Melatonin promotes equine cumulus-oocyte complex survival during in vitro maturation
2 Departamento de Reproducción, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, CDMX, Mexico, Email: boeta.myriam@unam.mx
3 Centre de Recherche en Reproduction et Fertilité, Faculté de Médecine Vétérinaire, Université de Montréal, St-Hyacinthe, Quebec, Canada, Email: rodrigo.lopezgonzalez@umassmed.edu
St-Hyacinthe, Quebec
, Canada, Email: mouhamadou.diaw@umontreal.caHilda Guerrero-Netro, Departamento de Reproducción, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, Ciudad Universitaria, Ciudad de México, 04510, Mexico, Email: hilda_morayma@hotmail.com
Mouhamadou Diaw, Département des Sciences Cliniques, Faculté́ de Médecine Vétérinaire Université́ de Montréal, Canada, Tel: +1 (450) 773-8521 ext. 8388, Email: mouhamadou.diaw@umontreal.ca
Received: 26-Oct-2019 Accepted Date: Jan 23, 2020; Published: 30-Jan-2020
Citation: Navarro LC, Boeta M, Lopez RG et al. Melatonin promotes equine cumulus-oocyte complex survival during in vitro maturation. Reprod biol April 2020;4(1):16-30.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Keywords
Melatonin; Oocyte; Equine; IVM; Apoptosis
Introduction
To achieve maturation, the oocyte undergoes nuclear and cytoplasmic changes, which requires adequate cumulus expansion. The synchrony between these changes is necessary for future fertilization and subsequent embryonic development [1]. In vivo, oocyte maturation, cumulus expansion, and ovulation of the cumulus-oocyte complex (COC) are induced by the pre-ovulatory increase of the luteinizing hormone levels [2]. The effects of luteinizing hormone in COC are mediated by epidermal growth factor-like ligands (EGF-L): amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC) [3]. In granulosa cells (GC), these EGF-L undergo proteolytic cleavage by members of the disintegrin and metalloproteinase (ADAM) family, which are subsequently released to activate EGF receptors (EGFR) on COC [4].
Upon activation, they stimulate meiotic resumption and transcription of genes involved in the regulation of cumulus expansion, including tumor necrosis factor-alpha-induced protein 6 (TNFAIP6), prostaglandinendoperoxide synthase 2 (PTGS2), and hyaluronan synthase 2 (HAS2) [4– 6]. In vitro maturation (IVM) environment can affect the above morphological and functional changes, as reported in several species [7,8]. The IVM environment can potentiate COC stress because of multiple nonphysiological stressors, such as temperature, toxicants, and oxidative stress [9].
Oxidative stress, if not controlled, can damage cell membrane lipids, and cause mitochondrial stress and DNA fragmentation, leading to apoptosis [10]. Apoptosis is activated by different pathways, e.g., mitochondrial damage. Mitochondrial damage is characterized by the accumulation of apoptotic signaling protein BAX, suppression of BCL2, and activation of caspases [11].
Further, in vivo, oxidative stress moderated by endogenously produced antioxidants secreted to the follicular fluid by GC, theca cells, and cumulus cells (CCs), creating optimal conditions for COC development [8,9]. A prominent antioxidant and anti-apoptotic agent in the follicle is melatonin (MT), a hormone which is synthesized by the pineal gland and is involved in numerous physiological functions in mammals [12].
The effects of MT are regulated by free-radical scavengers and by activation of specific membrane receptors (MT1 and MT2) [13,14]. The IVM environment can potentiate COC stress because of multiple nonphysiological stressors, such as temperature, toxicants, and oxidative stress [9]. Oxidative stress, if not controlled, can damage cell membrane lipids, and cause mitochondrial stress and DNA fragmentation, leading to apoptosis [10]. Apoptosis is activated by different pathways, e.g., mitochondrial damage. Mitochondrial damage is characterized by the accumulation of apoptotic signaling protein BAX, suppression of BCL2, and activation of caspases [11].
Further, in vivo, oxidative stress moderated by endogenously produced antioxidants secreted to the follicular fluid by GC, theca cells, and cumulus cells (CCs), creating optimal conditions for COC development [8,9]. A prominent antioxidant and anti-apoptotic agent in the follicle is melatonin (MT), a hormone which is synthesized by the pineal gland and is involved in numerous physiological functions in mammals [12].
The effects of MT are regulated by free-radical scavengers and by activation of specific membrane receptors (MT1 and MT2) [13,14]. The IVM environment can potentiate COC stress because of multiple nonphysiological stressors, such as temperature, toxicants, and oxidative stress [9]. Oxidative stress, if not controlled, can damage cell membrane lipids, and cause mitochondrial stress and DNA fragmentation, leading to apoptosis [10]. Apoptosis is activated by different pathways, e.g., mitochondrial damage. Mitochondrial damage is characterized by the accumulation of apoptotic signaling protein BAX, suppression of BCL2, and activation of caspases [11].
Further, in vivo, oxidative stress moderated by endogenously produced antioxidants secreted to the follicular fluid by GC, theca cells, and cumulus cells (CCs), creating optimal conditions for COC development [8,9]. A prominent antioxidant and anti-apoptotic agent in the follicle is melatonin (MT), a hormone which is synthesized by the pineal gland and is involved in numerous physiological functions in mammals [12]. The effects of MT are regulated by free-radical scavengers and by activation of specific membrane receptors (MT1 and MT2) [13,14].
Materials and Methods
Unless specified otherwise, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
In vitro maturation
To assess the effect of MT on cumulus expansion and COC apoptosis, ovaries from adult mares, regardless of the estrous cycle status during the reproductive season, were obtained from a local slaughterhouse and transported to the laboratory at the Université de Montreal (St-Hyacinthe campus) at room temperature (~22°C) in insulated containers. COCs were recovered by aspiration of all follicle walls smaller than 30 mm with a suction pump (suction of approximately 40 mL/min) and placing in an oocyte recovery medium (EquiPro OPU recovery medium; MOFA Global, Verona, WI, USA), as previously described by Diaw et al. [15].
