Metabolic diversity of microbial community associated with Rhizostoma pulmo (Scyphozoa: Rhizostomeae)
2 CoNISMa, Piazzale Flaminio, 9 - 00197 Roma, Italy
3 Istituto per l’Ambiente Marino Costiero, U.O.S. di Taranto, CNR, Via Roma, 3 – 70400 Taranto, Italy
Loredana Stabili, DiSTeBA, University of Salento, Via Prov.le Lecce-Monteroni - 73100 Lecce, Italy, Email: loredana.stabili@iamc.cnr.it
Received: 16-Aug-2017 Accepted Date: Sep 15, 2017; Published: 29-Sep-2017
Citation: Basso L, Rizzo L, Piraino S, et al. Metabolic diversity of microbial community associated with Rhizostoma pulmo (Scyphozoa: Rhizostomeae) J Mar Microbiol. 2017; 1(1):5-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The jellyfish Rhizostoma pulmo is one of the largest and most distributed scyphozoan species in the Mediterranean Sea, characterized by seasonal and inter-annual fluctuations in population size, with alternance of high abundance and rarity periods. In spite of a substantial number of studies on R. pulmo biology and ecology, the diversity and abundance of the jellyfish-associated microbiome is largely unknown. In the present study, we investigated the abundance and metabolic diversity of bacteria associated with three fractions of the R. pulmo jellyfish, namely two distinct body fractions, i.e. the umbrella and the oral arms, and the mucus secretion. Different bacterial metabolic pathways have been identified among the above mentioned fractions, with the highest value of abundance and metabolic activity in the mucus compared to the umbrella and oral arms. These findings are discussed in the framework of the ecology of the species.
Keywords
Bacterial metabolic diversity; Rhizostoma pulmo; Jellyfish bloom
Introduction
Several jellyfish taxa may play a paramount role in marine biological processes, because of their potential to undergo seasonal and inter-annual fluctuations by alternation of population outbreaks with rarity periods [1]. Jellyfish proliferations (or ‘’blooms’’) are regarded as natural components of healthy pelagic ecosystems [2,3] but, in recent decades, jellyfish are occurring at greater frequency and abundance in many coastal areas worldwide [4,5]. This trend has been related to human-derived, multiple impacts on marine ecosystems, including overfishing, eutrophication, and global warming [6]. When exceedingly abundant, jellyfish can cause substantial ecological impacts on marine biodiversity, interfere with economic and recreational human activities, and may be harmful to public health [7,8]. Due to their seasonal high biomass, jellyfish proliferations may represent nutrientrich direct food source for fish species [9]; also, jellyfish blooms or their decaying tissues (jelly-falls) may drive significant changes of the functional and structural microbial diversity and impact the food web structure of both seafloor and plankton communities [10-15].
Moreover, investigations on the structural and metabolic diversity of jellyfishassociated microbial communities may increase our understanding of the ecological impact of jellyfish on marine ecosystem functioning. The present study focuses on the analysis of the abundance and metabolic diversity of heterotrophic culturable bacteria isolated from one of the most common jellyfish species in the Mediterranean Sea, the scyphomedusa Rhizostoma pulmo (Macri, 1778), characterized by seasonal and inter-annual fluctuations in population density [16-19]. Different body fractions of jellyfish were separately investigated, namely two body fractions (the umbrella and the oral arms) and the mucus secretion from whole medusa specimens.
