Mesenchymal dependent mode of cartilage growth in post-hatching birds
2 Department of Anatomy and Embryology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt
Received: 15-Sep-2017 Accepted Date: Oct 10, 2017; Published: 20-Oct-2017
Citation: Soliman SA, Madkour FA, Abdelhakeim F. Mesenchymal dependent mode of cartilage growth in post-hatching birds. J Histol Histopathol Res. 2017;1(1):10-14.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Cartilage growth depends on two types of resident cells; the chondrocytes and the perichondrial stem cells. Chondrocytes proliferation commits interstitial growth of cartilage while the appositional growth is driven by differentiation of the perichondrial stem cells. A third type of cartilage growth depending on mesenchymal cells has been recently described in the embryo. The current study aims to investigate the occurrence of mesenchymal-dependent cartilage growth in postembryonic life. We used samples of the oropharyngeal cartilage of both duck and goose in different ages. Mesenchymal-like cells were identified in the oropharyngeal cartilage of both duck and goose. They appeared spindle or flattened in shape with an oval nucleus and connected by cytoplasmic processes. Mesenchymal cells were observed in the cartilage of the middle nasal conchae, laryngeal cartilages of duck and arytenoid cartilage and entoglossal cartilage in the goose. Mesenchymal-like cells were organized as cellular streaks or as individual cells. Multiple areas of mesenchymal-like cells in the cartilage could be recognized. Mesenchymal-like cells are derived from the perichondrium. They undergo differentiation, acquired a spherical profile and were surrounded by the little amount of basophilic cartilage matrix. In conclusion, Mesenchymal-like cells contributed to the interstitial growth of the cartilage in the postembryonic period.
Tissue development and growth is a well-regulated physiological process and primarily dependent on signaling molecules and factors which control cell proliferation, differentiation, and death [1]. Organ development achieved through cellular interacting between the three embryonic germ layers. Tissue growth is accomplished by stimulation of the terminally differentiated to re-enter the cell cycle to produce new progeny. Organ growth could be also dependent on activation of the stem cells reservoir to transform into differentiated progeny [2].
It is well-known that two fundamental types of cells participate in the growth of cartilage; chondrocytes and undifferentiated perichondrial cells. Chondrocyte capable to generate new cell progeny which produce cartilage matrix. Chondrocytes promote the interstitial type of cartilage growth. The perichondrial cells are considered as chondrogenic progenitor cells. They have the potential to differentiate into chondrocytes and produce circumferential layers of cartilage matrix “appositional growth” [3]. A third type of cartilage growth has been recently described in different species including aquatic, avian and mammalian species. This type is a mesenchymal-dependent mode of cartilage growth. Cd117 expressing mesenchymal cells penetrate the growing cartilage. These cells are derived from the developing perichondrium or from the surrounding mesenchyme where the perichondrium is not established. Mesenchymal cells express MMP-9 which commit to degrade extracellular matrix components. Mesenchymal cells acquire chondrogenic cell lineage and express collagen type II [4-7].
The mesenchymal-dependent mode of cartilage growth is described in embryonic stages [5]. The current study aims to investigate involvement of mesenchymal-like cells in growth of cartilage in postembryonic life.
We used two species of birds of different ages to identify mesenchymal-like cell in the cartilage of the respiratory passage.
Materials and Methods
To achieve the study purpose, we used bird oropharynx that is primarily supported by cartilaginous tissue. We focused on the entoglossal cartilage of the tongue and laryngeal cartilages.
Birds were obtained from the College farm of the faculty of Agriculture, Assiut University. The samples were collected from 15 days and 1-week Muscovy duck [Cairina moschata] and two months old Egyptian Goose [Alopochen Egyptian]. All birds were euthanized by decapitation. The whole oropharynx was carefully dissected and fixed in neutral buffered formalin.
