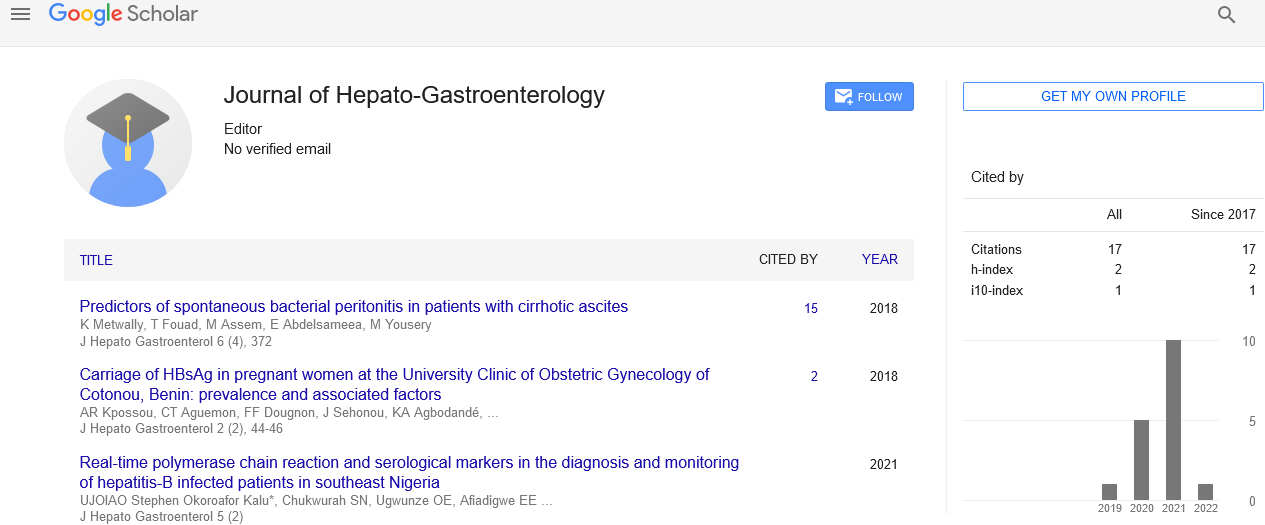
Liver failure after major hepatic resection, a persistent clinical conundrum
Received: 23-May-2018 Accepted Date: Jun 06, 2018; Published: 11-Jun-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
INTRODUCTION: Post-hepatectomy liver failure (PHLF) is a significant complication which consumes considerable resource, principally due to the need for intensive care involvement during an extensive and prolonged post-operative recovery. The incidence of PHLF varies from 0.7% to 34% and remains the primary cause of mortality following liver resection. The associated mortality rate varies from 0 to 5% and it remains a contributory factor in 18-75% of fatal cases. METHODS: This is a narrative review of the current literature focusing on the epidemiology, definition, risk factors, pathophysiology, prediction, prevention and management of PHLF. RESULTS: In patients with an increased risk of developing PHLF etiological factors are related to the patients’ co-morbidity and/or the surgical procedure. Patient risk factors include cirrhosis, steatosis, chemotherapy associated steatohepatitis (CASH), sinusoidal injury, cholestasis and cholangitis. Surgical risk factors include the extent of liver resection, the regenerative capacity of the future liver remnant (FLR), sepsis, ischemia reperfusion injury and ‘small for size syndrome’ (SFSS). Pre-operative work up including clinical scoring criteria, volumetric analysis and measurement of hepatocyte uptake and elimination are reviewed. DISCUSSION: PHLF remains a challenging clinical condition which is difficult to treat, and prevention and early recognition remains vitally important. The lack of a single accepted definition hinders the study of PHLF due to the difficulty of cross-comparison. Improved pre-operative planning and the early recognition and treatment of PHLF will improve patient care, morbidity and ultimately the mortality from this complex postoperative complication.
Keywords
Post-hepatectomy liver failure; Recognition; Preoperative planning
Hepatic resection remains the gold standard for the treatment of liver tumours [1]. Balancing the excision of all clinically detectable disease with adequate clear margins and the necessity to retain adequate future liver remnant remains a significant challenge. In the majority of patient’s substantial resections can be safely performed however in those who require very extensive resections, or where there is impaired hepatic parenchymal function and regenerative capacity, surgery is associated with a significant morbidity and mortality.
Literature Review
Post-hepatectomy liver failure (PHLF) is a significant complication which consumes considerable resource, principally due to the need for intensive care involvement during an extensive and prolonged post-operative recovery. With the increasing incidence of parenchymal disease PHLF remains a sequela of liver resection which necessitates further study [2]. This review will discuss the epidemiology, definition, risk factors, pathophysiology, prediction, prevention and management of PHLF.
Epidemiology
The incidence of PHLF varies from 0.7 to 34%, although the majority of studies suggest an incidence between 5 and 10% [3-8]. The lack of a universally accepted definition has resulted in other pathologies being described as PHLF. This coupled with a heterogeneous patient population makes comparisons between studies problematic [2,9]. PHLF remains the primary cause of mortality post hepatic resection, with a mortality rate between 0 and 5% [2,9] and contributes to mortality in 18-75% of fatal cases [10-12].
Definition
A universally accepted definition for PHLF does not exist. Rahbari defined PHLF as ‘a post-operative acquired deterioration in the ability of the liver to maintain its synthetic, excretory and detoxifying functions, which are characterised by and increased International normalised ratio (INR) and concomitant hyperbilirubinaemia on or after post-operative day 5’ [13,14]. Attempts have been made to assign numerical values to the abnormalities of liver function and a serum bilirubin above 50umol/l and a prothrombin time (PT) less than 50% below the patient’s baseline value (or and INR greater than 1.7) on day 5 post-hepatectomy have been suggested [2,15].
Balzan referred to this as the ‘50-50 criteria’ and when these criteria are met, patients have a 59% mortality risk compared to 1.2% when they are not met [15]. In a study by Mullen in 2007, these criteria were shown to have a sensitivity of 50% and a specificity of 96.6% for PHLF as a cause of death for patients without underlying parenchymal disease [16].
Risk Factors
In patients with an increased risk of developing PHLF aetiological factors are related to the patients’ co-morbidity and/or the surgical procedure.
Patient related risk factors
Pre-existing liver parenchymal disease is a significant risk factor and includes cirrhosis, steatosis, chemotherapy associated steatohepatitis (CASH), sinusoidal injury, cholestasis and cholangitis. Cirrhotic patients are known to have a significantly higher mortality rate following resection and studies report mortality rates between 5% and 20% [17-19]. In patients with cirrhosis, resection of up to 50% is considered safe in the absence of functional impairment or portal hypertension. However, in Child-Pugh grade B or C, any significant resection can result in PHLF [20,21]. Cirrhotic livers demonstrate reduced levels of hepatocyte growth factor [22] and reduced transcription factors [23], meaning that in patients with cirrhosis capacity for regeneration is affected [24] with impaired function post-operatively and reduced functional reserve [25]. CASH and sinusoidal injury are increasingly prevalent conditions as more patients with colorectal liver metastases are treated with neoadjuvant chemotherapy [2]. Sinusoidal injury has been demonstrated with oxaliplatin chemotherapy which is partially reversible on cessation of treatment [26]. CASH is associated with 5-flurouracil and irinotecan treatment which reduces the regenerative capacity of the liver remnant and increases post-resection liver dysfunction [26,27].