Material and Method
Rhizostoma pulmo medusae were sampled in Ginosa Marina (Ionian Sea 40°25.7’ N, 16°53.1’ E; Italy) in July 2016 by scuba diving. The animals were transported immediately to the laboratory, were washed in filter-sterilized (0.2 μm, Millipore) seawater to remove the mucus layer produced during transport and the bacteria settled on surfaces of umbrella and the oral arms. The newly secreted mucus was collected in sterile containers. Successively, the umbrella was separated from the oral arms using a sterile blade and the different fractions were homogenized in a sterile Waring. To enumerate the culturable bacteria, 1 mL of each homogenated sample and its appropriate decimal dilutions (10-1-10-5) was plated onto Marine Agar 2216. After incubation for 7 days at 25 °C the bacteria were counted according to the colony forming units (CFU) method [20]. The analysis of the metabolic profiles was performed by using the Biolog system-EcoplatesTM (BIOLOG Inc., Hayward, Calif.). Among the available methods used to identify environmental bacteria, the Biolog EcoPlate system offers a standardized rapid method for determining bacterial oxidation of 31 ecologically relevant carbon substrates (including two synthetic polysorbate polymers, Tween 40, Tween 80, and two naturally occurring carbohydrate polymers, α-cyclodextrin and glycogen) with a redox-sensitive tetrazolium indicator of microbial respiration [21]. Three replicas for each homogenate fraction were prepared using three BIOLOG ECO plates. The inoculation volume was 150 µL in each well. The plates were incubated at 30 °C for 1 week. The optical density (OD) values were measured at a wavelength of 590 nm with a plate reader (Microplate Reader model 3550; Bio-Rad, Richmond, Calif.). The difference between the OD values at the beginning and at the end of incubation was regarded as the increase in OD values for the well [22-24].
Results and Conclusion
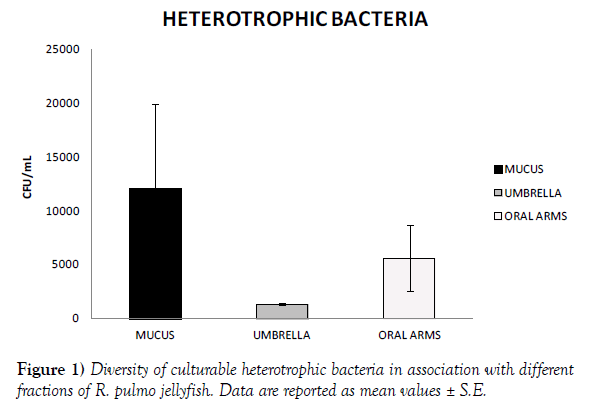
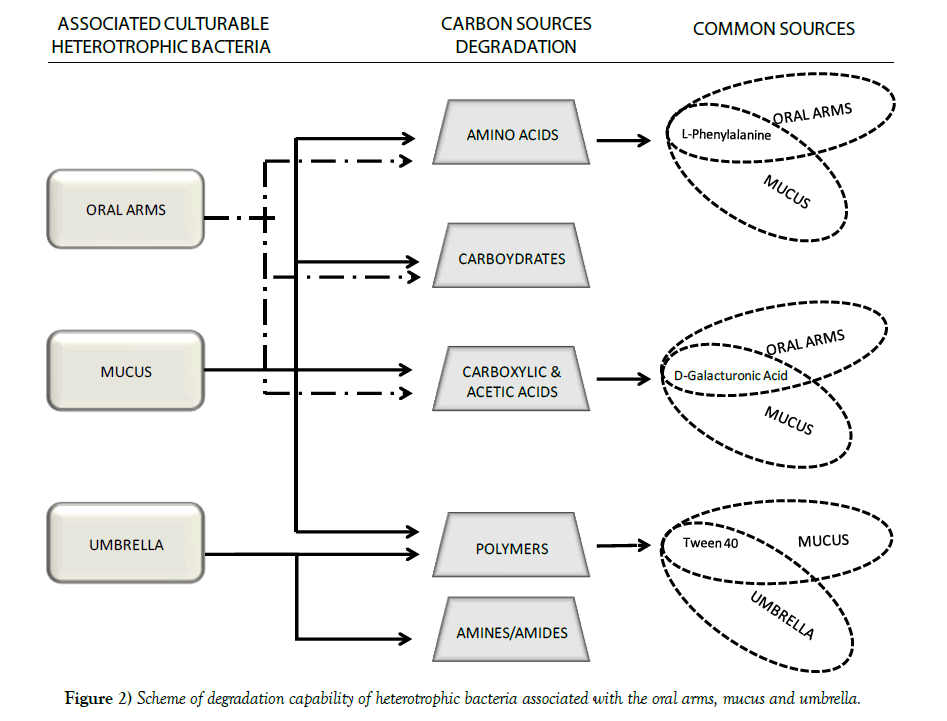
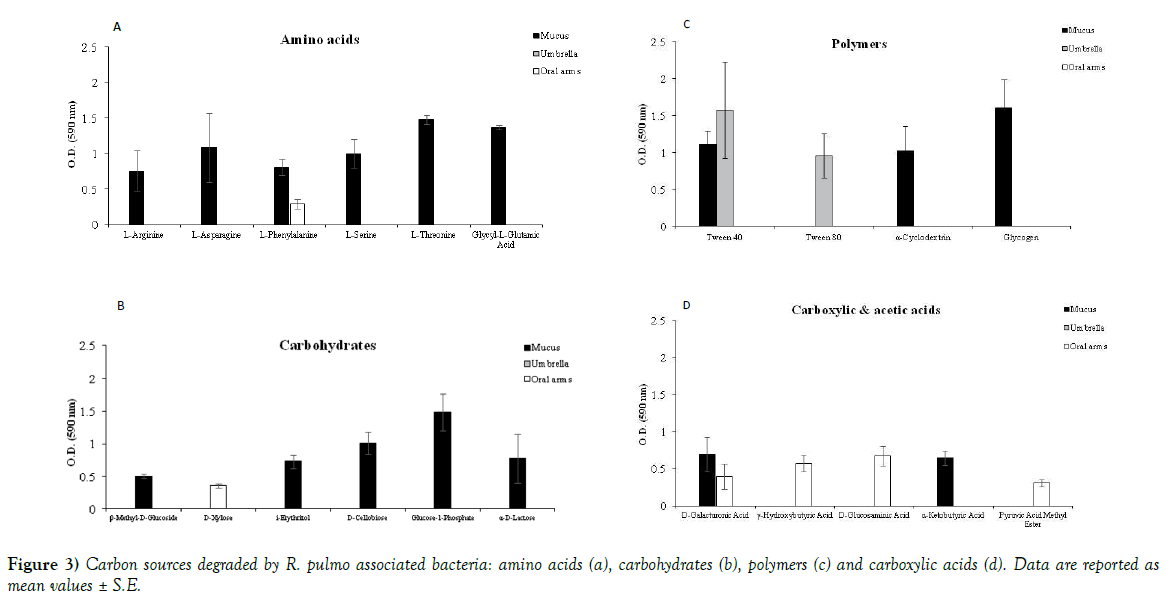
Jellyfish specimens of R. pulmo (mean umbrella diameter 25.38 ± 7.41 cm) were used for analysis of the associated microbiome. Bacterial concentrations were lower in the umbrella (1.3x103 CFU/mL) and the oral arms homogenates (5.6x103 CFU/mL) than in the newly secreted mucus fraction (1.21x104 CFU/mL) (Figure 1). The metabolic activities recorded by 72 h incubation BIOLOG ECO plates indicated amino acids, carbohydrates and carboxylic acids as preferential categories of carbon sources for the oral arms and mucus associated bacteria (Table 1, Figure 2). In addition, mucus associated bacteria were also capable to utilize three out of the four BIOLOG ECO polymers (Table 1). Preferred categories of carbon sources for the umbrella-associated microbes were only polymers and amine/amides (Figure 2). The highest activity was observed