The content was then stereomicroscopically examined; only oocytes with homogeneous cytoplasm were used in subsequent experiments. COCs were transferred to pre-warmed (~38.2°C) 35-mm Petri dishes and washed with equilibrated maturation medium [M199 medium with Earle ’ s salts (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen), 5 mU follicular stimulating hormone (Sioux Biochemicals, Sioux Center, IA, USA), and 25 mg/mL of gentamicin (Gibco, Life Technologies, NY, USA)]. Later, groups of 10 COCs were randomly placed to 100-mL drops of equilibrated maturation medium. Maturation medium was supplemented with MT (N-acetyl-5- methoxytryptamine; 10, 100, or 1000 ng/mL) or vehicle (ethanol). Drops were covered with mineral oil (Sage, In-Vitro Fertilization, Inc., Trumbull, CT, USA) and incubated at 38.2°C in a humidified atmosphere of 5% CO2 in air, for 10, 20, or 30 h, depending on the experiment.
Evaluation of cumulus expansion by stereomicroscopy. The cumulus expansion of COCs was evaluated by stereomicroscopy before and after 20- h and 30-h in vitro culture (IVC), to assess cumulus transition from compact (CP) to expanded (EX). The degree of cumulus expansion was evaluated based on a scoring system, as previously described by Hinrichs et al. [16]. COCs were classified as CP or EX; the cumulus transition from CP to EX was determined based on the difference between the number of CP at the end of culture and the number of CP at the beginning of culture.
Gene expression analysis
After 10, 20, and 30 h of IVC, COCs were denuded by pipetting in phosphate-buffered saline. RNA was extracted from CCs using PicoPure RNA extraction kit (Thermo Fischer Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. The concentration of total RNA was determined by measuring sample absorbance at 260 nm using a NanoDrop 2000 (Thermo Scientific, Wilmington, DE, USA). Reverse transcription of 100 ng RNA was carried out using SuperScript IV VILO master mix (Invitrogen). Real-time polymerase chain reaction (PCR) was performed in a reaction volume of 15°L, using 2° SsoAdavanced universal SYBR Green Supermix and a CFX-96 real-time PCR detection system (Bio- Rad Laboratories Ltd., CA, USA). The equine-specific primers used for expression analysis of target genes are given in Table 1. The primer amplification efficiencies were between 95% and 108%, depending on the primer pair. The reference genes used, ACTB and GAPDH, were determined by using the geNorm software [17-36]. The quantitation cycle (Cq) values did not differ under treatments reported in the current study. The thermal cycling conditions were 3 min at 95°C; followed by 40 cycles of 15 s at 95°C, 30 s at 60°C, and 30 s at 72°C.
| Gene | Primer sequence | ||||
|---|---|---|---|---|---|
| Forward (5´-3´) | Reverse (5´-3´) | Fragment size (bp) | |||
| AREG | TCC TCG GCT CAG CCC ATT AT | ACAGGGGAGATCTCACTTCCTGA | 129 | ||
| EREG | ACA ATC CAC GTG TGG CTC AA | AAC CCA CTT CAC ACC TGC AA | 126 | ||
| EREG | ACA ATC CAC GTG TGG CTC AA | AAC CCA CTT CAC ACC TGC AA | 126 | ||
| PTGS2 | CCC CCA GGG CAC AAA TAT GA | TGA CTT AAA TCC ACC CCG TGA C | 126 | ||
| TNFAIP6 | GAA GGC GGT GTG TGA ATA CGA | GGC TTC ACA ATG GGG TAT CCA | 137 | ||
| HAS2 | ATT TTG GAA ACT GCC CGC CA | CAC AAT GCA TCT TGT TCA GCT CT | 91 | ||
| ADAMTS1 | TCT CAC CAA AGG ACA GGT GC | CCA TCT ACC ACC TTG GGC TG | 85 | ||
| BCL2 | GGA TTG GTG GAA TCT TTG CCT | GTC TAC TTC CTC TGT GAT GTT GTA T | 109 | ||
| BAX | ATC ATG AGC CAC CTC AGT TCC | TGG ATG AAA CCC TGA AGC AAA AGG | 137 | ||
| GAPDH | TGA TTC CAC CCA TGG CAA GT | CAT CGC CCC ATT TGA TGT TG | 122 | ||
| ACTB | GCA CCA GGG CGT GAT GG | TCG ATG GGG TAC TTG AGG GT | 89 | ||
Table 1:Primers used in real-time PCR.
The samples were analyzed in duplicate, and data were normalized to the geometric mean of the reference genes using the 2^(−ΔΔCq) method [37]. Four replicates of each experiment were analyzed on the same plate, and data for all samples were expressed relative to the control sample in the first replicate. The average coefficients of variation were 0.1% and 11% for Cq and ΔΔCq values, respectively.
Evaluation of cell survival and apoptosis by fluorescenceactivated cell sorting (FACS)
At 30-h IVC, CCs were recovered for assessment of cell survival and apoptosis by FACS. CCs were stained with propidium iodide and annexin (using annexin V-FITC apoptosis detection kit), following the manufacturer’s instructions. At least 500 cells per sample were evaluated using a FACSVantage SE system (BD Biosciences, Oakville, ON, Canada) and analyzed using Cell Quest Pro software (BD Biosciences).
Evaluation of apoptosis by cleaved caspase 3
immunofluorescence
Following 20-h or 30-h IVC, the oocytes were recovered to assess caspase 3 activation by immunofluorescence. The oocytes were fixed in 4% paraformaldehyde, washed in 2% Triton-X and 0.05% Tween, blocked with 5% bovine serum albumin, and incubated with rabbit polyclonal antibody against cleaved caspase 3 (dilution 1:300; 9661S, Cell Signaling Technology, Beverly, MA, USA). After incubation with the primary antibodies, the oocytes were washed in phosphate-buffered saline and incubated with Cy3- conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA). The oocytes were examined under a PALM MicroBeam Zeiss epifluorescent microscope. Fluorescence in digital images captured for each fluorescence field using Cy3 filters was quantified using ImageJ software (version 1.40; NIH of Health, Bethesda, MD, USA), Statistical analysis.