Cholestasis
Cholestasis also reduces the regenerative capacity of the liver [28] and increases the likelihood of post-hepatic liver dysfunction [29]. Some centres advocate pre-operative biliary drainage which is believed to improve remnant function [30] although there is no proven survival benefit and a significant increased risk of morbidity from sepsis [31]. Drainage can either be performed by means of external biliary drain or internal stenting. Cholestasis reduces portal venous flow because the bile duct, portal vein and hepatic artery are enclosed within the Glissonian capsule with a finite amount of available space, referred to as the space of Mall [32]. As the biliary tract dilates, the space of Mall is reduced with a concomitant reduction of portal venous flow [33]. This reduction in portal venous flow is further exacerbated by hepatectomy and this may contribute to an impaired regenerative capacity (Baer). Hepatic regeneration is also impaired by reduced expression of transcription factors such as cyclin E [34] and cytokines such as interleukin six (IL-6) and epidermal growth factor [28,34-37]. Elevated levels of bile salts have also been shown to induce hepatocyte apoptosis [38], and the absence of bile salts within small bowel lumen means their protective activity against bacterial translocation is lost [39].
Other patient related risk factors
Men are twice as likely to develop PHLF and post resection morbidity compared to females [16,40] which is in part due to the immunosuppressive effect of testosterones and oestrogen. The regenerative capacity of the liver has also been shown to reduce with age in animal models [41-44] and some studies report an increase in morbidity [16] and mortality [15] with advanced age, particularly after hepatic resection [45,46]. Conversely a number of other studies have reported safe hepatic resection in the elderly [47] with no increased morbidity, mortality [48] or the development of PHLF [49,50]. Diabetes is known to increase PHLF and the consequent morbidity and mortality following hepatic resection [51]. This is thought to be due to an immune dysfunction and the impaired regenerative capacity of the liver resulting from the absence of insulin or insulin resistance [52]. The likelihood of developing PHLF increases two-fold with diabetes as an independent prognostic factor [53]. Hepatic atrophy has also been demonstrated in insulin deficient animal models [54]. Patients with two or more metabolic disorders undergoing right hepatectomy (diabetes mellitus, obesity, hypertension and dyslipidaemia) have been shown to have a perioperative mortality of 30% [55]. Malnutrition is noted in 65-90% of patients with advanced liver disease [56] and 20-55% of colorectal cancer patients [57] and optimizing nutrition has been shown to reduce post-operative liver dysfunction and morbidity. Studies have demonstrated that malnutrition impairs the immune response [58,59] reduces hepatic protein synthesis and increases the risk of developing PHLF. This is possibly the result of impaired regenerative capacity [58,60] and/or secondary to disordered mitochondrial function [61].
Surgery related risk factors
The extent of hepatic resection correlates with PHLF and perioperative mortality with 80% of deaths occurring when more than 50% of the liver is resected [15,16,18,61-70]. A minimum liver remnant volume (LRV) in patients with normal liver parenchyma is generally considered to be between 20-30% of total liver volume [4,19,21,40,70]. In the presence of parenchymal disease without portal hypertension or hepatic insufficiency the minimum LRV is considered to be between 40-50% [4,21,71,72]. Where liver dysfunction occurs as a result of extensive resection this is termed ‘small for size’ liver remnant [9]. Intraoperative blood loss of greater than 1-1.2L and the need for blood transfusion have also been shown to be associated with PHLF and sepsis [3,18,73-75]. Significant intraoperative haemorrhage results in large fluid shifts, coagulopathy and bacterial translocation, with a significant immunosuppressive action [76-78]. Vascular reconstruction following hepatic and inferior vena cava resection is also associated with PHLF [79,80] while biliary reconstruction is associated with increased morbidity and mortality, although is not generally considered an independent risk factor for the development of PHLF [18,29,75].
Pathophysiology
Liver failure following excessive resection is usually characterised by coagulopathy, hyperbiliruninaemia and encephalopathy and accompanied by sepsis and/or multi-organ failure [32]. PHLF is multi factorial and the extent of liver resection and the regenerative capacity of the future liver remnant (FLR) are the crucial factors which increase the risk of developing PHLR, while sepsis, ischaemia reperfusion injury and ‘small for size syndrome’ (SFSS) are important secondary factors. It has been postulated that the lack of regeneration demonstrated in failing livers may be a consequence of excessive resection rather the cause of failure [32], a theory that is supported by significant apoptosis and hepatocyte loss in animal models following hepatectomy [81]. To avoid PHLF the liver must not only limit hepatocyte death but also resist metabolic stress and provide sufficient synthetic function [52,82,83].
Post-operative portal flow
Post operatively, there is a significant reduction in portal venous flow, with an increase in hepatic arterial resistance and an increase in portal venous pressure [32,84]. The resultant congestion of liver sinusoids produces parenchymal stress which is similarly exhibited in SFSS seen after liver transplantation [85]. The parenchymal congestion causes an increase in shear stress and is a important factor in initiating regeneration [32].
Shear stress and liver damage
Despite being an important initiating factor in liver regeneration, excessive shear stress can lead to hepatocyte loss and collapse of the hepatic microcirculation [86]. Approaches to reducing portal venous pressure include splenectomy and porto-systemic shunting [87]. Normalising portal pressure has been demonstrated to produce a survival benefit in animal studies [38,88]. While portocaval shunting has been shown to reduce hepatocyte necrosis, this comes at the cost of a delay in liver regeneration [88,89], possibly due to an over reduction of shear stress and a diversion of hepatotrophic factors into the systemic circulation [32]. Mesocaval shunting may be a suitable compromise [90] and pharmacological control can be useful in the short-term post operatively [32].
Intra-operative and post-operative ischaemia
Ischaemia both intra and post-operatively can significantly influence the development of PHLF. Many techniques have been described to limit intraoperative blood loss and the Pringle manoeuvre is commonly used either continuously or intermittently to limit intraoperative blood loss. This does not prevent hepatic venous bleeding, and some centres advocate total vascular exclusion (TVE), where the Pringle manoeuvre is accompanied by clamping of the supra and infra-hepatic vena cava [32]. Ischaemic preconditioning (IP) has been shown to reduce the risk of ischaemia reperfusion injury (IRI) in a rat model [91] and improve survival rates [92]. It involves short periods of ischaemia followed by longer periods of reperfusion, after which the Pringle manoeuvre can be continuously or intermittently applied. This promotes liver regeneration through the up regulation of cytokines such as IL-6 and tumour necrosis factor alpha (TNFα), coupled with down regulation of transforming growth factor beta (TGFβ) [93]. IRI is characterised by persistent post-operative parenchymal damage [2] and ischaemia activates the complement cascade and leads to Kupffer cell activation, endothelial cell damage and the generation of reactive oxygen species (ROS)2, Excessive IL-6, TNFα and nuclear factor beta exacerbate micro vascular injury, Kupffer cell mediated inflammation and ultimately hepatocyte death [94,95].