in the mucus-associated heterotrophic bacteria, able to degrade 16 carbon sources on the total of 31 relevant carbon substrates; differently, the umbrella and oral arms associated bacteria degraded only 3 and 6 of the total carbon substrates (Table 1, Figure 3). L-phenylalanine and D-galacturonic acid were degraded both by the mucus and oral arms associated bacteria; the Tween 40 was the only common substrate used by the mucus and umbrella associated bacteria. It is worth noting that L-phenylalanine is a common amino acid component of living organisms [25,26] used by methylotrophic bacteria as carbon, nitrogen and energy sources for their growth [27]. In particular, a phenylalanine hydroxylase has been identified in Pseudomonas species [28]. The use of Tween 40 and 80 is considered a key point for the identification of highly specialized bacteria involved in hydrocarbon and oil degradation [29]. D-galacturonic acid results among the utilized carboxylic acids by R. pulmo associated bacteria. Notably, carboxylic acids are important carbon sources for bacterioplankton [30,31] and are considered part of the labile pool of organic matter. Our results suggest that the secreted mucus hosts a larger and more diverse bacterial community compared with the other fractions. This is consistent with previous studies showing that mucusassociated microbiota in the scyphozoan Aurelia aurita s.l. is more variable than the microbiota of the gastric cavity [32]. In the Semeostomeae jellyfish Chrysaora quinquecirrha, the highest species richness of the associated bacterial community was found on the umbrella surface rather than on mouth arms, tentacles, and gonads [33]. This can be explained by the different morphology of the oral arms between Semeostomeae and Rhizostomeae jellyfish, the latter containing a mesh network of small ciliated grooves that may facilitate settlement and growth of a richer microbial community. Previous studies revealed that corals provide several microbial-specific habitats, such as tissue [34], the gastrovascular cavity [35], and the surface mucus layer [36,37]. In Cnidaria the mucus is secreted by ephitelio-muscular cells of the body wall [38,39] and its impressive array of functions plays an important role in the biology and survival of organisms. Mucus can be associated to egg-laying and may function as protective barrier against infection [40], physical shield [41] and slippery coating effective in preventing bacteria and debris accumulation on the body surface [42,43]. The mechanisms leading to the rapid enrichment of newly secreted mucus in R. pulmo is still unknown. However, on account of the above mentioned functions, we may speculate that mucus secretion is a key mechanism for Rhizostomeae jellyfish to keep clean and open the small ciliated grooves of their branched oral arms, to transfer the collected food into the gastric cavities.