Fixed samples were dehydrated in ascending grades of alcohols at 70%, 80%, 90% and 100% for 90 minutes at each concentration. The sampled were cleared using methyl benzoate. Dehydrated samples were then impregnated and embedded in Paraplast [Sigma Aldrich]. Serial sections of 3-5 μm were cut using a Richert Leica RM 2125 Microtome, Germany and mounted on glass slides. Sections were kept in an incubator at 40°C for dryness.
In the current study, we used H&E stained as a general histological stain [8] and Mallory trichrome as a specific stain for cartilage [9]. Stained sections were examined using DMLS light microscope [Leica, Germany] outfitted with MC120 HD camera [Leica, Germany].
Results
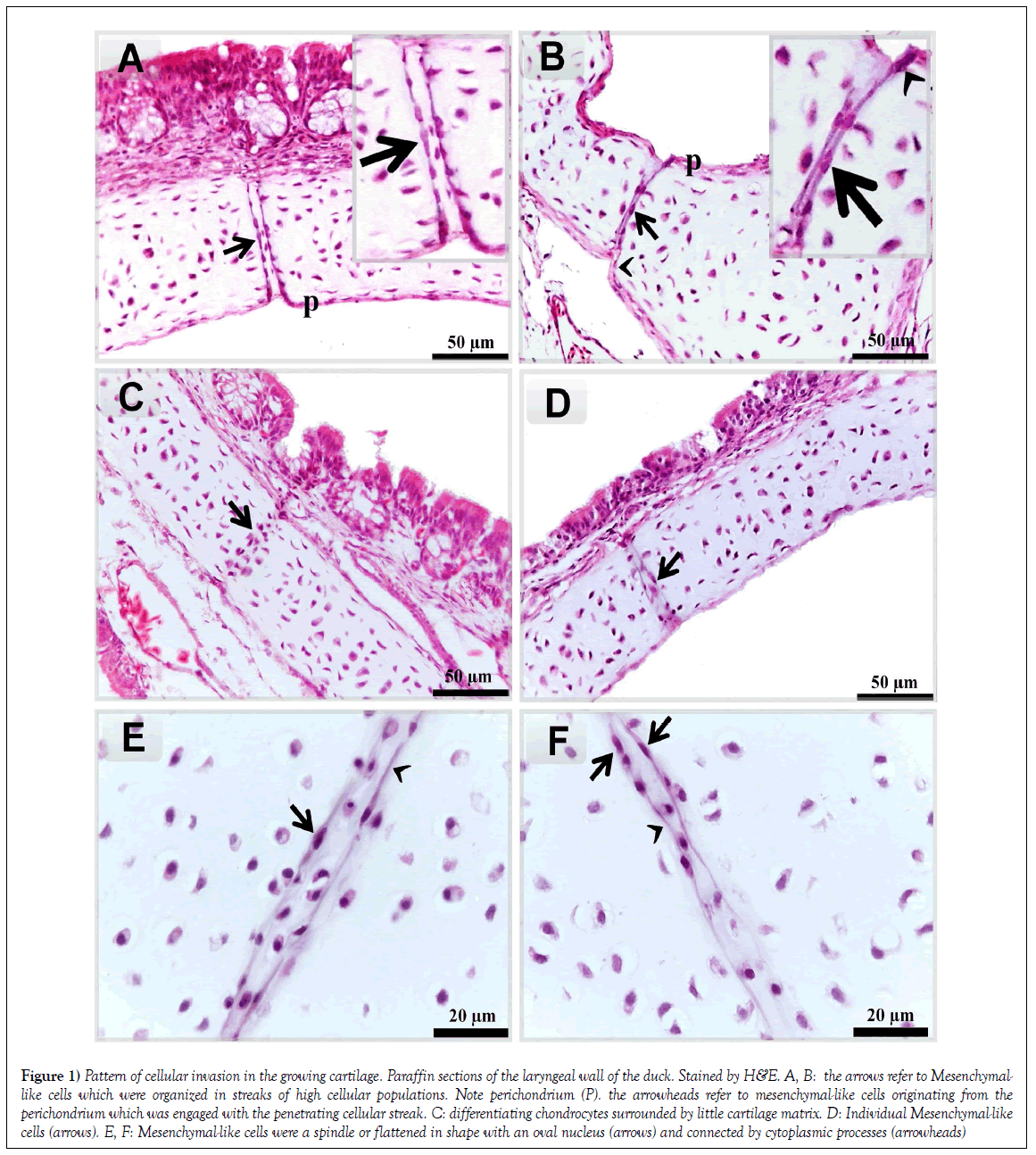
The current study aims to identify mesenchymal-like cells and their contribution to cartilage growth in the oropharyngeal cartilage of both duck and goose. Mesenchymal-like cells could be observed in the laryngeal cartilages of Duck. They appeared spindle or flattened in shape with an oval nucleus [arrows] and connected by cytoplasmic processes (Figures 1E and 1F). The distribution of these cells exhibited different forms. They may arrange in a streak of the high population (Figures 1A and 1B). Individual mesenchymal-like cells could be also observed (Figure 1D). Differentiating chondrocytes, which had a spherical profile, were surrounded by the little amount of basophilic cartilage matrix (Figure 1C). Mesenchymal cells seem to be derived from the perichondrium (Figure 1B).
