Microbial Production of Nattokinase Enzyme by Bacillus subtilis
Received: 17-Jun-2022, Manuscript No. PULJMBR-22-5038; Editor assigned: 20-Jun-2022, Pre QC No. PULJMBR-22-5038 (PQ); Accepted Date: Aug 17, 2022; Reviewed: 28-Jun-2022 QC No. PULJMBR-22-5038 (Q); Revised: 05-Jul-2022, Manuscript No. PULJMBR-22-5038 (R); Published: 02-Sep-2022, DOI: 10.37532/puljmbr.2022.5(5).48-52
Citation: Kumar V. Microbial production of nattokinase enzyme by Bacillus subtilis. J Mic Bio Rep. 2022;5(5):48-52.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The objective of the present study was production, purification & activity of the Nattokinase enzyme from Bacillus subtilis. Nattokinase is produced by Bacillus subtilis which act as fibrinolytic enzyme. This enzyme is used for the treatment of blood clot. The main interest about this enzyme is due to its direct fibrinolytic activity. Being stable enough in the gastrointestinal tract makes this enzyme a useful agent for the oral thrombolytic therapy. Nattokinase enzyme was produced in two type of production media the first is inorganic salt & Wheat bran, another media is Glucose & Wheat bran. Production of enzyme was carried out in 250 ml conical flask at 37°C for 18 hours. When the enzyme was produced crude enzyme centrifuged at 10000 rpm for 15 minutes at 4°C. After this we have done comparative study on both the production media. After the extraction of crude enzyme experiment performed on blood clot and also on casein protein to demonstrate the fibrinolytic activity.
Key Words
Nattokinase; Bacillus subtilis
Introduction
The Aim of this project is to produce Nattokinase enzyme by utilizing other than conventional media. In this project we have used different composition of chemical & raw material for the production of enzyme. Enzyme was produced in inorganic media also in wheat bran and glucose. We had used Wheat bran instead of other raw material because wheat is good source of nitrogenous compound. Second one benefits is that wheat bran is thrown as waste. So, we utilize the wheat bran as good nutritional component for bacteria to produce the enzyme. It is cheap and easily available.
Natto, a fermented soybean product, has been consumed as a traditional food in Japan dating back thousands of years. Nattokinase, a potent bloodclot-dissolving protein used for the treatment of heart disease, is produced by Bacillus subtilis as it ferments soybeans to produce Natto. Japanese, Korean, and Chinese scholars have studied extensively. Western medicine recently discovered that Nattokinase has fibrinolytic (anticlotting) properties. It has been investigated and evaluated by the National Science Foundation in the United States. For the prevention of atherothrombosis, it is currently undergoing a phase II clinical trial study in the USA. Different expression system studies have identified many genes, characterized them, and produced them. In the context of antithrombotic applications, recombinant technology represents a promising approach for the production of Nattokinase with high purity. In 1980, Hiroyuki Sumi, a Japanese researcher at the Chicago University Medical School, discovered that natto can dissolve artificial fibrin. Sumi and his team extracted an enzyme from natto that not only degraded fibrin but also a plasmin substrate. He named this novel, fibrinolytic enzyme “Nattokinase” [1].
Mechanism of action
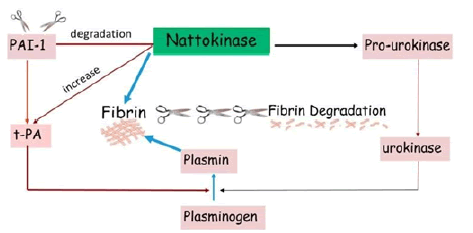
Nattokinase can break down blood clots by directly hydrolyzing fibrin and plasmin substrate, converts endogenous prourokinase to urokinase (uPA), degrades PAI-1 (Plasminogen Activator Inhibitor-1), and increases Tissue Plasminogen Activator (t-PA) which supports fibrinolytic activity [2]. Unlike common fibrinolytic proteases, such as t-PA and uPA, which can produce various side effects such as bleeding, Nattokinase exhibits little to no side effects. Studies also indicate that an oral administration of Nattokinase can be absorbed by the intestinal tract [3,4]. Nattokinase exhibits strong fibrinolytic activity after intraduodenal absorption. These characteristics make Nattokinase a versatile and potent fibrinolytic enzyme that can be used to combat blood clots (Figure 1).