| CARBON SOURCES | MUCUS | UMBRELA | ORAL ARMS | |
|---|---|---|---|---|
| AMINES/AMIDES | Phenylethyl-amine | - | - | - |
| Putrescine | - | + | - | |
| AMINO ACIDS | L-Arginine | + | - | - |
| L-Asparagine | + | - | - | |
| L-Phenylalanine | + | - | + | |
| L-Serine | + | - | - | |
| L-Threonine | + | - | - | |
| Glycyl-L-Glutamic Acid | + | - | - | |
| CARBOYDRATES | ß-Methyl-D-Glucoside | + | - | - |
| D-Xylose | - | - | + | |
| i-Erythritol | + | - | - | |
| D-Mannitol | - | - | - | |
| N-Acetyl-D-Glucosamine | - | - | - | |
| D-Cellobiose | + | - | - | |
| Glucose-1-Phosphate | + | - | - | |
| a-D-Lactose | + | - | - | |
| D, L-a-Glycerol Phosphate | - | - | - | |
| CARBOXYLIC &ACETIC ACIDS | D-Galactonic Acid γ-Lactone | - | - | - |
| D-Galacturonic Acid | + | - | + | |
| 2-Hydroxy Benzoic Acid | - | - | - | |
| 4-Hydroxy Benzoic Acid | - | - | - | |
| γ-Hydroxybutyric Acid | - | - | + | |
| D-Glucosaminic Acid | - | - | + | |
| Itaconic Acid | - | - | - | |
| a-Ketobutyric Acid | + | - | - | |
| D-Malic Acid | - | - | - | |
| POLYMERS | Pyruvic Acid Methyl Ester | - | - | + |
| Tween 40 | + | + | - | |
| Tween 80 | - | + | - | |
| a-Cyclodextrin | + | - | - | |
| Glycogen | + | - | - |
Table 1: Utilization of the carbon sources: Results from BIOLOG ECO plate assay indicating the utilization of the 31 substrates by the bacterial community on different compartments of R. pulmo jellyfish.
Figure 3) Carbon sources degraded by R. pulmo associated bacteria: amino acids (a), carbohydrates (b), polymers (c) and carboxylic acids (d). Data are reported as mean values ± S.E.
Further studies are needed to clarify the overall spatial and temporal composition of the bacterial communities associated with R. pulmo jellyfish as well as their role, the possible origin (lateral transfer, epibiosis, gut or food related), their maintenance and change during different life stages. Particularly, it will be mandatory to clarify whether there is selective enrichment of specific microbial groups or the jellyfish-associated microbiome reflects the abundance and diversity of the planktonic bacteria in the water column. The next steps of our investigation will be the isolation and characterization of the here investigated jellyfish associated culturable bacteria from both a genotypic and phenotypic point of view. Detailed knowledge on the composition of bacteria associated with jellyfish might also provide insight on the network of interactions between the jellyfish host and its associated microbial consortia, and the possible physiological pathways that may contribute to the host life history.