Figure 1: Pattern of cellular invasion in the growing cartilage. Paraffin sections of the laryngeal wall of the duck. Stained by H&E. A, B: the arrows refer to Mesenchymallike cells which were organized in streaks of high cellular populations. Note perichondrium (P). the arrowheads refer to mesenchymal-like cells originating from the perichondrium which was engaged with the penetrating cellular streak. C: differentiating chondrocytes surrounded by little cartilage matrix. D: Individual Mesenchymal-like cells (arrows). E, F: Mesenchymal-like cells were a spindle or flattened in shape with an oval nucleus (arrows) and connected by cytoplasmic processes (arrowheads)
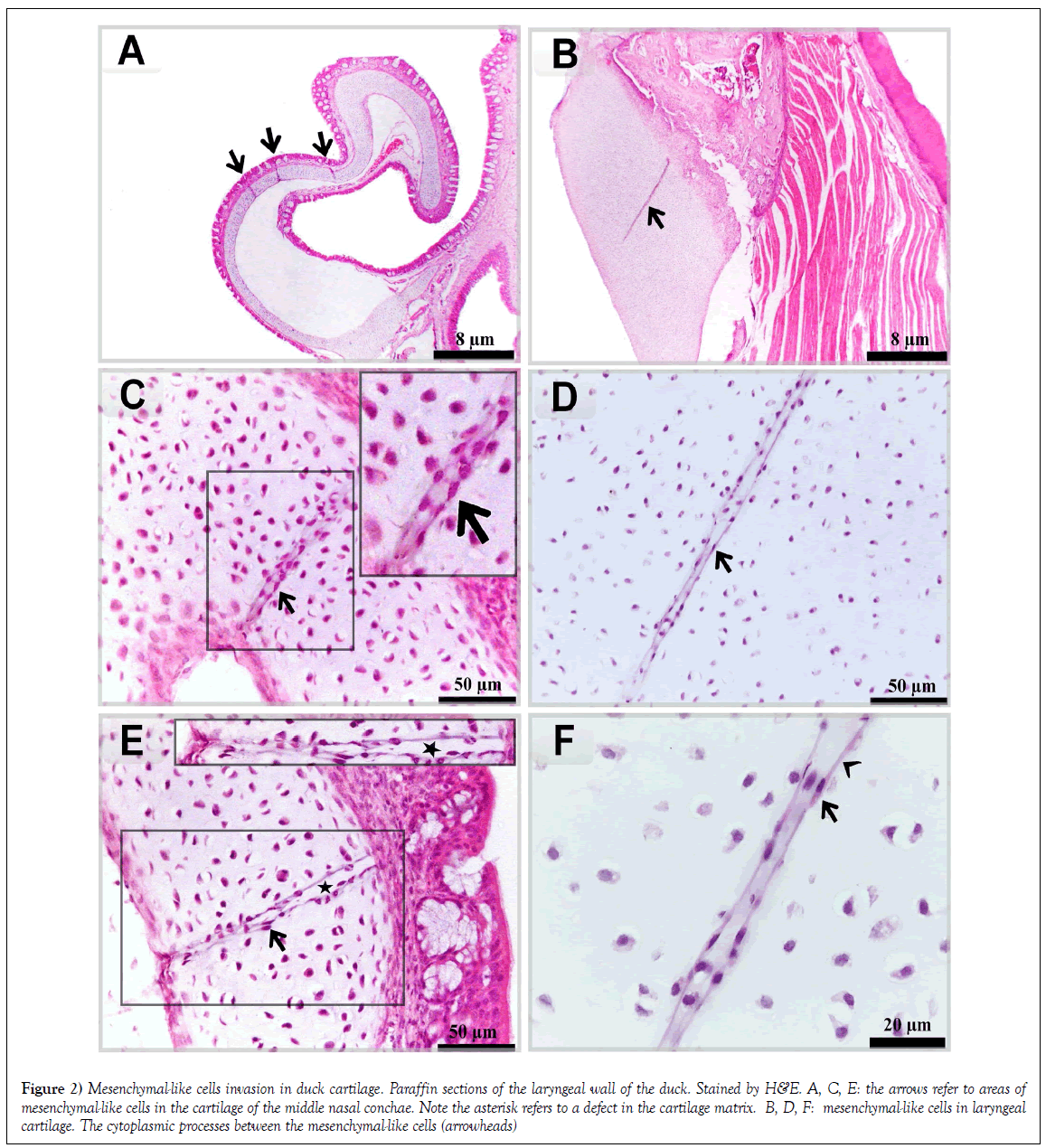
Multiple areas of mesenchymal-like cells in the cartilage of the middle nasal conchae. In certain areas, a defect in cartilage matrix could be observed (Figures 2A, 2C and 2E). Mesenchymal-like cells were connected by fine cytoplasmic processes in laryngeal cartilage (Figures 2B, 2D and 2F).