Review of Literature
Thrombosis, a serious disease caused by the mass coagulation of fibrin and platelets, threatens human health, and is often related to some other diseases, such as stroke, cerebral and myocardial infarctions [5]. The presence of thrombosis can lead to ischemic heart disease, stroke, and venous thromboembolism. Thrombolytic/fibrinolytic therapy is a treatment for infarcts of the heart, brain, and, in some cases, the lungs. The use of fibrinolytic therapy can lower mortality in many situations. This has resulted in an increase in the use of such treatments in recent years. Nattokinase was also innovatively used as the coating of medical devices, which was designed from a bio-functional Nattokinase–nanosilver–polyethyleneimine complex with potent antithrombotic and antimicrobial activity [6]. Nattokinase is regarded as a valuable dietary supplement or nutraceutical; proven safety and ease of mass production are other advantages of this enzyme. In addition to these valuable advantages, there are other applications attributed to Nattokinase including treatment of hypertension, Alzheimer’s disease, and vitreoretinal disorders. Microbial fibrinolytic protease is considered as highly purified fibrinolytic enzymes from microbial sources is the most stable and economic way to obtain protein with fibrinolytic activity [7].
In earlier days’ thrombosis was treated by the use of anticoagulants such as heparin and coumarin, which inhibit the fibrin clot formation. The in vivo lysis of fibrin that involves the conversion of inactive plasminogen into active plasmin led to an alternative approach based on enzyme [8]. With the development of molecular biotechnology, genetic and protein engineering have been considered as the effective strategies to improve the Nattokinase activity [9].
Compared to other fibrinolytic enzymes (urokinase, t-PA and streptokinase), Nattokinase shows the advantages of having no side effects, low cost and long life-time, and it has the potential to be used as a drug for treating cardiovascular disease and served as a functional food additive [10]. In adults with primary hypercholesterolemia, Nattokinase was well tolerated and had lowering effects on the serum cholesterol profile in combination with low cholesterol diet. Intravenous fibrinolytic agents such as urokinase and tissue plasminogen activator have been widely used in clinical practice for thrombolytic therapy. Microbial fibrinolytic enzymes have now attracted much more attention than typical thrombolytic agents because of the expensive prices and the undesirable side effects of the latter (Table 1 and 2) [11].
TABLE 1 Physiochemical properties of Nattokinase from various microorganism
| S. No | Organism | Molecular weight (KDa) | Optimum Temperature | Optimum pH | References |
|---|---|---|---|---|---|
| 1 | Bacillus subtilis | 40-45 | 370C | 9 | 13 |
| 2 | Bacillus subtilis RAJS19 | 35 | 500C | 9 | 14 |
| 3 | Bacillus subtilis | 28 | 370C -550C | 9 | 15 |
| 4 | Staphylococcus sciuri | 30 | 480C | 8 | 16 |
| 5 | PseudomonasÃÂ? aeruginosa CMSS | 21 | 250C | 7 | 17 |
| 6 | Bacillus subtilis YJ1 | 27.5 | 500C | 8.5 | 18 |
| 7 | Bacillus subtilis N1 | 46.5 | 550C | 8 | 19 |
| 8 | Pseudomonas sp. TKU015 | 21 | 500C | 7 | 20 |
| 9 | Bacillus subtilis TKU007 | 30 | 400C | 8 | 21 |
TABLE 2 Average content (g/100 g food) of bioactive compounds in wholegrain wheat and wheat bran and germ fractions [12].