Sepsis
Sepsis has been reported to occur in up to 50% of patients following hepatic resection [70]. Sepsis affects post-operative liver function and regenerative capacity and can be both a consequence of, and precipitate PHLF. Sepsis induced hypotension can prolong post-operative ischaemia leading to Kupffer cell dysfunction and hepatotoxic levels of circulating cytokines [32]. Although a relative increase in endotoxin delivery to the liver can stimulate regeneration, excessive or prolonged endotoxinaemia can have an inhibitory effect on hepatocyte proliferation [96,97] by impairing mitochondrial function and bile salt excretion [98-100]. Kupffer cell activation is instrumental in initiating liver regeneration and its interaction with leukocytes through an intracellular adhesion molecule ICAM-1 leads to a cytokine mediated pro-inflammatory response promoting hepatocyte proliferation [32]. Mice deficient in ICAM-1 demonstrated an impaired capacity of the liver to regenerate following a 70% liver resection [101]. It has also been shown that after a significant hepatic resection there is a reduction in the number of Kupffer cells, impairing the liver’s ability to eliminate bacteria from the blood which may persist for up to 2 weeks [102]. TNFα normally induces hepatocyte proliferation at physiological blood levels but during sepsis excess levels occur and can initiate apoptosis largely due to its activation of NF-kappaB [103].
Prediction
Clinical scoring systems
The Child-Pugh score and the model for end-stage liver disease (MELD) score are systems that are now widely used to predict the risk of hepatic resection in cirrhotic patients [104-109]. The Child-Pugh score was originally designed to predict mortality in cirrhotic patients undergoing shunting procedures [110], and incorporates five parameters; serum albumin, bilirubin, INR, clinical evidence of ascites and hepatic encephalopathy. The majority of hepatic resections are performed in Child-Pugh A patients; however an increasing number are being undertaken in Child Pugh B patients. The majority of centres do not advocate hepatic resection in Child-Pugh C, although a small number are beginning to be performed in selected cases (111). The MELD score is calculated using serum creatinine, bilirubin and INR and has the benefit of incorporating renal function. Some studies have suggested it’s superiority to the classically used Child-Pugh [14,109,112]. In the absence of cirrhosis, neither scoring system can be utilised resulting in the use of clinical and radiological assessment for patient selection [1].
Volume measurement
Establishing the LRV is of vital importance in operative planning. This is accomplished by pre-operative radiological assessment using computed tomography (CT) or magnetic resonance imaging (MRI) [113]. Crosssectional imaging also permits the calculation of LRV to total functioning liver volume ratio [40,114,115] and can assist in the diagnosis of underlying parenchymal disease [4,40,115-120]. Advanced CT volumetric analysis is increasingly accessible with software such as PhotoshopTM and ImageJTM demonstrating good correlation with predicted and actual volume of liver resection [116,121]. However, cross sectional imaging provides inadequate information when predicting the functioning hepatocyte mass post resection.
Measurement of hepatocyte uptake and elimination
Pre-operative assessment of liver function is crucial when planning hepatic resection and predicting the risk of PHLF and several techniques have been developed.
Indocyanine green retention rate
Indocyanine green (ICG) binds to albumin in plasma and is water soluble [1]. It is a fluorescent dye which is selectively taken up by hepatocytes but importantly not by the enterohepatic circulation and it is also not metabolised by the liver [9,122]. Following intravenous injection, ICG retention rate at 15 minutes (ICG-15) is a commonly used measurement to determine an index of functional hepatocyte mass, liver perfusion and energy reserve [25,123,124]. It has demonstrated a greater predictive accuracy when compared to the Child-Pugh [125] and MELD scores [126]. There is no definitive cut off for a “safe” resection, although patients with an ICG- 15 of above 10-20% are considered to have impaired hepatic reserve [127], and require an adequate liver remnant volume to avoid PHLF [68,128,129]. Those with ICG-15 between 10-20% may benefit from preoperative volume manipulation [20,21] As ICG absorption and emission spectrum are in the near infrared range, measurements can be taken non-invasively meaning ICG uptake can be monitored intra and post-operatively [8,124,130-136]. One large series reported only 1 mortality in 1429 hepatic resections [73], although it can be argued that cases with a with a borderline ICG-15 who may have safely tolerated surgery were possibly excluded [1].
Hepatobiliary Scintigraphy/Single positron emission CT (SPET-CT)
The uptake of various radiolabelled compounds allows for the assessment of hepatic anatomy and functional mass. Physiologically, asialoglycoproteins are exclusively taken up by receptors on hepatic sinusoidal membranes [137] and a decrease in number of receptors has been noted in chronic liver disease [1]. Technetium-99m-diethylenetriamine-pentacetic acid-galactosyl-human serum albumin 99 Tc-GSA is a compound that binds to asialoglyoprotein receptors allowing for volumetric assessment of the functional hepatocyte mass [1]. SPET-CT allows measurement of radiotracer-labelled compounds such as 99 Tc-GSA in combination with standard CT assessment and is considered superior to scintigraphy, which only uses a gamma camera [138,139].
Lidocaine metabolism (MEGX)
The ‘MEGX test’ refers to the conversion rate of lidocaine to monoethylglycinexylidide (MEGX) in hepatocytes by the cytochrome p4503A system [140]. This test is inconsistent when patients are taking medications which are also metabolised by the cytochrome system. MEGX levels are measured at intervals after administration of 1 mg/kg of 2% lidocaine [141] and MEGX correlates with the extent of cirrhosis and predicts prognosis in cirrhosis [142]. Although there is little evidence to support the use of postoperative MEGX testing, it has been shown pre-operatively to predict the risk of developing PHLF in non-cirrhotic patients, particularly when combined with resection volume [143].
Galactose elimination capacity (GEC)
Another means of measuring hepatic metabolism is by galactose elimination. Following intravenous administration the serial measurement of galactose in serum and urine allows for the calculation of galactose elimination. GEC is predictive of post-operative liver dysfunction and long-term survival after hepatic resection [144] although it is resource intensive requiring serial sampling.