All percentage data were subjected to arcsine transformation before statistical analysis. The effects of MT on cumulus expansion, cumulus expansion-related genes expression, and cleaved caspase 3 immunofluorescence levels at different evaluated times were analyzed by one-way ANOVA, followed by Tukey ’ s HSD. FACS data (evaluation of apoptosis) were analyzed by multiple t-tests, to permit simultaneous comparisons. All analyses and graphs were generated using GraphPad Prism 5.0 software package (San Diego, CA, USA). The results are presented as the mean + standard error of the mean (SEM), and all experiments were repeated at least four times. Differences were considered significant at *P< 0.05, **P< 0.01, and ***P< 0.001.
Results
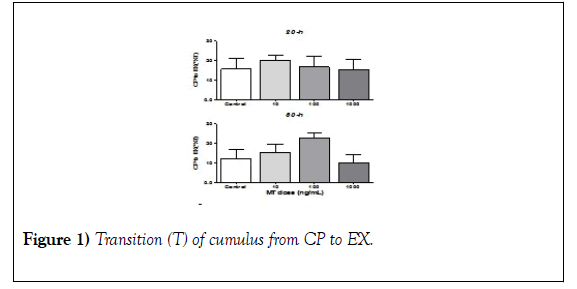
The effect of MT on cumulus transition from CP to EX Evaluation of the cumulus transition from CP to EX revealed no changes associated with MT dosing at any time evaluated (Figure 1; 20-h and 30-h). However, the highest MT dose used (1000 ng/mL) tended to reduce the percentage of transitions from CP to EX compared with the control (Figure 1; 1000 ng/mL).
Groups of COC (n=10) were cultured under different doses of MT (10, 100 and 1000 ng/ml) or without MT (control). Before starting the IVM and after 20 and 30 hours after the IVM began, the cumulus expansion was classified as CP or EX. The cumulus transition from CP to EX was determined by the difference between the number of CP at 20 and 30 h initiated by the MIV concerning the number of CP at the beginning of the MIV. Data were obtained from four (20-h) and seven (30-h) independent replicates and are presented with the mean + SEM. a´. Image of the control group at 20 hours started the MIV.
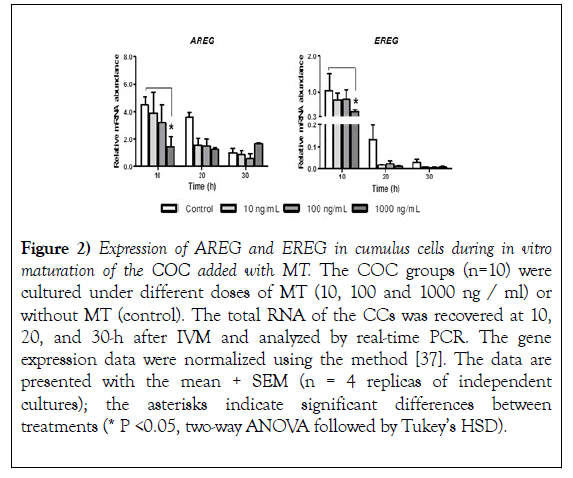
The effect of MT on AREG and EREG gene expression at 10, 20 and 30 hours after IVM, the expression of mRNA coding for AREG and EREG was evaluated in CCs, the evaluation showed that both AREG and EREG significantly reduce (P<0.05) their expression with the addition of 1000 ng/mL at 10 hours started the IVM with respect to the control group (Figure 2, AREG and EREG 10-h). At 20 hours, despite observing a reduction in the expression of AREG and EREG due to the addition of MT, no significant differences were found (Figure 2, AREG and EREG 20- h). At 30 hours, the addition of MT showed no changes concerning the control (Figure 2, AREG and EREG 20-h). Additionally, the study reveals that the expression of AREG and EREG is reduced after the time of MIV (Figure 2).
Figure 2): Expression of AREG and EREG in cumulus cells during in vitro maturation of the COC added with MT. The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng / ml) or without MT (control). The total RNA of the CCs was recovered at 10, 20, and 30-h after IVM and analyzed by real-time PCR. The gene expression data were normalized using the method [37]. The data are presented with the mean + SEM (n = 4 replicas of independent cultures); the asterisks indicate significant differences between treatments (* P <0.05, two-way ANOVA followed by Tukey’s HSD).
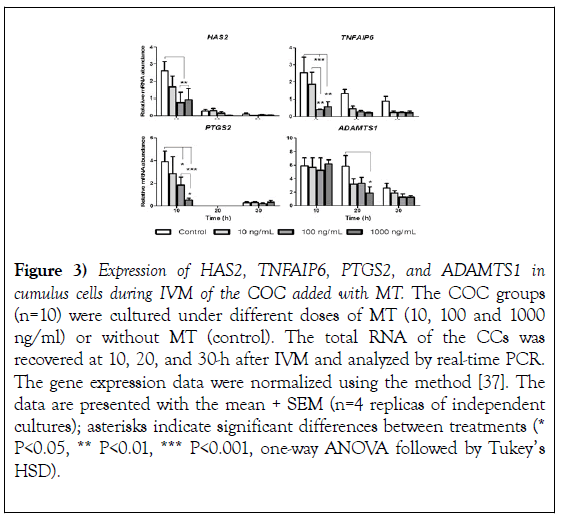
The effect of MT on the expression of cumulus expansion n-related genes After 10, 20 and 30 hours of IVM had elapsed, CCs recovered to evaluate the expression of cumulus expansion-related genes. The evaluation showed that the 10-h higher doses of MT 100 and 1000 ng/ml reduced the abundance of HAS2, TNFAIP6 and PTGS2 mRNA significantly (HAS2 P<0.01, TNFAIP6 P<0.01, PTGS2 P<0.05) concerning control group (Figure 3; HAS2, TNFAIP6, and PTGS2 10-h). The abundance of ADAMTS1 mRNA showed no significant changes at 10-h but was significantly reduced at 20-h by the addition of 1000 ng/ml of MT (Figure 3; ADAMTS1 20-h).