Acknowledgements
This work was supported by the project “PULMO - Population dynamics, trophic interactions, and human exploitation of a novel nutraceutical and pharmaceutical marine resource: the Mediterranean sea lung jellyfish, Rhizostoma pulmo” funded from the European Community’s Marie Sklodowska-Curie individual Fellowships (H2020-MSCA-IF-2015) under Grant Agreement No. 708698, and by the project CERES (Climate Change and European Aquatic Resources, grant n. 678193, Horizon 2020 programme).
REFERENCES
- Boero F, Bouillon J, Gravili C, et al. Gelatinous plankton: irregularities rule the world (sometimes). Mar Ecol Prog Ser. 2008;356:299-310.
- Boero F. Review of jellyfish blooms in the Mediterranean and Black Sea. Food and Agriculture Organization of the United Nations (FAO), editor General Fisheries Commission for the Mediterranean Studies and Reviews FAO; Rome, Italy 2013;92:53.
- Graham WM, Pagès F, Hamner WM. A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia. 2001;451:199-212.
- Canepa A, Fuentes V, Sabatés A, et al. Pelagia noctiluca in the Mediterranean Sea. Jellyfish Blooms Springer. 2014;237-266.
- Uye S. The giant jellyfish Nemopilema nomurai in East Asian marginal seas. Jellyfish Blooms Springer. 2014;185-205.
- Purcell JE. Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann Rev Mar Sci. 2012;4:209-235.
- Bosch BM, Azzurro E, Pulis K, et al. Jellyfish blooms perception in Mediterranean finfish aquaculture. Marine Policy. 2017;76: 1-7.
- De Donno A, Idolo A, Bagordo, et al. Impact of Stinging Jellyfish Proliferations along South Italian Coasts: Human Health Hazards, Treatment and Social Costs. Int. J. Environ. Res. Public Health. 2014;11:2488-2503.
- Milisenda G, Rosa S, Fuentes VL, et al. Jellyfish as Prey: Frequency of Predation and Selective Foraging of Boops boops (Vertebrata, Actinopterygii) on the Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa). PLoS One. 2014;9:94600.
- Manzari C, Fosso B, Marzano M, et al. The influence of invasive jellyfish blooms on the aquatic microbiome in a coastal lagoon (Varano, SE Italy) detected by an Illumina-based deep sequencing strategy. Biol Invasions. 2015;17:923-940.
- Condon RH, Steinberg DK, del Giorgio PA, et al. Jellyfish blooms result in a major microbial respiratory sink of carbon in marine systems. Proceedings of the National Academy of Sciences. 2011;108:10225-10230.
- Riemann L, Titelman J, Båmstedt U. Links between jellyfish and microbes in a jellyfish dominated fjord. Mar Ecol Prog Ser. 2006;325:29-42.
- Blanchet M, Pringault O, Bouvy M, et al. Changes in bacterial community metabolism and composition during the degradation of dissolved organic matter from the jellyfish Aurelia aurita in a Mediterranean coastal lagoon. Environ Sci Pollut Res. 2014;18:13638-653.
- Tinta T, Malej A, Kos M, et al. Degradation of the Adriatic medusa Aurelia sp by ambient bacteria. Hydrobiologia. 2010;645:179-191.
- Tinta T, Kogovsek T, Malej A, Turk V. Jellyfish Modulate Bacterial Dynamic and Community Structure. Plos One. 2012;7.
- Mills CE. Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia. 2001;451:55-68.
- Graham WM, Martin DL, Felder DL, et al. Ecological and economic implications of a tropical jellyfish invader in the Gulf of Mexico. Biol Invasions 2003;5:53-69.
- Lilley MKS, Houghton JDR, Hays GC. Distribution, extent of inter-annual variability and diet of the bloom-forming jellyfish Rhizostoma in European waters. J Mar Biol Assoc U K. 2009;89:39-48.
- Fuentes V, Straehler-Pohl I, Dachaet A, et al. Life cycle of the jellyfish Rhizostoma pulmo (Scyphozoa: Rhizostomeae) and its distribution, seasonality and inter-annual variability along the Catalan coast and the Mar Menor (Spain, NW Mediterranean). Mar Biol. 2011;158:2247-66.
- Stabili L, Gravili C, Tredici SM, et al. Epibiotic Vibrio luminous bacteria isolated from some Hydrozoa and Bryozoa species. Microb Ecol. 2008;56:625-36.