Prevention
Patient optimisation
Prior to surgery it is important to improve any modifiable co-morbidity. In patients with biopsy proven steatosis, weight reduction of 5% has been shown to improve the steatosis [145,146] but no concomitant improvement in post-operative recovery has been demonstrated [147]. Patients are often malnourished pre-operatively [57] and improving their nutritional status has been shown to reduce complications particularly in cirrhotic patients [58,148]. However, these studies have failed to demonstrate a link between malnutrition and PHLF [3] and importantly there is no evidence to support delaying surgery while nutritional status is addressed unless the patient is significantly malnourished [18,149,150]. Pre-operative screening for diabetes mellitus is essential and oral carbohydrate loading should be considered to limit post-operative insulin resitance [151]. Studies have failed to show a benefit from pre-operative percutaneous transhepatic drainage (PTD) in the setting of cholestasis. However, complication rates are significant and PTD has been shown to prolong hospital stay [31,152,153]. PTD is only advocated to reduce morbidity when cholestasis is accompanied by segmental cholangitis in patients with a biliary carcinoma [154].
Improve FLR
Portal vein embolization (PVE) is a percutaneous procedure that occludes a branch of the portal vein. This induces apoptosis of the ipsilateral lobe and stimulates growth of the contralateral lobe, thus increasing FLR volume [155]. It is indicated in patients with normal liver function and a FLR volume of less than 25-30% [4], or where liver function is impaired (ICG- 15 of 15-20%) when FLR volume is less than 40-45% (155-157]. PVE can offer a FLR volume increase of between 20-46% and this is dependent on the patient’s comorbidity and extent of hepatic parenchymal disease with peak growth at 2-4 weeks post treatment [155,157-159]. Patients who do not demonstrate a significant increase in liver volume after PVE are likely to have impaired regenerative capacity and therefore are unlikely to tolerate extensive resection [159.) However, there is some evidence to suggest that PVE can cause a tumour flare in the ipsilateral lobe resulting from an increase in hepatic arterial flow to that lobe [160,161]. To combat this, neoadjuvant chemotherapy can be used to reduce tumour growth prior to resection [162,163]. Portal vein ligation (PVL) is occasionally preferred to PVE especially when bi-lobar tumour invasion occurs, necessitating a twostage resection to maintain an adequate FLR [164,165]. In this approach, the contralateral portal vein is ligated during the initial surgery followed by interval of between 3-6 weeks, after which the second, and often more extensive resection is performed [9]. PVE and PVL were shown to be comparable in a meta-analysis that demonstrated no significant difference in FLR volume achieved by either technique [166]. There is evidence that, in selected cases, two-stage hepatectomy in combination with PVE, PVL or neoadjuvant radiotherapy can increase FLR and overall survival rates [21,164,167,168].
Operative considerations
As previously discussed, excessive blood loss is a risk factor for PHLF and surgery should be performed with a central venous pressure (CVP) of less than 5mmHg limits bleeding without affecting renal function [169-171]. Ischaemic preconditioning (IP) reduces hepatic parenchymal damage and is used prior to either intermittent or continuous portal triad clamping to reduce intra-operative blood loss. Intermittent clamping is preferred to continuous clamping typically in a 15-minute clamp to 5-minute unclamp ratio [94,172,173]. Intermittent portal triad clamping is preferable to total vascular exclusion, which has been shown to induce more haemodynamic instability and a higher complication rate [154].
Management
Post-operatively, patients should be monitored for clinical or biochemical evidence of liver failure, particularly ascites, hepatic encephalopathy, coagulopathy and hyperbilirubinaemia. Close attention must also be paid to nutrition, haemodynamic status, renal function and early signs of infection should warrant a low threshold for treatment [174,175]. Sepsis can exacerbate PHLF and bacterial infection is present in 80% of patients with PHLF [70]. Sepsis should always be considered in any acute deterioration and should be managed with microbiology involvement [176]. Antibiotic prophylaxis has not been demonstrated to reduce the incidence of sepsis or PHLF [177], however antibiotics may be of benefit once PHLF is established [178,179].
Current practice for the management of PHLF largely mimics that of acute liver failure with a focus on goal-directed therapy and end organ support in an intensive care setting [180,181]. As gastrointestinal bleeding is a recognized complication of PHLF, proton pump inhibitors or H2-receptor antagonists are routinely administered in mechanically ventilated patients [182,183].
Extracorporeal Liver Support (ELS)
ELS devices detoxify existing plasma or replace it with fresh frozen plasma, allowing the administration of plasma components such as albumin and clotting factors while removing toxic compounds such as ammonium which is water soluble and when in excess results in hepatic encephalopathy. ELS has been shown to improve clinical condition but not survival [184,185].
Molecular Absorbent Recirculating System (MARS®)
MARS® is an extracorporeal system which dialyses plasma albumin and albumin bound toxins against an albumin enriched dialysate [186,187]. Although MARS® has provided promising results for the treatment of acute liver failure, [188] it is yet to demonstrate a survival benefit for PHLF [189- 192].
Modified fractionated plasma separation and adsorption (Prometheus®)
Like MARS®, Prometheus® utilises fractionated plasma separation and albumin dialysate to remove albumin-bound toxins through a semi-permeable membrane, after which the detoxified albumin is returned to the patient [186,187]. Although the detoxifying ability of Prometheus® appears superior to that of MARS® there is a lack of evidence to suggest any advantage in the management for PHLF [193].
Liver transplantation
Rescue hepatectomy and emergency liver transplantation is a last resort when supportive methods have failed. Many patients with PHLF are not candidates for further major surgery and there is a lack of criteria directing the selection of patients for transplantation. Van den Broek proposed that patients with favourable tumour characteristics without significant comorbidities limiting life expectancy should be considered [3].
Discussion and Conclusion
PHLF is still a challenging clinical condition that is difficult to treat. The prevention and early recognition remain the mainstays of management. The lack of a universally accepted definition hinders the study of PHLF owing to the difficulty of cross-comparison. With the increasing prevalence of parenchymal disease such as cirrhosis, non-alcoholic fatty liver disease and CASH, it is becoming an increasing health and economic burden and further studies are required to help identify those patients at risk, methods of pre-operative optimisation and the effective management of PHLF. This will improve patient care, morbidity and ultimately mortality from this complex post-operative complication.
REFERENCES
- Garcea G, Ong SL, Maddern GJ. Predicting liver failure following major hepatectomy. Dig Liver Dis. 2009;41:798-806.
- Hammond JS, Guha IN, Beckingham IJ, et al. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188-200.
- Van Den Broek MAJ, Olde Damink SWM, Dejong CHC, et al. Liver failure after partial hepatic resection: Definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-80.
- Ribero D, Chun YS, Vauthey J-N. Standardized liver volumetry for portal vein embolization. Semin Intervent Radiol. 2008;25:104-9.
- Filicori F, Keutgen XM, Zanello M, et al. Prognostic criteria for postoperative mortality in 170 patients undergoing major right hepatectomy. Hepatobiliary Pancreat Dis Int. 2012;11:507-12.
- Kamiyama T, Nakanishi K, Yokoo H, et al. Analysis of the risk factors for early death due to disease recurrence or progression within 1 year after hepatectomy in patients with hepatocellular carcinoma. World J Surg Oncol. 2012;10:107.