Figure 3): Expression of HAS2, TNFAIP6, PTGS2, and ADAMTS1 in cumulus cells during IVM of the COC added with MT. The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng/ml) or without MT (control). The total RNA of the CCs was recovered at 10, 20, and 30-h after IVM and analyzed by real-time PCR. The gene expression data were normalized using the method [37]. The data are presented with the mean + SEM (n=4 replicas of independent cultures); asterisks indicate significant differences between treatments (* P<0.05, ** P<0.01, *** P<0.001, one-way ANOVA followed by Tukey’s HSD).
At 20-h the evaluation of the expression of HAS2 and TNFAIP6 showed a negative tendency on the addition of MT, but it was not significant (Figure 3, HAS2, TNFAIP6 20-h), curiously the expression of PTGS2 was not detected at 20-h (Figure 3; PTGS2 20-h). At 30 hours, the addition of MT showed no changes in the expression of coding mRNA for HAS2, TNFAIP6, PTGS2, and ADAMTS1 for the control (Figure 3; 30-h). Furthermore, the mRNA expression of all the evaluated genes showed a reduction in advancing the MIV time (Figure 3) response also observed in the expression of AREG and EREG (Figure 2).
The effect of MT on BAX and BCL2 gene expression
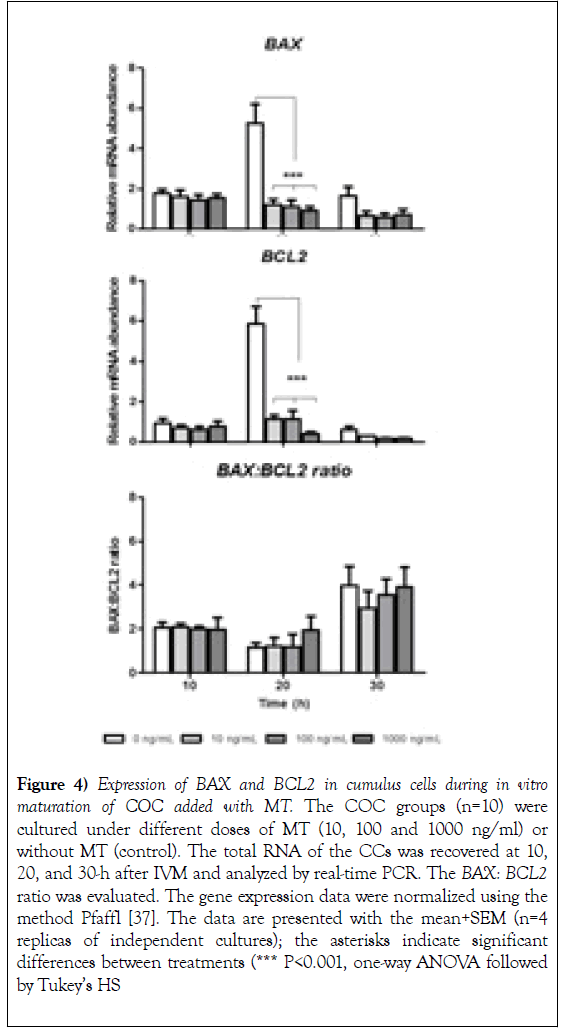
The expression of mRNA coding for BAX and BCL2 did not show changes with the addition of MT concerning the control group at 10 and 30-h initiated IVM (Figure 4, 10h, and 30h). However, the addition of MT (10, 100 and 1000 ng/mL) significantly reduced (P<0.001) the expression of mRNA coding for BAX and BCL2 at 20 hours compared to the control group (Figure 4; 20h). The evaluation of the BAX: BCL2 ratio did not suggest changes related to the dose of MT at any time evaluated, only a slight non-significant decrease was observed for the control in the group of 10 ng/ml at 30-h initiated the IVM (Figure 4, BAX: BCL2 ratio 20h). In addition, an increase in the BAX: BCL2 ratio was noticeable at 30h compared to the other moments evaluated (Figure 4, BAX: BCL2 ratio 30h).
Figure 4): Expression of BAX and BCL2 in cumulus cells during in vitro maturation of COC added with MT. The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng/ml) or without MT (control). The total RNA of the CCs was recovered at 10, 20, and 30-h after IVM and analyzed by real-time PCR. The BAX: BCL2 ratio was evaluated. The gene expression data were normalized using the method Pfaffl [37]. The data are presented with the mean+SEM (n=4 replicas of independent cultures); the asterisks indicate significant differences between treatments (*** P<0.001, one-way ANOVA followed by Tukey’s HS.
The effect of MT on cell survival and apoptosis
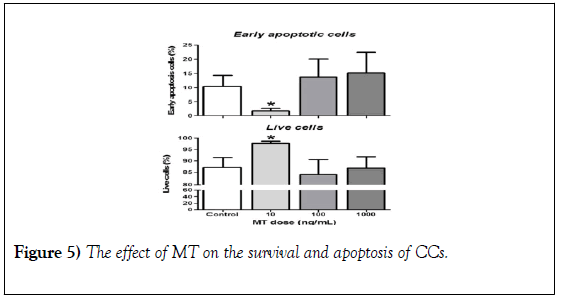
The evaluation of the effect of MT on the survival and apoptosis of the CCs at 30-h initiated the MIV of the COC revealed that the addition of 10 ng/ml of MT significantly increases the proportion of living CCs and reduces the proportion of CCs with early apoptosis compared to all groups (Figure 5, 10 ng/mL). A pattern similar to that of early apoptosis was observed in the BAX: BCL2 ratio at 30 hours after IVM (Figure 4, BAX: BCL2 ratio 30h).
Figure 5): The effect of MT on the survival and apoptosis of CCs. The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng/ml) or without MT (control). The CC was mechanically separated from the oocytes and recovered at 30-h for analysis by FACS. The data are presented with the mean + SEM (n=6 replicas of independent cultures); the asterisks indicate significant differences between treatments (* P<0.05, multiple tests of t).