| S. No | Bioactive compounds | Wholegrain wheat | Wheat bran | Wheat germ |
|---|---|---|---|---|
| 1 | α-Linoleic acid (18:3n–3) | - | 0.16 | 0.53 |
| 2 | Sulphur compounds | 0.5 | 0.7 | 1.2 |
| 3 | Total free glutathione | 0.007 | 0.038 | 0.27 |
| 4 | Fiber (as AOAC) | 13.2 | 44.6 | 17.7 |
| 5 | Lignin | 1.9 | 5.6 | 1.5 |
| 6 | Oligosaccharides | 1.9 | 3.7 | 10.1 |
| 7 | Phytic acid | 0.9 | 3.39 | 1.8 |
| 8 | Minerals and trace elements | 1.12 | 33.9 | 2.51 |
| 9 | B vitamins | 0.0091 | 0.0095 | 0.0123 |
| 10 | Vitamin E (tocopherols and tocotrienols) | 0.0047 | 0.0095 | 0.0271 |
| 11 | Carotenoids | 0.00034 | 0.00072 | - |
| 12 | Polyphenols | 0.15 | 1.1 | >0.37 |
| 13 | Phenolic acids | 0.11 | 1.07 | >0.07 |
| 14 | Flavonoids | 0.037 | 0.028 | 0.3 |
| 15 | Lignans | 0.0004 | 0.005 | 0.0005 |
| 16 | Alkyl resorcinol | 0.07 | 0.27 | - |
| 17 | Phytosterols | 0.08 | 0.16 | 0.43 |
Brief chronology of natto and its relatives
1051-1083: The origin of natto is obscure. According to legend, it was discovered accidentally in northeast Japan by Minamoto (Hachimantaro) Yoshiie when warm, cooked soybeans, placed in a rice-straw sack on the back of a horse, turned into natto[12]. The warmth of the horse helped the fermentation [13].
1906 Aug: “On the microorganisms of natto,” by S. Sawamura published in a scientific journal in Japan. He found two bacteria in natto. He was the first to isolate Bacillus natto from natto and to give that name to the newlydiscovered microorganism, and to show that it was responsible for the natto fermentation [14].
1912: Dr. Shinsuke Muramatsu of the Morioka College of Agriculture publishes “On the Preparation of Natto” in English. He found that three Bacillus species or strains produced fine natto with strong viscosity and good aroma at 45°C, but that Bacillus No. 1 produced the best product; he recommended its use as a pure culture. He concluded by giving the first nutritional analysis of fresh natto and of natto that was several days old. Soon Dr. Muramatsu started producing his “College Natto” at the College of Agriculture. His students helped to make and sell it, as a source of income, and it became very popular [15].
1919: Dr. Jun Hanzawa, of Hokkaido University’s Department of Agriculture, published the first of three key reports which helped to bring natto production in Japan out of the “Dark Ages.” Serving simultaneously as a microbiologist, and extension worker, and a pilot plant operator, Dr. Hanzawa began by making a pure-culture bacterial inoculum for natto; this enabled commercial natto manufacturers, for the first time, to discontinue the use of rice straw as a source of inoculum. Secondly, disliking the use of rice straw even as a wrapper, he developed a simple, low-cost method for packing, incubating, and selling natto wrapped in paper-thin sheets of pine wood (kyogi) or small boxes of pine veneer (oribako). A third important improvement followed shortly; the development of a new incubation room design (buNattokinasea muro), which had an air vent on the ceiling and substantially decreased the natto failure rate [16].
These three developments laid the basis for modern industrial, sanitary, scientific natto manufacture. Commercial natto makers filled his classes and he worked as a consultant for them. Like Dr. Muramatsu before him, Dr. Hanzawa sold his “University Natto” from his research lab, promoting it as a rival to cheese. He was given the appellation of “the father of modern natto production.” In 1971 he was given the honor of addressing the emperor of Japan on the subject of natto.
2001 April: An article by M. Kaneki et al. in the journal Nutrition is the first to point out that natto is one of the most concentrated sources of vitamin K1 (MK7). Conclusion: “... natto consumption may contribute to the relatively lower fracture risk in Japanese women [17].
2003 April: An article by Kasahara and Kato, published in the prestigious journal Nature (London) confirms that PQQ (pyrroloquinoline quinone), a substance discovered in 1979, can be classified as a vitamin. More specifically, it is a new B vitamin, joining niacin/nicotinic acid (vitamin B3 ) and riboflavin (vitamin B2 ) – first new vitamin in 55 years. The most concentrated known source of PQQ is natto.