- Won ACM, Testa G. Chronic obstructive uropathy due to uretero-inguinal hernia: A case report. Int J Surg Case Rep. 2012;3:379-81.
- Ren Z, Xu Y, Zhu S. Indocyanine green retention test avoiding liver failure after hepatectomy for hepatolithiasis. Hepatogastroenterology. 2012;59:782-4.
- Lafaro K, Buettner S, Maqsood H, et al. Defining post hepatectomy liver insufficiency: Where do we stand? J Gastrointest Surg. 2015;19:2079-92.
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:98--99.
- Bolder U, Brune A, Schmidt S, et al. Preoperative assessment of mortality risk in hepatic resection by clinical variables: A multivariate analysis. Liver Transplant Surg. 1999;5:227-37.
- Detroz B, Sugarbaker PH, Knol JA, et al. Causes of death in patients undergoing liver surgery. In: Springer, Boston, MA. 1994:241-57.
- Schreckenbach T, Liese J, Bechstein WO, et al. Posthepatectomy liver failure. Dig Surg. 2012;29:79-85.
- Rahbari NN, Reissfelder C, Koch M, et al. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: A validation analysis in 807 Patients. Ann Surg Oncol. 2011;18:3640-349.
- Balzan S, Belghiti J, Farges O, et al. The and quot criteria and quot; on postoperative day 5: An accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-8.
- Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-62.
- Farges O, Malassagne B, Flejou JF, et al. Risk of major liver resection in patients with underlying chronic liver disease: A reappraisal. Ann Surg. 1999;229:210-15.
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: Analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708.
- Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-9.
- Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: How I do it. J Hepatobiliary Pancreat Surg. 2005;12:31-7.
- Clavien P-A, Petrowsky H, De Oliveira ML, et al. Strategies for Safer Liver Surgery and Partial Liver Transplantation. N Engl J Med. 2007;356:1545-59.
- Kaibori M, Inoue T, Sakakura Y, et al. Impairment of activation of hepatocyte growth factor precursor into its mature form in rats with liver cirrhosis. J Surg Re. 2002;106:108-114.
- Zhao G, Nakano K, Chijiiwa K, et al. Inhibited Activities in CCAAT/Enhancer-Binding Protein, Activating Protein-1 and Cyclins after Hepatectomy in Rats with Thioacetamide-Induced Liver Cirrhosis. Biochem Biophys Res Commun. 2002; 292:474-81.
- Yamanaka N, Okamoto E, Oriyama T, et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg. 1994;219:342-46.
- Hemming AW, Scudamore CH, Shackleton CR, et al. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. J Surg. 1992;163:515-18.
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-24.
- Aloysius MM, Zaitoun AM, Beckingham IJ, et al. The pathological response to neoadjuvant chemotherapy with FOLFOX-4 for colorectal liver metastases: a comparative study. Virchows Arch. 2007;451:943-48.
- Makino H, Shimizu H, Ito H, et al. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol. 2006;12:2053-59.
- Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302-8.
- Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB Oxford. 2005;7:252-53.
- Sewnath ME, Karsten TM, Prins MH et al. A meta-analysis on the efficacy of preoperative biliary drainage for tumors causing obstructive jaundice. Ann Surg. 2002;236:17-27
- Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg. 2009;16:145-55.
- Kanda H, Nimura Y, Yasui A, et al. Hepatic blood flow after acute biliary obstruction and drainage in conscious dogs. Hepatogastroenterology. 1996;43:235-40.
- Nakano K, Chijiiwa K, Tanaka M. Lower Activity of CCAAT/Enhancer-Binding Protein and Expression of Cyclin E, but Not Cyclin D1, Activating Protein-1 and p21WAF1, after Partial Hepatectomy in Obstructive Jaundice. Biochem Biophys Res Commun. 2001;280:640-5.
- Baer HU, Guastella T, Wheatley AM, et al. Acute effects of partial hepatectomy on liver blood flow in the jaundiced rat. J Hepatol. 1993;19:377-82.
- Bissig KD, Marti U, Solioz M, et al. Epidermal growth factor is decreased in liver of rats with biliary cirrhosis but does not act as paracrine growth factor immediately after hepatectomy. J Hepatol. 2000;33:275-81.
- Fujiwara Y, Shimada M, Yamashita Y, et al. cytokine characteristics of jaundice in mouse liver. Cytokine. 2001;13:188-192.
- Wang H, Ohkohchi N, Enomoto Y, et al. Effect of Portocaval Shunt on Residual Extreme Small Liver after Extended Hepatectomy in Porcine. World J Surg. 2006; 30:2014-22.
- Sano T, Ajiki T, Takeyama Y, et al. Internal biliary drainage improves decreased number of gut mucosal T lymphocytes and MAdCAM-1 expression in jaundiced rats. Surgery 2004;136:693-99.
- Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg. 2003;7:325-30.
- Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495-506.
- Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171-76.
- Simon-Santamaria J, Malovic I, Warren A, et al. Age-Related Changes in Scavenger Receptor-Mediated Endocytosis in Rat Liver Sinusoidal Endothelial Cells. Journals Gerontol Ser A. 2010;65:951-60.
- Le Couteur DG, Warren A, Cogger VC, et al. Old age and the hepatic sinusoid. Anat Rec Adv Integr Anat Evol Biol. 2008;291:672-83.
- Alfieri S, Carriero C, Caprino P, et al. Avoiding early postoperative complications in liver surgery. A multivariate analysis of 254 patients consecutively observed. Dig Liver Dis. 2001;33:341-46.
- Koperna T, Kisser M, Schulz F. Hepatic resection in the elderly. World J Surg. 1998;22:406-12.
- Tietz NW, Shuey DF, Wekstein DR. Laboratory values in fit aging individuals--sexagenarians through centenarians. Clin Chem. 1992;38:1167-85.
- Aldrighetti L, Arru M, Caterini R, et al. Impact of Advanced Age on the Outcome of Liver Resection. World J Surg. 2003;27:1149-54.
- Nanashima A, Abo T, Nonaka T, et al. Prognosis of patients with hepatocellular carcinoma after hepatic resection: Are elderly patients suitable for surgery? J Surg Oncol. 2011;104:284-91.
- Kim JM, Cho BI, Kwon CHD, et al. Hepatectomy is a reasonable option for older patients with hepatocellular carcinoma. Am J Surg. 2015;209:391-97.
- Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg 6:88-94.
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300.
- Huo T-I, Lui W-Y, Huang Y-H, et al. Diabetes mellitus is a risk factor for hepatic decompensation in patients with hepatocellular carcinoma undergoing resection: a longitudinal study. Am J Gastroenterol. 2003;98:2293-8.
- Bucher NL. Insulin, glucagon, and the liver. Adv Enzyme Regul 1976;15:221-30.
- Zarzavadjian Le Bian A, Costi R, Constantinides V, et al. Metabolic disorders, non-alcoholic fatty liver disease and major liver resection: An underestimated perioperative risk. J Gastrointest Surg. 2012;16:2247-55.