The effect of MT on cleaved caspase 3 levels
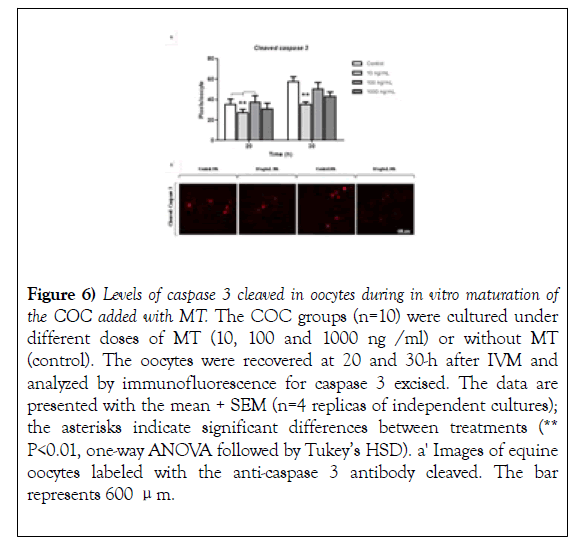
The evaluation of the effect of MT on the levels of caspase 3 cleaved in oocytes revealed that, like early apoptosis in CCs (Figure 5; Early apoptotic cells 10 ng/mL), the levels of cleaved caspase 3 are significantly reduced with the addition of-of 10 ng/ml of MT at 20 and 30 hours after IVM compared with the control group (Figure 6, 20 and 30-h). Also, the levels of cleaved caspase 3 show an increase at 30-h of the MIV compared to 20 hours (Figure 6) response similar to that observed in the BAX: BCL2 (Figure 5, BAX: BCL2 ratio).
Figure 6): Levels of caspase 3 cleaved in oocytes during in vitro maturation of the COC added with MT. The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng /ml) or without MT (control). The oocytes were recovered at 20 and 30-h after IVM and analyzed by immunofluorescence for caspase 3 excised. The data are presented with the mean + SEM (n=4 replicas of independent cultures); the asterisks indicate significant differences between treatments (** P<0.01, one-way ANOVA followed by Tukey’s HSD). a' Images of equine oocytes labeled with the anti-caspase 3 antibody cleaved. The bar represents 600 μm.
The COC groups (n=10) were cultured under different doses of MT (10, 100 and 1000 ng/ml) or without MT (control). The CC was mechanically separated from the oocytes and recovered at 30-h for analysis by FACS. The data are presented with the mean + SEM (n=6 replicas of independent cultures); the asterisks indicate significant differences between treatments (* P<0.05, multiple tests of t).
Discussion
In the current study, for the first time, we revealed the anti-apoptotic effect of 10 ng/mL of MT on equine COC; and the down-regulation of AREG and EREG expression, and cumulus expansion-related genes by 100 and 1000 ng/mL of MT. We established that a low MT dose (10 ng/mL) might promote successful maturation of equine COC in vitro by improving cell survival, as previously observed in bovine, pig, and rodent COC [38–41]. Further, we observed that higher MT doses (100 and 1000 ng/mL) caused down-regulation of the expression of AREG and EREG genes and cumulus expansion-related genes (Figures 2 and 3).
This down-regulation could lead to a failure in the COC maturation and consequently, reduce future embryonic development. Mehaisen and Saeed (2015) observed that high doses of MT (10–3 M) negatively affect the rate of embryonic development in rabbit, in contrast to moderate doses (10–6 M), which positively promote this rate. Other studies involving cattle, pig, and22 rodents showed that the positive effect of MT on oocyte maturation and embryonic development could be achieved with doses of 10–7 M to 10–9 M [17,22,27,37-43]. In the case of equine COC, in the current study, we showed that 10 ng/mL of MT (4.3–8 M) could benefit COC maturation, opening the door for future research into assisted reproduction techniques.
The described effects of MT on COC during IVM, together with the presence of melatonin receptor MT1 in the antral follicle and equine luteal cells reported by Pedreros et al. [23], suggest that MT plays an important role during follicular development, the formation of the corpus luteum, and oocyte maturation. Even though the presence of this hormone in the follicle has not been verified, we suggest that MT can be an important component of the follicular fluid, as observed in several other mammals [15,16,18]. MT is present in the follicular fluid of woman and sow and contributes to the establishment of a follicular microenvironment that is crucial for the protection, nourishment, and promotion of oocyte maturation [16,44]. Several studies have shown that one of the main roles of MT in the follicle is protecting the oocyte, mainly by acting as an antioxidant and anti-apoptotic agent, and by modulating the expression of various genes [45,46]. Such activities are regulated by MT membrane receptors MT1 and MT2, and by a direct effect of MT on free radical levels [12,13].
Considering the above, it is clear that removing the oocyte from the follicular microenvironment and subjecting it to IVM increases nonphysiological stress and eliminates the protection provided by MT, which can cause oocyte damage and activate apoptosis [9]. In the current study, we demonstrated that 10 ng/mL of MT promote COC survival. Accordingly, we suggest the following model explaining how MT could prevent apoptosis. We observed that MT reduces the expression of the pro-apoptotic BAX gene, and the anti-apoptotic and antioxidant BCL2 gene in CCs (Figure 4). We propose that MT reduces cellular stress mainly at 20-h of culture, thus reducing the production of BAX [47]. Consequently, the activity of BCL2 would be reduced because of the reduction of cellular stress and increased permeability (damage) of the outer mitochondrial membrane caused by the formation of pores by active BAX protein [11], suggesting that MT can help to maintain a balance between the functions of these proteins [48]. This balance can be altered by culture duration, as evidenced by the BAX: BCL2 ratio increases at 30-h IVM compared with other culture times. The latter indicates that most likely, greater number of CCs experience stress and undergo apoptosis initiation at 30-h IVM than cells cultured for shorter periods (Figure 4 B). Evaluation of the BAX: BCL2 ratio as an indicator of cellular apoptosis in the oocyte has been suggested by Yang and Rajamahendran [49]. We did not observe changes in the BAX: BCL2 ratio caused by MT; however, the ratio showed a negative trend when cells were exposed to 10 ng/mL of MT.