Figure 2: Mesenchymal-like cells invasion in duck cartilage. Paraffin sections of the laryngeal wall of the duck. Stained by H&E. A, C, E: the arrows refer to areas of mesenchymal-like cells in the cartilage of the middle nasal conchae. Note the asterisk refers to a defect in the cartilage matrix. B, D, F: mesenchymal-like cells in laryngeal cartilage. The cytoplasmic processes between the mesenchymal-like cells (arrowheads)
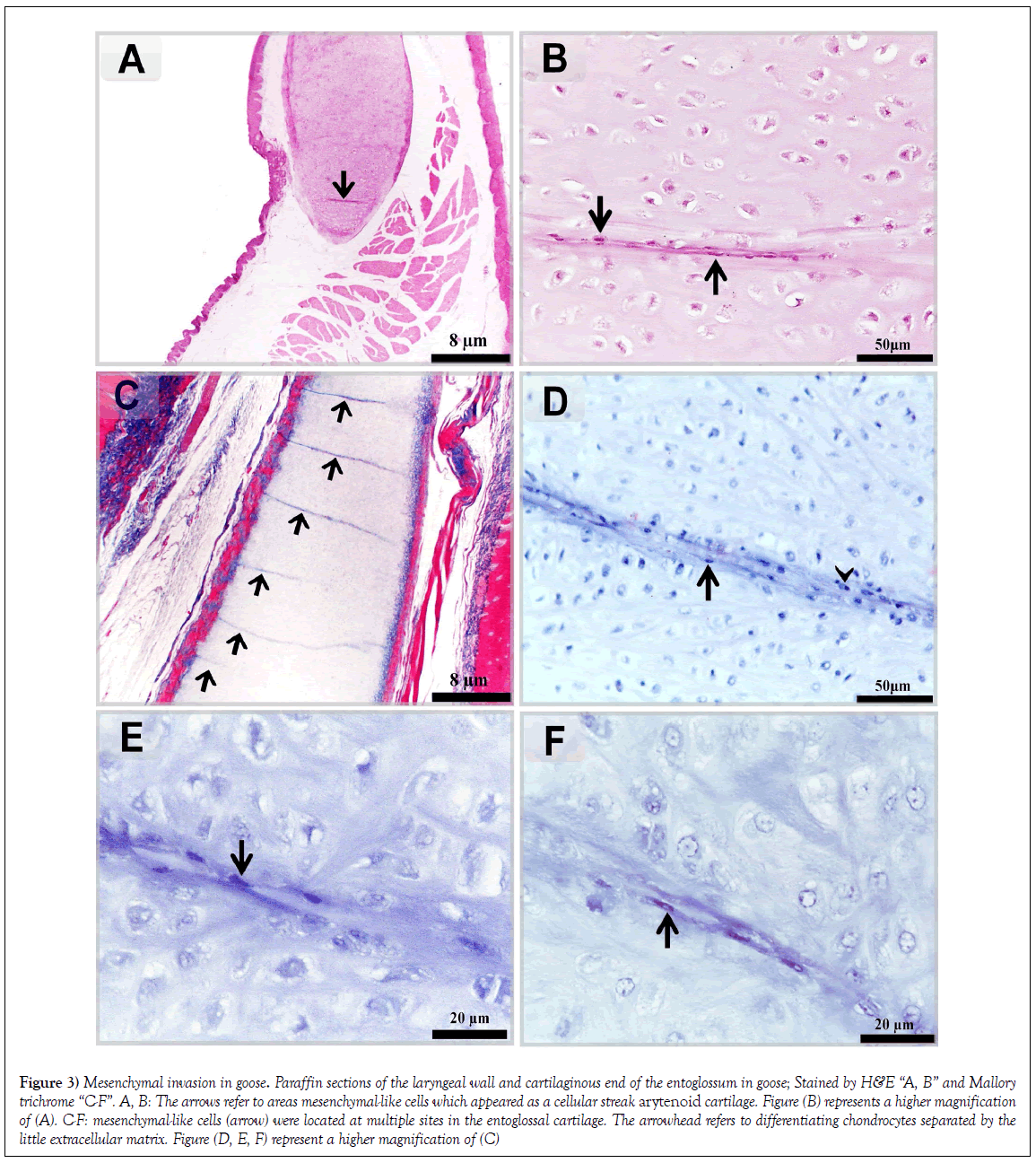
In the laryngeal mound of Goose, mesenchymal-like cells were distributed as a cellular streak arytenoid cartilage (Figures 3A and 3B). Mesenchymal-like cells were located at multiple sites in the entoglossal cartilage (Figures 3C-3F).
Figure 3: Mesenchymal invasion in goose. Paraffin sections of the laryngeal wall and cartilaginous end of the entoglossum in goose; Stained by H&E “A, B” and Mallory trichrome “C-F”. A, B: The arrows refer to areas mesenchymal-like cells which appeared as a cellular streak arytenoid cartilage. Figure (B) represents a higher magnification of (A). C-F: mesenchymal-like cells (arrow) were located at multiple sites in the entoglossal cartilage. The arrowhead refers to differentiating chondrocytes separated by the little extracellular matrix. Figure (D, E, F) represent a higher magnification of (C)
Discussion
The aim of the current study was to explore the contribution of mesenchymallike cells in the growth of cartilage in postembryonic development. We investigated the oropharyngeal cartilages of duck and goose to distinguish mesenchymal-like cell.
In the current study, we recognized mesenchymal-like cells for the first time in the oropharyngeal cartilages of post-hatching duck and goose. They exhibited a different pattern of the organization either individual cells, cellular streaks. Multiple sites of mesenchymal-like cells could be recognized.
Previous studies speculated involvement of mesenchymal cells in the growth of cartilage. This type of growth is identified in the cartilage of different skeletal element in quail [5,10] and camel embryos [7]. Mesenchymal cells were recognized morphologically by histological techniques, TEM, SEM and immunologically using C-KIT. C-KIT or CD117 is a type of tyrosine kinase receptor specific for the undifferentiated mesenchymal cell. C-KIT regulates cell proliferation, differentiation, adhesion, chemotaxis, and apoptosis [11-13].