- Lautz HU, Selberg O, Körber J, et al. Protein-calorie malnutrition in liver cirrhosis. Clin Investig. 1992;70:478-86.
- Read JA, Boris Choy ST, Beale PJ, et al. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer. 2006;55:78-85.
- Fan S-T, Lo C-M, Lai E, et al. Perioperative nutritional support in patients undergoing hepatectomy for hepatocellular carcinoma. N Engl J Med. 1994;331:1547-52.
- Bozzetti F. Rationale and indications for preoperative feeding of malnourished surgical cancer patients. Nutrition. 18:953-959.
- Fan ST. Review: Nutritional support for patients with cirrhosis. J Gastroenterol Hepatol. 1997;12:282-6.
- Awad S, Constantin-Teodosiu D, Macdonald IA, et al. Short-term starvation and mitochondrial dysfunction - A possible mechanism leading to postoperative insulin resistance. Clin Nutr. 2009;28:497-509.
- House MG, Ito H, Gönen M, et al. Survival after Hepatic Resection for Metastatic Colorectal Cancer: Trends in Outcomes for 1,600 Patients during Two Decades at a Single Institution. J Am Coll Surg. 2010;210:744-52.
- Virani S, Michaelson JS, Hutter MM, et al. Morbidity and mortality after liver resection: Results of the patient safety in surgery study. J Am Coll Surg. 2007;204:1284-92.
- Rees M, Plant G, Bygrave S. Late results justify resection for multiple hepatic metastases from colorectal cancer. Br J Surg. 1997;84:1136-40.
- Midorikawa Y, Kubota K, Takayama T, et al. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126:484-91.
- Akashi K, Mizuno S, Isaji S. Comparative study of perioperative management of hepatic resection. Dig Dis Sci. 2000;45:1988-95.
- McCall J, Koea J, Gunn K, et al. Liver resections in Auckland: Mortality, morbidity and blood product use. N Z Med J. 2001;114:516-19.
- Das BC, Isaji S, Kawarada Y. Analysis of 100 consecutive hepatectomies: risk factors in patients with liver cirrhosis or obstructive jaundice. World J Surg. 2001;25:266-73.
- Sun H-C, Qin L-X, Wang L, et al. Risk factors for postoperative complications after liver resection. Hepatobiliary Pancreat Dis Int. 2005;3:370-34.
- Schindl MJ, Redhead DN, Fearon KCH, et al. Edinburgh liver surgery and transplantation experimental research group eLISTER. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-96.
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving Resectability of Hepatic Colorectal Metastases: Expert Consensus Statement. Ann Surg Oncol. 2006;13:1271-80.
- Fan ST. Methods and related drawbacks in estimation of surgical risks in cirrhotic patients undergoing hepatectomy. Hepatogastroenterology. 2002;49:17-20.
- Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: Decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12:16-22.
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860-70.
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: Analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406.
- Jensen LS, Andersen AJ, Christiansen PM, et al. Postoperative infection and natural killer cell function following blood transfusion in patients undergoing elective colorectal surgery. Br J Surg. 1992;79:513-6.
- Silva MA, Muralidharan V, Mirza DF. The management of coagulopathy and blood loss in liver surgery. Semin Hematol. 2004;411 Suppl 1:132-9.
- Luyer MDP, Buurman WA, Hadfoune M, et al. Pretreatment with high-fat enteral nutrition reduces endotoxin and tumor necrosis factor-?? and preserves gut barrier function early after hemorrhagic shock. Shock. 2004;21:65-71.
- Azoulay D, Andreani P, Maggi U, et al. Combined liver resection and reconstruction of the supra-renal vena cava. Ann Surg. 2006;244:80-8.
- Sarmiento JM, Bower TC, Cherry KJ, et al. Is combined partial hepatectomy with segmental resection of inferior vena cava justified for malignancy? Arch Surg. 2003;138:624.
- Sakamoto T, Liu Z, Murase N, et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403-11.
- Jin X, Zhang Z, Beer-Stolz D, et al. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology 2007;46:802-12.
- Morita T, Togo S, Kubota T, et al. Mechanism of postoperative liver failure after excessive hepatectomy investigated using a cDNA microarray. J Hepatobiliary Pancreat Surg. 2002;9:352-9.
- Kin Y, Nimura Y, Hayakawa N, et al. Doppler analysis of hepatic blood flow predicts liver dysfunction after major hepatectomy. World J Surg. 1994;18:143-9.
- Palmes D, Budny TB, Dietl K-H, et al. Detrimental effect of sinusoidal overperfusion after liver resection and partial liver transplantation. Transpl Int. 2004;17:862-71.
- Fukuchi T, Hirose H, Onitsuka A, et al. Effects of portal-systemic shunt following 90% partial hepatectomy in rats. J Surg Res. 2000;89:126-31.
- Ito K, Ozasa H, Horikawa S. Effects of prior splenectomy on remnant liver after partial hepatectomy with Pringle maneuver in rats. Liver Int. 2005;25:438-44.
- Iida T, Yagi S, Taniguchi K, et al. Improvement of morphological changes after 70% hepatectomy with portocaval shunt: Preclinical study in porcine model. J Surg Res. 2007;143:238-46.
- Hata Y, Yoshikawa Y, Une Y, et al. Liver regeneration following portacaval shunt in rats: 3’,5’-cyclic AMP changes in plasma and liver tissue. Res Exp Med Berl. 1992;192:131-6.
- Pouyet M, Méchet I, Paquet C, et al. Liver regeneration and hemodynamics in pigs with mesocaval shunt. J Surg Res. 2007;138:128-34.
- Szijártó A, Hahn O, Lotz G, et al. Effect of ischemic preconditioning on rat liver microcirculation monitored with laser Doppler flowmetry. J Surg Res. 2006;131:150-7.
- Yin DP, Sankary HN, Chong AS, et al. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152-7.
- Gomez D, Homer Vanniasinkam S, Graham AM, et al. Role of ischaemic preconditioning in liver regeneration following major liver resection and transplantation. World J Gastroenterol. 2007;13:657-70.
- Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology 2003;125:917-36.
- Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol - Gastrointest Liver Physiol. 2003; 284:G15-6.
- Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-9.
- Boermeester MA, Straatsburg IH, Houdijk AP, et al. Endotoxin and interleukin-1 related hepatic inflammatory response promotes liver failure after partial hepatectomy. Hepatology. 1995;22:1499-506.
- Akita K, Okuno M, Enya M, et al. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002; 123:352-64.
- Roelofsen H, van der Veere CN, Ottenhoff R, et al. Decreased bilirubin transport in the perfused liver of endotoxemic rats. Gastroenterology. 1994;107:1075-84.
- Nolan JP. Endotoxin, reticuloendothelial function, and liver injury. Hepatol. 1981;1:458-65.
- Selzner N, Selzner M, Odermatt B, et al. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-α/IL-6 in mice. Gastroenterol. 2003;124:692-700.