Based on the down-regulation of BAX and BCL2 expression at 20-h culture by MT, and the slight reduction of the ratio at 30-h culture in the 10 ng/mL of MT group, we moved to determine whether these changes could modify CCs survival and apoptosis at the end of IVM (30-h). Accordingly, we used FACS to evaluate (i) cell exposure of PtdSer as an early apoptosis marker; (ii) the integrity of cell membrane, indirectly, by staining DNA as a marker of late apoptosis/necrosis; and (iii) the absence of both these markers to identify living cells. The analysis revealed that 10 ng/mL of MT reduced the incidence of early apoptosis and increased CCs survival. Interestingly, a response pattern similar to that observed for the BAX: BCL2 ratio at 30-h IVC was observed. A possible relationship between apoptotic markers and the BAX/BCL2 ratio led us to propose that MT modulates apoptosis mediated by mitochondrial stress. According to the hypothetical model, 10 ng/mL of MT prevents the damage caused by BAX to membrane integrity, reducing cytochrome c release, and consequently limiting the activation of apoptosis effector proteases, i.e., caspases [49-51]. Effector caspases are responsible for DNA fragmentation, membrane blebbing, nucleotide release, and PtdSer exposure. In mouse and human cells, PtdSer exposure on the cell surface during apoptosis is caspase 3-dependent, and proceeds by the inactivation of ATP11C flippase and simultaneous activation of the XKR8 scramblase [52]. It should be noted that caspase 3 is also involved in the apoptotic pathway mediated by death receptors [10,48]. This would explain the slightly negative trend at 30-h of the BAX: BCL2 ratio, contrasting with the significant reduction of the incidence of early apoptosis and increase of cell survival, in the 10 ng/mL of MT group compared with the control (Figure 5). However, further studies should be performed to determine whether MT acts on the apoptotic pathway mediated by death receptors in equines.
Considering the increased CCs survival, and the current knowledge that CCs health directly influences the health of the oocyte and vice versa [53], we evaluated cleaved caspase 3 levels in the oocyte, to identify a possible health-associated relationship between CCs and the oocyte. As anticipated, the data corroborated such a relationship. Namely, at 20 and 30-h of IVC, the levels of cleaved caspase 3 were reduced in the presence of the lowest MT dose tested (10 ng/mL; Figure 6), similarly to what has been observed in CCs at-30 h of IVM (Figures 4 and 5). Further, similarly to the changes of the BAX: BCL2 ratio in CCs, the cleaved caspase 3 levels in the oocyte apparently increased at 30-h in all groups. This suggested that long culture time promoted COC apoptosis. The above findings confirm what has been already reported in multiple studies, i.e., that the CCs and oocyte function as a unit that is finely regulated by signals from both cells [1,53]. The observation of a response pattern in the oocyte at 20 h (Figure 6), followed by a response pattern in CCs at 30-h (Figures 4 and 5), suggests that the oocyte can enter the apoptotic state before CCs, and therefore regulates the apoptosis of CCs. Consequently, the assessment of the CCs survival could serve as an indirect indicator of oocyte health. We also observed that even though the time is a determining factor for the onset of apoptosis during IVM, MT delays apoptosis onset, possibly preventing cellular stress and COC aging, as reported in cattle and mouse [39–41,54]. The findings of the current study corroborate those reported for other species, i.e., that MT reduces apoptosis of the oocyte and CCs [27,42,55].
We next asked whether MT could modify the expression of genes involved in the maturation and cumulus expansion of equine COC. We started by evaluating the expression of genes encoding the two main follicle EGF-L: AREG and EREG [56] because of (i) their suggested importance in the dominant equine follicle in vivo [57]; and (ii) the notion that local COC production of these EGF-L during IVM in various mammals is required for COC progression to a competent stage of maturation [7]. We observed that none of the MT supplementation doses used increased the expression of AREG and EREG genes relative to the control. By contrast, in the highest MT dose group (1000 ng/mL), MT reduced the expression of both genes at 10-h, compared with the control group (Figure 2). Reduced AREG and EREG expression could limit the activation of maturation and adequate cumulus expansion by impairing the activation of signaling pathways regulated by EGFR [3]. Shimada et al. [6]. proposed a model whereby autocrine regulation of AREG and EREG in CCs leads to expression of PTGS2, HAS2, and TNFAIP6, genes involved in the cumulus expansion.
Because of the importance of the signaling pathway regulated by (AREG/ EREG)/EGFR in the expression of cumulus expansion related-genes, we asked whether the down-regulation of AREG and EREG described above could modify the expression of PTGS2, HAS2, and TNFAIP6. Similarly to AREG and EREG expression, the expression of PTGS2, HAS2, and TNFAIP6 was reduced at10-h in the presence of 1000 ng/mL of MT, also decreasing in the presence of 100 ng/mL of MT, compared with the control group. This suggested that the regulation of PTGS2, HAS2, and TNFAIP6 in the equines, as well as in rodents, pig, and bovine, possibly relies on the (AREG/EREG)/EGFR pathway, and this could potentiate the MT effect [2,6,58-62]. Based on these findings and those presented by others, we propose that the reduction of AREG, EREG, and PTGS2 gene expression can reduce the expression of PTGS2, HAS2, and TNFAIP6. This compromises cumulus expansion of equine COC by, possibly, decreasing the synthesis of the main ECM component (HA) by down-regulating HAS2 expression, by leading to inefficient joining of HA chains that stabilize ECM, to down-regulate TNFAIP6 and additionally by the ineffectiveness organization of cumulus ECM due to down-regulation ADAMTS1 expression [4,6,58 – 60]. The reduction of HAS2, TNFAIP6, and PTGS2 mRNA levels might corroborate the proposal of Davis et al. [50]. who suggested that PTGS2 plays an important role in HA production, and the recruitment of factors related to cumulus stabilization and expansion.