In the current study, mesenchymal-like cells transformed to spherical cells which surrounded by cartilage matrix. This result may indicate mesenchymal-like cells have a chondrogenic potential and support the previous researchers in quail and camel embryos. By using histochemical and immunohistochemically techniques, the authors found that mesenchymal cells during differentiation express type II collagen and produce proteoglycanrich matrix. They also speculated that mesenchymal cell participated in the production of the new interstitial matrix, and consider this type as an interstitial type of cartilage growth [7,10]. Two prevalent types of cartilage growth are known for long decades. The first type depends on perichondrial stem cells which secrete new matrix adding to the outer circumference of the cartilage to increase the diameter. Hence, this type is known as appositional growth. The second type is a chondrocytes-dependent, in which chondrocytes propagate and the daughter cells produce interstitial matrix [an interstitial type of cartilage growth] [14,15]. In the current study, areas of a defect in cartilage matrix appeared where mesenchymal-like cells were localized. This may regard to secretion of proteolytic enzymes during penetration of the mesenchymal cells in the cartilage matrix. Mesenchymal cells express MMP-9 during the invasion in the cartilage in quail embryos. MMPs or matrixes are a family of metal-dependent endopeptidases that degrade extracellular matrix components. MMPs are involved in tissue remodeling during physiological or pathological conditions [16]. MMP-9 is contributed in angiogenesis, the migration of immune cells, the activation of cytokines and chemokines, and cancer progression [17]. MMP- 9 break down collagen types IV, V, XIk´, XIVl´, elastin, aggrecan, link protein, decorinr, lamininn, entactin, SPARCq, myelin basic proteinm,∞2Mn, ∞1Pli, IL-1βj, proTNF-∞k [18].
Penetration of mesenchymal cells in the cartilage templates of prospective long bone has been described as an initial step in endochondral ossification. Mesenchymal cells acquire an osteogenic cell lineage to serve in bone formation [19]. Mesenchymal cells may be also as a constituent of the cellular elements of the cartilage canals. These canals are derived from perichondrial papillae to provide a nutritional support to the cartilage [20]. Two different cell lineage is described in the cartilage canals; type II collagen expressing cells [21,22] and type I collagen and, periostin expressing cells [23-25]. Our results were quite similar to those obtained in quail and camel embryos [5-7] that mesenchymal-like cells were not limited to a specific area in the cartilage. Unlike cartilage canals which are only developed in the epiphysis and associated with vascular invasion
In a previous study, mesenchymal cells are physiologically invading the cartilage template of the growing femur and tibia in quail embryos. The mesenchymal cells were limited to the central hypertrophic zone in a random manner [26]. Unlike results of the current study, mesenchymal-like cells had a focal distribution which may organize at multiple sites. Mesenchymal dependent mode of cartilage growth also occurs in fish. Mesenchymal cells invade the cartilage of the air-breathing organ catfish. The invading cells acquired chondrogenic properties. They secrete type II collagen. The authors concluded that the aim of mesenchymal invasion is to grow, renew, and replace the cartilaginous tissue [4-6].
Conclusion
Mesenchymal-like cell contributed to interstitial growth of the cartilage in the postembryonic period.
Clinical Correlation
Several scientists study mechanism of development of the mesodermderived organs and tissues. Using of mesenchymal stem cells in regenerative medicine remain a promising target. Several attempts have been made aiming to enhance differentiation of stem cells into target cell types. This study introduces the chondrogenic potential of mesenchymal cells and their contribution to cartilage growth. This study should motivate Stem cell Technologies Scientist to consider that under special circumstances, articular cartilage repair may be as a clinical possibility. Future researchers should be carried out to explore factors and signals attract mesenchymal-like cells and govern their chondrogenic differentiation. The current study presented avian cartilage in the postembryonic period as a model to study this phenomenon.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- Thiriet M. Tissue growth, repair, and remodeling. biology and mechanics of blood flows: Part I: Biology. New York: Springer 2008:435-88.