- Gross K, Katz S, Dunn SP, et al. Bacterial clearance in the intact and regenerating liver. J Pediatr Surg 1985;20:320-3.
- Chaisson ML, Brooling JT, Ladiges W, et al. Hepatocyte-specific inhibition of NF-κB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193-202.
- Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatol. 2000;31:864-71.
- Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85.
- Schroeder RA, Marroquin CE, Bute BP, et al. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373-9.
- Ercolani G, Grazi GL, Callivà R, et al. The lidocaine MEGX test as an index of hepatic function: its clinical usefulness in liver surgery. Surg. 2000;127127:464-71.
- Huang L, Li J, Yan J-J, et al. Prealbumin is predictive for postoperative liver insufficiency in patients undergoing liver resection. World J Gastroenterol. 2012;18:7021-25.
- Huo T-I, Lee S-D, Lin H-C. Selecting an optimal prognostic system for liver cirrhosis: The model for end-stage liver disease and beyond. Liver Int. 2008;28:606-13.
- Angermayr B, Cejna M, Karnel F, et al. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879-85.
- Wu CC, Ho WL, Lin MC, et al. Is hepatic resection absolutely contraindicated for hepatocellular carcinoma in Child-Pugh class C cirrhotic patients? Hepatogastroenterology. 1999;4626:635-39.
- Cucchetti A, Ercolani G, Vivarelli M, et al. Impact of model for end-stage liver disease MELD score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transplant 2006;12:966-71.
- Ulla M, Ardiles V, Levy-Yeyati E, et al. New surgical strategy to induce liver hypertrophy: role of MDCT-volumetry to monitor and predict liver growth. Hepatogastroenterol. 2013;60:337-42.
- Vauthey J-N, Chaoui A, Do K-A, et al. Standardized measurement of the future liver remnant prior to extended liver resection: Methodology and clinical associations. Surg. 2000;127:512-519.
- Kubota K, Makuuchi M, Kusaka K, et al. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatol. 1997;26:1176-81.
- Dello SAWG, Van Dam RM, Slangen JJG, et al. Liver volumetry plug and play: Do it yourself with image. World J Surg. 2007;31:2215-21.
- D’Onofrio M, De Robertis R, Demozzi E, et al. Liver volumetry: Is imaging reliable? Personal experience and review of the literature. World J Radiol. 2014;6:62-71.
- Yamanaka N, Okamoto E, Kawamura E, et al. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatol. 1993;18:79-85.
- Uchiyama K, Mori K, Tabuse K, et al. Assessment of liver function for successful hepatectomy in patients with hepatocellular carcinoma with impaired hepatic function. J Hepatobiliary Pancreat Surg. 2008;15:596-602.
- Du Z-G, Li B, Wei Y-G, et al. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:265-9.
- Lu Y, Wu Z, Liu C, et al. Hepatic volumetry with PhotoShop in personal computer. Hepatobiliary Pancreat Dis Int 2004;3:82-5.
- Faybik P, Hetz H. Plasma Disappearance Rate of Indocyanine Green in Liver Dysfunction. Transplant Proc. 2006;38:801-2.
- Chijiiwa K, Watanabe M, Nakano K, et al. Biliary indocyanine green excretion as a predictor of hepatic adenosine triphosphate levels in patients with obstructive jaundice. Am J Surg. 2000;179:161-6.
- De Liguori Carino N, O’Reilly DA, Dajani K, et al. Perioperative use of the LiMON method of indocyanine green elimination measurement for the prediction and early detection of post-hepatectomy liver failure. Eur J Surg Oncol. 2009;35:957-62.
- Fan ST. Liver functional reserve estimation: State of the art and relevance for local treatments. J Hepatobiliary Pancreat Sci. 2010;17:380-4.
- Zipprich A, Kuss O, Rogowski S, et al. Incorporating indocyanin green clearance into the model for end stage liver disease MELD-ICG improves prognostic accuracy in intermediate to advanced cirrhosis. Gut. 2010;59:963-8.
- Lam CM, Fan ST, Lo CM, et al. Major hepatectomy for hepatocellular carcinoma in patients with an unsatisfactory indocyanine green clearance test. Br J Surg. 1999;86:1012-17.
- Poon RT-P, Fan S-T. Hepatectomy for hepatocellular carcinoma: Patient selection and postoperative outcome. Liver Transplant. 2004;10:S39-45.
- Lau H, Man K, Fan ST, et al. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-59.
- Oussoultzoglou E, Jaeck D, Addeo P, et al. Prediction of mortality rate after major hepatectomy in patients without cirrhosis. Arch Surg. 2010;145:1075.
- Ohwada S, Kawate S, Hamada K, et al. Perioperative real-time monitoring of indocyanine green clearance by pulse spectrophotometry predicts remnant liver functional reserve in resection of hepatocellular carcinoma. Br J Surg. 2006;93:339-46.
- Yokoyama Y, Nishio H, Ebata T, et al. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97:1260-68.
- Derpapas MK, Contis J, Fragulidis GP, et al. Correlation of the ICG test with risk factors and postoperative outcomes following hepatic resection. J BUON. 2013;18:703-7.
- Tralhao JG, Hoti E, Oliveiros B, et al. Study of perioperative liver function by dynamic monitoring of ICG-clearance. Hepatogastroenterol. 2012;59:1179-83.
- Okochi O, Kaneko T, Sugimoto H, et al. ICG pulse spectrophotometry for perioperative liver function in hepatectomy. J Surg Res. 2002;103:109-13.
- Sugimoto H, Okochi O, Hirota M, et al. Early detection of liver failure after hepatectomy by indocyanine green elimination rate measured by pulse dye-densitometry. J Hepatobiliary Pancreat Surg. 2006;13:543-8.
- Ashwell G, Harford J. Carbohydrate specific receptors of the liver. Annu Rev Biochem 1982;51:531-54.
- Kwon A-H, Matsui Y, Kaibori M, et al. Preoperative regional maximal removal rate of technetium-99m-galactosyl human serum albumin GSA-Rmax is useful for judging the safety of hepatic resection. Surg. 2006;140:379-86.
- Satoh K, Yamamoto Y, Nishiyama Y, et al. 99mTc-GSA liver dynamic SPECT for the preoperative assessment of hepatectomy. Ann Nucl Med. 2003;17:61-7.
- Bargetzi MJ, Aoyama T, Gonzalez FJ, et al. Lidocaine metabolism in human liver microsomes by cytochrome P450IIIA4. Clin Pharmacol Ther. 1989;46:521-7.
- Oellerich M, Burdelski M, Ringe B, et al. Lignocaine metabolite formation as a measure of pre-transplant liver function. Lancet London, England. 1989;1:640-2.
- Conti F, Dousset B, Cherruau B, et al. Use of lidocaine metabolism to test liver function during the long-term follow-up of liver transplant recipients. Clin Transplant. 2004;18:235-41.