- Truu M, Juhanson J, Truu J. Microbial biomass, activity and community composition in constructed wetlands. Sci Total Environ. 2009;407:3958-71.
- Garland JL, Mills AL. Classification and Characterization of Heterotrophic Microbial Communities on the Basis of Patterns of Community-Level Sole-Carbon-Source Utilization. Appl Environ Microbiol 1991;57:2351-59.
- Stabili L, Rizzo L, Pizzolante G, et al. Spatial distribution of the culturable bacterial community associated with the invasive alga Caulerpa cylindracea in the Mediterranean Sea. Mar Environ Res. 2017;125:90-98.
- Rizzo L, Pusceddu A, Stabili L, et al. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci Rep. 2017;7.
- Lachnit T, Wahl M, Harder T. Isolated thallus associated compounds from the macroalga Fucus vesiculosus mediate bacterial surface colonization in the field similar to that on the natural alga. Biofouling. 2010;26:247-55.
- Rajasulochana P, Krishnamoorthy P, Dhamotharan R. Investigations on marine red and brown algae with respect to biochemical composition and nutraceuticals-an overview. Int J Pharm Techn. 2013; 4(4):5019-27.
- De Boer L, Harder W, Dijkhuizen L: Phenylalanine and tyrosine metabolism in the facultative methylotroph Nocardia sp. 239. Archives of Microbiology. 1988, 149(5):459-65.
- Guroff G, Ito T: Phenylalanine hydroxylation by Pseudomonas species (ATCC 11299a). JBC. 1965;240:1175-84.
- Yakimov M, Timmis K, Golyshin P. Obligate oil-degrading marine bacteria. Curr Opin Biotechnol. 2007;18:257-66.
- Pullin MJ, Bertilsson S, Goldstone JV, et al. Effects of sunlight and hydroxyl radical on dissolved organic matter: Bacterial growth efficiency and production of carboxylic acids and other substrates. Limnol and Oceanogr. 2004;49:2011-22.
- Sala MM, Marta E, Josep MG. Seasonal changes in the functional diversity of bacterioplankton in contrasting coastal environments of the NW Mediterranean. Aquat Microbl Ecol. 2006;44:1-9.
- Weiland-Bräuer N, Neulinger SC, Pinnow N, et al. Composition of bacterial communities associated with Aurelia aurita changes with compartment, life stage, and population. Appl Environ Microbiol 2015;81:6038-52.
- Hao W. Bacterial community associated with Jellyfish. PhD thesis, University of Bremen, 2014.
- Lesser M, Mazel C, Gorbunov M, et al. Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science. 2004;305: 997-1000.
- Herndl GJ, Velimirov B. Bacteria in the coelenteron of Anthozoa: control of coelomic bacterial density by the coelenteric fluid. J Exp Mar Biol Ecol. 1985;93:115-130.
- Rohwer F, Seguritan V, Azam F, et al. Diversity and distribution of coral- associated bacteria. Mar Ecol Prog Ser. 2002;243:1-10.
- Kooperman N, Ben-Dov E, Kramarsky-Winter E, Barak Z, Kushmaro A. Coral mucus-associated bacterial communities from natural and aquarium environments. FEMS Microbiol. 2007;276:106-113.
- Burnett AL. A model of growth and cell differentiation in Hydra. Am Nat. 1966; 100:165-190.
- Davis LE. Histological and ultrastructural studies of the basal disc of Hydra. The glandulomuscular cell. Zeilforsch. 1973;139:1-27.
- Davis JM, Viney C. Water-mucin phases: Conditions for mucus liquid crystallinity. Thermochim Acta. 1998;315:39-49.
- Martin R, Walther P. Protective mechanisms against the action of nematocysts in the epidermis of Cratena peregrina and Flabellina affinis (Gastropoda, Nudibranchia). Zoomorphology. 2003;122:25-35.
- Baier RE, Gucinski H, Meenaghan A,et al. Biophysical studies of mucosal surfaces. In Oral Interfacial Reactions of Bone, Soft Tissue and Saliva IRL Press: Oxford, UK. 1985;83-95.
- Bythell JC, Wild C. Biology and ecology of coral mucus release. J Exp Mar Biol Ecol. 2011;408: 88-93.