Finally, in the current study, we showed that the expression of AREG, EREG, PTGS2, HAS2, TNFAIP6, and ADAMTS1 genes is reduced with culture time, independent of MT. The PTGS2 mRNA levels were undetected at 20-h, which was associated with very low gene expression at that particular time. These observations suggest that early induction of the expression of these genes is essential for leading the COC through the expansion process and maturation, as described by Shimada et al. [6]. The function of cumulus expansion is not limited to COC recovery by the infundibulum and its subsequent fertilization in the oviduct; it also requires oocyte meiotic maturation as it enables adequate filtration of molecules that contribute to the establishment of appropriate cell signaling [61]. Further, Yokoo et al. [62]. reported that in pig, HA during cumulus expansion is important for the activation of the CD44 receptor that phosphorylates gap junction proteins, closes the cellular connection, and interrupts the cAMPcGMP flow from the CCs to the oocyte. However, it has been suggested that although cumulus expansion is necessary for oocyte maturation, it is not sufficient by itself. In addition to cumulus expansion, an adequate number of healthy CCs layers is required for the success of oocyte IVM and their viability. Therefore, it is not uncommon for the viability of CCs and cumulus expansion to be used as quality markers of COC, important for assisted reproduction techniques [60]. Early evaluation (˂10-h) of the cumulus expansion related-genes expression and late evaluation of apoptosis (˃30-h), might bring novel data of MT effects. However, the overall validity of the information provided is not compromised.
Conclusion
In summary, incorporation of 10 ng/mL of MT into IVM protocol for equine COC promotes cell survival of CCs and the oocyte, delaying apoptosis onset, possibly by modulating the apoptotic pathway mediated by mitochondrial damage. The specified dose does not impair the expression of cumulus expansion- and maturation-related genes. On the other hand, higher MT doses (100 and 1000 ng/mL) do not delay apoptosis and downregulate the expression of cumulus expansion- and maturation-related genes. Based on the data presented herein and previously published information, we suggest a hypothetical model of MT activity as a modulator of an apoptotic pathway regulated by mitochondrial damage and propose a model for the regulation of cumulus expansion-related genes. We conclude that the incorporation of 10 ng/mL of MT into IVM protocol promotes equine COC survival and competent maturation state.
Declaration Of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by the Equine health fund from the Faculty of Veterinary Medicine of the Université de Montréal supported by Zoetis; PAEP-Universidad Nacional Autónoma de México and CONACYT-México
Acknowledgement
The authors thank Dr. Christopher Price and Dr. Anthony Estienne for the use of Centre de Recherche en Reproduction et Fertilité facilities (University of Montreal, Canada) and their technical assistance.
REFERENCES
- Landim-Alvarenga FC, Maziero RRD. Control of oocyte maturation. Anim Reprod. 2014:150–8.
- Procházka R, Petlach M, Nagyová E. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: Comparison with gonadotropins. Reproduction. 2011;141:425–35.
- Park J-Y. EGF-Like Growth Factors As Mediators of LH Action in the Ovulatory Follicle. Science. (300 ) 2004;303:682–4.
- Ben-Ami I, Freimann S, Armon L. Novel function of ovarian growth factors: Combined studies by DNA microarray, biochemical and physiological approaches. Mol Hum Reprod. 2006;12:413–9.
- Ashkenazi H, Cao X, Motola S. Epidermal growth factor family members: Endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84.
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I. Paracrine and Autocrine Regulation of Epidermal Growth Factor-Like Factors in Cumulus Oocyte Complexes and Granulosa Cells: Key Roles for Prostaglandin Synthase 2 and Progesterone Receptor. Mol Endocrinol. 2006;20:1352–65.
- Brown HM, Dunning KR, Sutton-McDowall M, et al. Failure to launch: Aberrant cumulus gene expression during oocyte in vitro maturation. Reproduction. 2017;153:R109–20.
- Virant-Klun I, Bauer C, Ståhlberg A. Human oocyte maturation in vitro is improved by co-culture with cumulus cells from mature oocytes. Reprod Biomed Online. 2018;36:508–23.
- Kala M, Shaikh MV, Nivsarkar M. Equilibrium between anti-oxidants and reactive oxygen species: a requisite for oocyte development and maturation. Reprod Med Biol. 2017;16:28–35.
- Tiwari M, Prasad S, Tripathi A, et al. Apoptosis in mammalian oocytes : a review. Apoptosis. 2015;20:1019–25.
- Susnow N, Zeng L, Margineantu D. Bcl-2 family proteins as regulators of oxidative stress. Semin Cancer Biol. 2009;19:42–9.
- Tamura H, Takasaki A, Taketani T. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:1–9.
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10.
- Cruz MHC, Leal CLV, Cruz JF da. Role of melatonin on production and preservation of gametes and embryos: A brief review. Anim Reprod Sci. 2014;145:150–60.
- Diaw M, Salgado RM, Canesin HS. Effect of different shipping temperatures (∼22 °C vs. ∼7 °C) and holding media on blastocyst development after overnight holding of immature equine cumulus-oocyte complexes. Theriogenology. 2018;111:62–8.
- Hinrichs K. The equine oocyte: Factors affecting meiotic and developmental competence. Mol Reprod Dev. 2010;77:651–61.
- Brzezinski A, Seibel MM, Lynch HJ. Melatonin in human preovulatory follicular fluid. J Clin Endocrinol Metab. 1987;64:865–7.
- Shi JM, Tian XZ, Zhou G Bin. Melatonin exists in porcine follicular fluid and improves in vitro maturation and parthenogenetic development of porcine oocytes. J Pineal Res. 2009;47:318–23.
- Tian X, Wang F, He C. Beneficial effects of melatonin on bovine oocytes maturation: a mechanistic approach. J Pineal Res. 2014;57:239–47.
- 20.El-Raey M, Geshi M, Somfai T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev. 2011;78:250–62.
- Yie SM, Niles LP, Younglai EV. Melatonin receptors on human granulosa cell membranes. J Clin Endocrinol Metab. 1995;80:1747–9.
- Soares JM, Masana MI, Erşahin C. Functional melatonin receptors in rat ovaries at various stages of the estrous cycle. J Pharmacol Exp Ther. 2003;306:694–702.
- Wang SJ, Liu WJ, Wu CJ. Melatonin suppresses apoptosis and stimulates progesterone production by bovine granulosa cells via its receptors (MT1 and MT2). Theriogenology. 2012;78:1517–26.