- Lorf T, Schnitzbauer AA, Schaefers SKH, et al. Prognostic value of the monoethylglycinexylidide MEGX-test prior to liver resection. Hepatogastroenterol. 2008;55:539-43.
- Redaelli CA, Dufour J-F, Wagner M, et al. Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg. 2002;235:77-85.
- Hwang S, Lee S-G, Jang S-J, et al. The effect of donor weight reduction on hepatic steatosis for living donor liver transplantation. Liver Transplant. 2004;10:721-5.
- Nakamuta M, Morizono S, Soejima Y, et al. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplant.2005;80:608-12.
- Cucchetti A, Cescon M, Ercolani G, et al. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br J Surg. 2011;98:1147-54.
- Merli M, Nicolini G, Angeloni S, et al. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18:978-86.
- Shirabe K, Matsumata T, Shimada M, et al. A comparison of parenteral hyperalimentation and early enteral feeding regarding systemic immunity after major hepatic resection--the results of a randomized prospective study. Hepatogastroenterol. 1997;44:205-9.
- Richter B, Schmandra TC, Golling M, et al. Nutritional support after open liver resection: A systematic review. Dig Surg. 2006;23:139-45.
- Awad S, Constantin-Teodosiu D, Constantin D, et al. Cellular mechanisms underlying the protective effects of preoperative feeding. Ann Surg. 2010;252:247-53.
- Pitt HA, Gomes AS, Lois JF, et al. Does preoperative percutaneous biliary drainage reduce operative risk or increase hospital cost? Ann Surg. 1985;201:545-53.
- Hatfield AR, Tobias R, Terblanche J, et al. Preoperative external biliary drainage in obstructive jaundice. A prospective controlled clinical trial. Lancet London, England. 1982;2:896-9.
- Kanai M, Nimura Y, Kamiya J, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surg. 1996;119:498-504.
- Madoff DC, Abdalla EK, Vauthey J-N. Portal Vein Embolization in Preparation for Major Hepatic Resection: Evolution of a New Standard of Care. J Vasc Interv Radiol. 2005;16:779-90.
- Imamura H, Shimada R, Kubota M, et al. Preoperative portal vein embolization: An audit of 84 patients. Hepatol 1999;29:1099-105.
- Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480-6.
- Azoulay D, Castaing D, Krissat J, et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232:665-72.
- Farges O, Belghiti J, Kianmanesh R, et al. Portal Vein Embolization Before Right Hepatectomy. Ann Surg. 2003;237:208-17.
- Heinrich S, Jochum W, Graf R, et al. Portal vein ligation and partial hepatectomy differentially influence growth of intrahepatic metastasis and liver regeneration in mice. J Hepatol. 2006;45:35-42.
- Pamecha V, Levene A, Grillo F, et al. Effect of portal vein embolisation on the growth rate of colorectal liver metastases. Br J Cancer. 2009;100:617-22.
- Selzner N, Pestalozzi BC, Kadry Z, Selzner M, Wildermuth S, Clavien P-A. Downstaging colorectal liver metastases by concomitant unilateral portal vein ligation and selective intra-arterial chemotherapy. Br J Surg. 2006;93:587-92.
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-57-8.
- Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000; 232:777-85.
- Tsai S, Marques HP, De Jong MC, et al. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB 2010;12:262-9.
- Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery. 2015;157:690-8.
- Tanaka K, Shimada H, Matsuo K, Ueda M, Endo I, Togo S. Remnant liver regeneration after two-stage hepatectomy for multiple bilobar colorectal metastases. Eur J Surg Oncol. 2007;33:329-35.
- Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber J-C, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-49-51.
- Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620-5.
- Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058-60.
- Vassiliou I, Arkadopoulos N, Stafyla V, et al. The introduction of a simple maneuver to reduce the risk of postoperative bleeding after major hepatectomies. J Hepatobiliary Pancreat Surg. 2009;16:552-6.
- Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1999;229:369-75.
- Belghiti J, Noun R, Zante E, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155-61.
- Bernal W, Wendon J. Acute Liver Failure. N Engl J Med 2013;369:2525-34.
- Singanayagam A, Bernal W. Update on acute liver failure. Curr Opin Crit Care. 2015;21:134-41.
- Szakmany T, Pugh R, Kopczynska M, et al. Defining sepsis on the wards: Results of a multi-centre point-prevalence study comparing two sepsis definitions. Anaesthesia. 2018;73:195-204.
- Wu CC, Yeh DC, Lin MC, et al. Prospective randomized trial of systemic antibiotics in patients undergoing liver resection. Br J Surg. 1998;85:489-3.
- Jin S, Fu Q, Wuyun G, et al. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983-91.
- Rolando N, Gimson A, Wade J, et al. Prospective controlled trial of selective parenteral and enteral antimicrobial regimen in fulminant liver failure. Hepatol. 1993;17:196-201.
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368-77.
- Jalan R. Acute liver failure: current management and future prospects. J Hepatol. 2005;42:S115-23.
- Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998;338:791-7.
- Levy MJ, Seelig CB, Robinson NJ, et al. Comparison of omeprazole and ranitidine for stress ulcer prophylaxis. Dig Dis Sci. 1997;42:1255-9.
- Liu JP, Gluud LL, Als-Nielsen B, et al. Artificial and bioartificial support systems for liver failure. In: Liu JP, ed. cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd. 2004;CD003628.
- Onodera K, Sakata H, Yonekawa M, et al. Artificial liver support at present and in the future. J Artif Organs. 2006; 9:17-28.
- Sen S, Williams R, Jalan R. Emerging Indications for Albumin Dialysis. Am J Gastroenterol 2005;100:468-475.
- Krisper P, Stauber RE. Technology Insight: artificial extracorporeal liver support—how does Prometheus® compare with MARS®? Nat Clin Pract Nephrol. 2007;3:267-276.
- Heemann U, Treichel U, Loock J, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: A prospective, controlled study. Hepatology. 2002;36:949-58.
- Rittler P, Ketscher C, Inthorn D, et al. Use of the molecular adsorbent recycling system in the treatment of postoperative hepatic failure and septic multiple organ dysfunction - preliminary results. Liver Int. 2004;24:136-141.
- van de Kerkhove M-P, de Jong KP, Rijken AM, et al. MARS treatment in posthepatectomy liver failure. Liver Int 2003;23 Suppl 3:44-51.
- Kellersmann R, Gassel H-J, Bühler C, et al. Application of Molecular Adsorbent Recirculating System in patients with severe liver failure after hepatic resection or transplantation: initial single-centre experiences. Liver.2002;22 Suppl 2:56-58.
- Chiu A, Chan LMY, Fan ST. Molecular adsorbent recirculating system treatment for patients with liver failure: the Hong Kong experience. Liver Int. 2006;26:695-702.
- Krisper P, Haditsch B, Stauber R, et al. In vivo quantification of liver dialysis: Comparison of albumin dialysis and fractionated plasma separation. J Hepatol. 2005;43:451-7.