Materials and Methods
Preparation of inoculum
Preparation of inoculum medium
• Here we have used liquid media (broth) for the inoculation of Bacillus subtilis. The bacteria were inoculated from pure strain which was available in HCST Lab.
• We have taken two flasks of 250 ml each.
• Took 100 ml of distilled water and add 2.5 gram LB, All the chemical compositions were weight through Denver Instrument analytical balance.
• Now transfer 50 ml in each flask and add 1% agar.
• Autoclave at 121°C for 15 psi for 15 minutes.
• We will add Bacillus subtilis in the broth and place it for two days on incubator at 37°C (Table 3).
TABLE 3 Tryptone Yeast Extract Glucose Medium (TYG) composition
| S. No | Suubstance | Amount |
|---|---|---|
| 1 | Tryptone | 10 g/L |
| 2 | Yeast Extract | 5 g/L |
| 3 | NaCl | 10 g/L |
| 4 | Agar | 15 g/L |
| 5 | Glucose | 5 g/L |
Preparation of production medium for Nattokinase enzyme
Here we have taken two production media by changing the composition
• Inorganic production medium: Medium was prepared as per given in Table 4.
• Organic production medium: Glucose 1%, wheat bran 5% in 100 ml distilled water taken for organic production medium.
• 2% broth culture was inoculated in each production medium.
• Each medium was transferred to shaking incubator for 30 hours– 40 hours
• Now we have extracted Nattokinase enzyme from the production medium (Table 4).
TABLE 4 Chemicals composition for production media
| S. No | Chemicals | Amount |
|---|---|---|
| 1 | Glucose | 5 g/L |
| 2 | K2HPO4 | 1 g/L |
| 3 | MgSO4.7H2O | 2 g/L |
| 4 | MnSO4.7H2O | 0.02 g/L |
| 5 | FeSO4.7H2O | 0.05 g/L |
| 6 | CoCl2.6H2O | 0.01 g/L |
| 7 | Wheat bran | 5 g/L |
Purification of Nattokinase from the production medium
The extracellular Nattokinase of Bacillus subtilis was produced. The culture supernatant was obtained by centrifugation at 10,000 rpm for 15 minutes. The culture was centrifuged through Hermle Z 233 M-2 Centrifuge.
Enzyme test
• Lowry’s method for estimation of protein concentration.
• Blood hydrolysis test.
Lowry’s method for estimation of protein concentration
Enzyme assay for Nattokinase:
Chemicals used in enzyme assay for Nattokinase
• 0.1 M potassium phosphate buffer (pH 7.5)
• 0.1 ml of 2% casein
• 0.1 ml of enzyme sol
• 0.1 ml of 1.5 M trichloroacetic acid
The Nattokinase activity was determined using caseinolytic assay by using casein as a substrate. Caseinolytic activity was assayed using the following procedure:
• A mixture (1ml) containing 0.7 ml of 0.1 M potassium phosphate buffer (pH7.5).
• 0.1 ml of 2% casein, and 0.1 ml of enzyme solution was incubated for 5 minutes at RT.
• Mixed with 0.1 ml 0f 1.5 M trichloroacetic acid, allowed to stand at 4 degrees centigrade for 30 minutes and then centrifuged at RT.
• Folin - Lowry assay was done for the supernatant, to determine the amount of tyrosine residues released and the absorbance at 560 nm was measured and converted to the amount of enzyme releasing 1 micromole of tyrosine equivalent/minute.
Folin-Lowry’s reagents:
• 2% Na2CO3 in 0.1 N NaOH
• 1% Potassium sodium tartrate tetrahydrate in distilled water.
• 0.5% CuSO4 .5H2 O in distilled water.
Reagent I: 48 ml of A, 1 ml of B, 1 ml C.
Reagent II: 1-part Folin Phenol (2N):1-part water.
To 100 µl of protein, add 225 µl of Reagent I, incubate for 10 minutes at room temperature, take the OD at 560 nm. The OD was taken with help of UV spectrophotometer of UV 1700 Shimadzu.
Blood clot hydrolysis test
• Blood from healthy person were transferred on three slides and tissue paper, place it for 45 minutes at room temperature.