- Sanchez AA, Yamanaka S. Rethinking differentiation: stem cells, regeneration, and plasticity. Cell 2014;157(1):110-19.
- Eroschenko VP, Fiore M. DiFiore's atlas of histology with functional correlations. Philadelphia: Lippincott Williams & Wilkins; 2013:114.
- Soliman S. New aspect in cartilage growth “The invasive interstitial type”. Journal of Aquaculture Research and Development 2014;5:253
- Soliman SA, Abd-Elhafeez HH. Mesenchymal cells in cartilage growth. Germany: Lambert Academic Publishing; 2014:49.
- Soliman SA, Abd-Elhafeez HH. Are C-KIT, MMP-9 and type II collagen positive undifferentiated cells involved in cartilage growth? A description of unusual interstitial type of cartilage growth. J Cytol Histol 2016;7:440.
- Soliman SA, Abd-Elhafeez HH, Enas A. A new mechanism of cartilage growth in mammals “Involvement of CD117 positive undifferentiated cells in interstitial growth”. M J Cyto 2017;1(1):1.
- Bancroft JD, Layton C, Suvarna SK. Bancroft's theory and practice of histological techniques. 7th edn. US: Elsevier Health Sciences; 2013.
- Mallory FB. The aniline blue collagen stain. Stain Technol 1936;11:101-10.
- Soliman SA, Abd-Elhafeez HH. Mesenchymal cells in cartilage growth and regeneration “An immunohistochemical and electron microscopic study”. J Cytol Histol 2016;7:437.
- Miettinen M, Lasota J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 2005;13(3):205-20.
- Arnhold S, Gluer S, Hartmann K, et al. Amniotic-fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int 2011.
- Margaritescu C, Pirici D, Simionescu C, et al. The utility of CD44, CD117 and CD133 in identification of cancer stem cells (CSC) in oral squamous cell carcinomas (OSCC). Rom J Morphol Embryol 2011;52(3):985-93.
- Marjit B. General anatomy genetics histology and embryology. India: Academic Publishers; 2009:26.
- Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater 2008;5:100-14.
- Ozet OG. Matrix metalloproteinase enzyme family. Archives Medical Review Journal 2013;222:209-20.
- Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011;41(2):271-90.
- Clendeninn NJ, Appelt K. Matrix metalloproteinase inhibitors in cancer therapy. New York: Humana Press; 2001.
- Carlevaro MF, Cermelli S, Cancedda R, et al. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J Cell Sci 2000;113(1):59-69.
- Gabner S, Hausler G, Bock P. Vascular channels in hyaline cartilage: development of channels. and removal of matrix degradation products. Anat Histol Embryol 2014;43:29.
- Le Guellec D, Mallein-Gerin F, Treilleux I, et al. Localization of the expression of type I, II and III collagen genes in human normal and hypochondrogenesis cartilage canals. Histochem J 1994;26(9):695-704.
- Claassen H, Kirsch T, Simons G. Cartilage canals in human thyroid cartilage characterized by immunolocalization of collagen types I, II, pro-III, IV and X. Anat Embryol (Berl) 1996;194(2):147-53.
- Blumer MJ, Fritsch H, Pfaller K, et al. Cartilage canals in the chicken embryo: ultrastructure and function. Anat Embryol (Berl) 2004;207(6):453-62.
- Blumer MJ, Longato S, Fritsch H. Cartilage canals in the chicken embryo are involved in the process of endochondral bone formation within the epiphyseal growth plate. Anat Rec A Discov Mol Cell Evol Biol 2004;279(1):692-700.
- Blumer MJ, Schwarzer C, Perez MT, et al. Identification and location of bone-forming cells within cartilage canals on their course into the secondary ossification centre. J Anat 2006;208(6):695-707.
- Soliman SA. Histomorphological and morphometrical studies on endochondral ossification in the Japanese Quail. Germany: LAP LAMBERT Academic publishing; 2013:84.