal to the oblique anastomosis forming the left brachiocephalic vein. The oblique anastomosis itself formed the right brachiocephalic vein. The persistent left anterior cardinal vein proximal to the anastomosis and the left common cardinal vein together formed the left SVC. Left horn of sinus venosus formed coronary sinus. The proximal part of the persistent left supracardinal vein along with the proximal part of the posterior cardinal vein formed the accessory hemiazygos vein draining into the left SVC.
With the regression of rest of right posterior cardinal vein, the azygos vein via the post-aortic anastomotic channels (Figure 6) drained into the accessory hemiazygos vein.
In 1954, 46 cases of persistent left SVC were reviewed, out of which, 65% cases showed double SVC, 39% showed transposition of thoracic and abdominal viscera and many cases had associated conditions like Fallot’s tetralogy, tricuspid atresia and Eisenmenger’s complex [3].
In 1998 double SVC was detected during central venous catheterization of a 3-year-old boy with no other related structural variations observed [1].
A case of persistent left SVC was discovered in 2001 after insertion of a pulmonary artery catheter [4].
In 2004 a study was carried out on 10 patients of persistent left SVC where 30% cases were found to be having associated congenital heart disease, 30% cases had absent right SVC and a dilated coronary sinus and 50% cases showed absence of left brachiocephalic vein [5].
A study carried out in 2007 on 152 cases undergoing surgery for congenital heart disease, reported 3% cases having a persistent left SVC and dilated coronary sinus, one of these cases showing absence of right SVC [6].
Finding of persistent left SVC was reported in 2007 in a 60-year-old cadaver where a narrow communicating vein connected it to the right superior vena cava. The left SVC drained via dilated coronary sinus into the right atrium [7].
A left SVC with dilated coronary sinus was detected in an accident victim with thoracic injuries in 2008 [8].
Conclusion
In the present case, a left SVC was observed with the absence of right sided SVC, presence of right brachiocephalic vein crossing to the left and joining the left brachiocephalic vein to form the left SVC, accessory hemiazygos vein draining into the left SVC, right internal thoracic vein draining into the right brachiocephalic vein and left internal thoracic vein draining into left SVC. There were no associated congenital cardiac malformations nor situs inversus.
The present case is clinically important because left SVC can impede manipulation of a cardiac venous catheter in or through the coronary sinus which can result in hypotension, angina or cardiac arrest [1]. Left SVC can also cause difficulties in placing of a pulmonary artery catheter or pacemaker in the right ventricle because of the orientation of the coronary sinus [1]. There may be higher incidences of cardiac arrhythmias in these patients [7]. The cardiac catheter may pass through the dilated coronary sinus during exploration of the right atrium thus appearing to pass right across the heart and being mistaken for presence of an atrial septal defect [3]. Venous variations often complicate mediastinal surgeries with intraoperative haemorrhage due to damage to variant large vessels having life-threatening consequences [9]. Occurrence of left SVC can be indicative of congenital heart malformations. Left SVC may not always produce symptoms, therefore going undetected [3].
Despite the clinical importance of these venous variations they often go undetected because there is no alteration in the hemodynamics of patients with agenesis of right SVC and persistent left SVC and such patients being asymptomatic. [10] In cases where there is double SVC the persistence of left SVC may go undetected as most central catheters are placed on the right [1].
These variations can be detected by a radiological picture of heart showing straight borders on both sides and a broad pedicle [3] and by angiocardiography.
An attempt has been made in this article to explain the possible embryological basis for the venous variations observed and their clinical significance. Study of such cases not only increases awareness of their clinical implications in the present scenario but also has high educational value for students of embryology.
References
- Higgs AG, Paris S, Potter F. Discovery of left sided superior vena cava during central venous catheterization. Br J Anaesth. 1998; 81: 260–261.
- Sadler TW. Langman’s Medical Embryology. 10th Ed., Philadelphia, Lippincott Williams & Wilkins. 2006; 186–189.
- Campbell M, Deuchar DC. The left-sided superior vena cava. Br Heart J. 1954; 16: 423–439.
- Masuda Y, Imaizumi H, Satoh M, Hazama K, Nakamura M, Chaki R, Asai Y. Persistent left-sided superior vena cava diagnosed after flow-directed pulmonary artery catheterization; report of a case. Masui. 2001; 50: 1109–1112. (Japanese)
- Gonzalez-Juanatey C, Testa A, Vidan J, Izquierdo R, Garcia-Castelo A, Daniel C, Armesto V. Persistent left superior vena cava draining into the coronary sinus: report of 10 cases and literature review. Clin Cardiol. 2004; 27: 515–518.
- Erdogan M, Karakas P, Uygur F, Mese B, Yamak B, Bozkir MG. Persistent left superior vena cava the anatomical and surgical importance. West Indian Med J. 2007; 56: 72–76.
- Paval J, Nayak S. A persistent left superior vena cava. Singapore Med J. 2007; 48: e90–e93.
- Goyal SK, Punnam SR, Verma G, Ruberg FL. Persistent left superior vena cava:a case report and review of literature. Cardiovasc Ultrasound. 2008; 6: 50.
- Pyrzowski J, Spodnik JH, Lewicka A, Poplawska A, Wojcik S. A case of multiple abnormalities of the azygos venous system: a praeaortic interazygos vein. Folia Morphol (Warsz). 2007; 66: 353–355.
- Palinkas A, Nagy E, Forster T, Morvai Z, Nagy E, Varga A. A case of absent right and persistent left superior vena cava. Cardiovasc Ultrasound. 2006; 4: 6.