• After clot formation, added few drops of Nattokinase enzyme on blood clot from both production media.
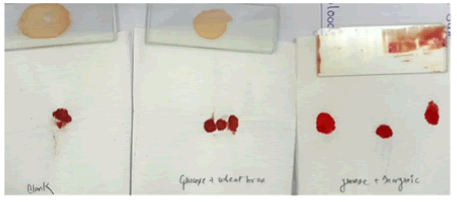
• On one blood clot we added distilled water after 20 minutes we got the following result shown in the images below (Figure 2).
Results and Discussion
The results of the experiment found clear support for the “Microbial production of Nattokinase enzyme by Bacillus subtilis”. We found that the media which we had used for this experiment are found to be effective.
Preparation of inoculum
In TYG medium bacteria strain was revived (Figure 3).
Production of Nattokinase enzyme
Two type of media we had used for this experiment one of the two media is inorganic salt with mixture of wheat bran. Second media is Glucose & wheat bran [18]. We found that enzyme which was produced in Glucose & wheat bran had more efficient than inorganic salt with mixture of wheat bran (Figure 4).
Purification of Nattokinase from the production medium
The extracellular Nattokinase of Bacillus subtilis was produced. The culture supernatant was obtained by centrifugation at 10,000 rpm for 15 minutes. We decarded the pallet and kept supernatant as crude enzyme for further analysis (Figure 5).
Enzyme test
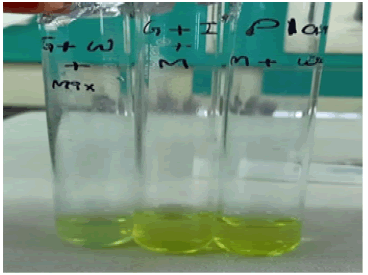
The enzyme test was done by utilizing Folin-Lowry’s method for estimation of protein concentration [19]. Blue color observed when enzyme is present. The OD was taken at 560 nm. Absorbance of Glucose and Wheat bran sample is 0.121 & absorbance of Inorganic salt and wheat bran sample is 0.078 (Figure 6).
Blood clot hydrolysis test
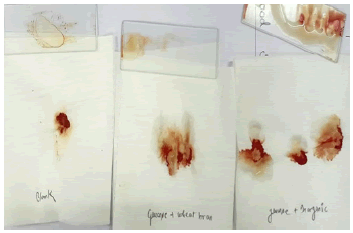
When we did hydrolysis test of blood, we found that enzyme produced by both of the media are equally efficient in work instead of their production [20]. Hydrolysis test on Blood sample is found to be effective within the moments blood clot started dissolving. Image on the left-hand taken as blank Nattokinase sample and is treated with distilled water whereas the other two treated with Nattokinase enzyme extracted from two different production media (Figure 7).
Summary and Conclusion
There are many serious blood clot related diseases like myocardial infarction, stroke in which there is urgent need of blood hydrolase enzyme. In our project we are producing Nattokinase enzyme from Bacillus subtilis which can play an important role in hydrolysis of blood clot due to its fibrinolytic activity. Here we are using two different production media the first is inorganic salt and wheat bran, another media is Glucose and wheat bran to optimize the growth of microbes. After the growth of microbes, we extracted the crude enzyme by centrifugation at 10,000 rpm for 15 minutes and performed the enzyme assay for Nattokinase. The Nattokinase activity was determined using caseinolytic assay by using casein as a substrate. Folin Lowry assay was done for the supernatant, to determine the amount of tyrosine residues released and the absorbance at 560 nm was measured and converted to the amount of tyrosine equivalent. Absorbance of inorganic salt and wheat bran sample is 0.121 whereas absorbance of glucose and wheat bran sample is 0.078.
At last, we performed test for blood clot hydrolysis, we took sample of blood from a healthy person and placed it for few minutes to let it clot. When we added few drops of Nattokinase enzyme to this blood clot it actually worked and we crossed checked the difference from blank blood clot sample on which we used distilled water. As Nattokinase have not as such side effects on human and people in Japan use it in meal yet there are many works yet to be done before implementing it on human like the amount which is to be used. We have to check dose which we can give and other precaution.