- Kang JT, Koo OJ, Kwon DK. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J Pineal Res. 2009;46:22–8.
- Pedreros M, Ratto M, Guerra M. Expression of functional melatonin MT1 receptors in equine luteal cells: In vitro effects of melatonin on progesterone secretion. Reprod Fertil Dev. 2011;23:417–23.
- Adriaens I, Jacquet P, Cortvrindt R. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006;228:333–43.
- Manjunatha BM, Devaraj M, Gupta PSP. Effect of taurine and melatonin in the culture medium on buffalo in vitro embryo development. Reprod Domest Anim. 2009;44:12–6.
- Vázquez MI, Abecia JA, Forcada F. Effects of exogenous melatonin on in vivo embryo viability and oocyte competence of undernourished ewes after weaning during the seasonal anestrus. Theriogenology. 2010;74:618–26.
- Wang F, Tian X, Zhang L. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J Pineal Res. 2013;55:267–74.
- Fu Y, He CJ, Ji PY. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int J Mol Sci. 2014;15:21090–104.
- Mehaisen GMK, Saeed AM. In vitro development rate of preimplantation rabbit embryos cultured with different levels of melatonin. Zygote. 2014;23:111–5.
- Pereira GR, Lorenzo PL, Carneiro GF. The involvement of growth hormone in equine oocyte maturation, receptor localization and steroid production by cumulus-oocyte complexes in vitro. Res Vet Sci. 2013;95:667–74.
- Lorenzo PL, Liu IKM, Carneiro GF. Equine oocyte maturation with epidermal growth factor. Equine Vet J. 2002;34:378–82.
- Scott TJ, Carnevale EM, Maclellan LJ. Embryo development rates after transfer of oocytes matured in vivo, in vitro, or within oviducts of mares. Theriogenology. 2001;55:705–15.
- Hinrichs K, Schmidt AL, Friedman PP. In vitro maturation of horse oocytes: characterization of chromatin configuration using fluorescence microscopy. Biol Reprod. 1993;48:363–70.
- Ramakers C, Ruijter JM, Lekanne Deprez RH. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–6..
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:45e – 45.
- Li Y, Zhang ZZ, He CJ. Melatonin protects porcine oocyte in vitro maturation from heat stress. J Pineal Res. 2015;59:365–75.
- Dai X, Lu Y, Zhang M. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum Reprod. 2017;32:598–606.
- Liang S, Guo J, Choi JW. Effect and possible mechanisms of melatonin treatment on the quality and developmental potential of aged bovine oocytes. Reprod Fertil. Dev 2017;29:1821–31.
- Yang Q, Dai S, Luo X. Melatonin attenuates postovulatory oocyte dysfunction by regulating SIRT1 expression. Reproduction. 2018:1–51.
- Rodrigues-Cunha MC, Mesquita LG, Bressan F. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology. 2016;86:1685–94.
- Zhao XM, Wang N, Hao HS. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J Pineal Res. 2018;64:1–15.
- Jing T, Shile S, Sun Y. Melatonin levels in follicular fluid as markers for IVF outcomes and predicting ovarian reserve. Reproduction. 2017;153:443–51.
- Tamura H, Takasaki A, Taketani T. The role of melatonin as an antioxidant in the follicle. J Ovarian Res. 2012;5:5.
- Cruz MHC, Leal CL, Cruz JF. Essential actions of melatonin in protecting the ovary from oxidative damage. Theriogenology. 2014;82:925–32.
- Westphal D, Dewson G, Czabotar PE. Molecular biology of Bax and Bak activation and action. BBA - Mol Cell Res. 2011;1813:521–31.
- Cory S, Adams JM. The BCL2 family: Regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56.
- Yang MY, Rajamahendran R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim Reprod Sci. 2002;70:159–69.
- Eray M, Mttö M, Kaartinen M. Flow cytometric analysis of apoptotic subpopulations with a combination of Annexin V-FITC, propidium iodide, and SYTO 17. Cytometry. 2001;43:134–42.
- Morita Y, Tilly JL. Oocyte apoptosis:like sand through an hourglass. DevBiol. 1999;213:1–17.
- Segawa K, Nagata S. An Apoptotic “Eat Me” Signal: Phosphatidylserine Exposure. Trends Cell Biol. 2015;25:639–50.
- Zuccotti M, Merico V, Cecconi S. What does it take to make a developmentally competent mammalian egg? Hum Reprod Update. 2011;17:525–40.
- Lord T, Nixon B, Jones KT. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013;88:67.
- Takada L, Junior AM, Mingoti GZ. Research in Veterinary Science Effect of melatonin on DNA damage of bovine cumulus cells during in vitro maturation ( IVM ) and on in vitro embryo development. Res Vet Sci. 2012;92:124–7.
- Conti M, Hsieh M, Musa Zamah A. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73.
- Lindbloom SM, Farmerie TA, Clay CM. Potential involvement of EGF-like growth factors and phosphodiesterases in initiation of equine oocyte maturation. Anim Reprod Sci. 2008;103:187–92.
- Portela VM, Zamberlam G, Gonçalves PBD. Role of Angiotensin II in the Periovulatory Epidermal Growth Factor-Like Cascade in Bovine Granulosa Cells In vitro1. Biol Reprod. 2011;85:1167–74.
- Davis BJ, Lennard DE, Lee CA. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology. 1999;140:2685–95.
- Nevoral J, Orsák M, Klein P. Cumulus Cell Expansion, Its Role in Oocyte Biology and Perspectives of Measurement: A Review. Sci Agric Bohem. 2015;45:212–25.
- Dunning KR, Watson LN, Sharkey DJ. Molecular Filtration Properties of the Mouse Expanded Cumulus Matrix: Controlled Supply of Metabolites and Extracellular Signals to Cumulus Cells and the Oocyte. Biol Reprod. 2012;87:1–10.
- Yokoo M, Shimizu T, Kimura N. Role of the hyaluronan receptor CD44 during porcine oocyte maturation. J Reprod Dev. 2007;53:263–70.