References
- Sumi H, Hamada H, Tsushima H, et al. A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia. 1987;43(10):1110-1.
- Yatagai C, Maruyama M, Kawahara T, et al. Nattokinase-promoted tissue plasminogen activator release from human cells. Pathophysiol Haemost Thromb. 2007;36(5):227-32.
- Fujita M, Hong K, Ito Y, et al. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995;18(9):1194-6.doi: 10.1248/bpb.18.1194.
- Fujita M, Ohnishi K, Takaoka S, et al. Antihypertensive effects of continuous oral administration of nattokinase and its fragments in spontaneously hypertensive rats. Biol Pharm Bull. 2011;34(11):1696-701.
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938-49.
- Kamiya S, Hagimori M, Ogasawara M, et al. In vivo evaluation method of the effect of nattokinase on carrageenan-induced tail thrombosis in a rat model. Acta Haematologica. 2010;124(4):218-24.
- Dabbagh F, Negahdaripour M, Berenjian A, et al. Nattokinase: production and application. Appl microbiol biotechnol. 2014 Nov;98(22):9199-206.
- Vijayaraghavan P, Vincent SG, Arasu MV. Purification, characterization of a novel fibrinolytic enzyme from Paenibacillus sp. IND8, and it’s in vitro thrombolytic activity. South Indian J Biol Sci. 2016; 2:434.
- Fu LL, Xu ZR, Li WF, et al. Protein secretion pathways in Bacillus subtilis: implication for optimization of heterologous protein secretion. Biotechnology advances. 2007;25(1):1-2.
- Cai D, Zhu C, Chen S. Microbial production of nattokinase: current progress, challenge and prospect. World J Microbiol Biotechnol. 2017;33(5):1-7.
- Wu DJ, Lin CS, Lee MY. Lipid-lowering effect of nattokinase in patients with primary hypercholesterolemia. Acta Cardiologica Sinica. 2009;25(1):26-30.
- Stevenson LE, Phillips F, O'sullivan K, et al. Wheat bran: its composition and benefits to health, a European perspective. Int j food sci nutr. 2012;63(8):1001-13.
- Taneja K, Bajaj BK, Kumar S, et al. Production, purification and characterization of fibrinolytic enzyme from Serratia sp. KG-2-1 using optimized media. 3 Biotech. 2017 Jul;7(3):1-5.
- Kumar DJ, Rakshitha R, Vidhya MA, et al. Production, optimization and characterization of fibrinolytic enzyme by Bacillus subtilis RJAS19. Pak J Biol Sci: PJBS. 2014;17(4):529-34.
- Dubey R, Kumar J, Agrawala D, et al. Isolation, production, purification, assay and characterization of fibrinolytic enzymes (Nattokinase, Streptokinase and Urokinase) from bacterial sources. Afr J Biotechnol. 2011;10(8):1408-20.
- Choubey D, Dhusia K, Gupta N, Viswan NA, et al. Identification and characterization of Nattokinase producing bacteria and optimization of enzyme production.2016.
- Chandrasekaran SD, Vaithilingam M, Shanker R, et al. Exploring the in vitro thrombolytic activity of nattokinase from a New Strain Pseudomonas aeruginosa CMSS. Jundishapur j microbiol. 2015;8(10).
- Yin LJ, Lin HH, Jiang ST. Bioproperties of potent nattokinase from Bacillus subtilis YJ1. J agric food chem. 2010;58(9):5737-42.
- Lin HT, Wu GJ, Hsieh MC, et al. Purification and characterization of Nattokinase from cultural filtrate of red alga porphyra dentata fermented by Bacillus subtilis N1. J Mar Sci Technol. 2015;23(2):13.
- Wang SL, Chen HJ, Liang TW, et al. A novel nattokinase produced by Pseudomonas sp. TKU015 using shrimp shells as substrate. Process Biochemistry. 2009;44(1):70-6.
- Wang SL, Wu YY, Liang TW. Purification and biochemical characterization of a nattokinase by conversion of shrimp shell with Bacillus subtilis TKU007. New biotechnology. 2011;28(2):196-202.