Microphytobenthic biomass, diversity and exopolymeric substances in a shallow dystrophic coastal lagoon
2 CNR– IAMC, National Research Council, Institute for Coastal Marine Environment, Italy
3 Department of Biology, Laboratory for Biology of Algae, University of Rome, Italy, Email: roberta.congestri@uniroma2.it
Received: 30-Jan-2018 Accepted Date: Feb 15, 2018; Published: 23-Feb-2018
Citation: Di Pippo F, Magni P, Congestri R. Microphytobenthic biomass, diversity and exopolymeric substances in a shallow dystrophic coastal lagoon. J Mar Microbiol. 2018;2(1):6-12.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objective: This study is aimed to provide a first insight into the microphytobenthos (MPB) species composition, biomass and exopolymeric substances (EPS) of the organic enriched Cabras lagoon (Western Mediterranean Sea, Italy), due to the lack of information on the presence and distribution of microphytobenthic assemblages in this system, although their importance has been shown in other organic-enriched coastal lagoons.
Methods: Surface sediment samples were collected at three sites of the lagoon (C1, C2, C3), differing in sediment granulometry, on 4 occasions, over one year. MPB biomass, expressed as chlorophyll a and organic matter, was evaluated as well as the quantity of two operationally distinct EPS fractions. Sampled communities were also characterized in light and confocal microscopy to assess the spatial distribution and composition of microphytobenthic species in relation with sediment characteristics. Alcian Blue cytochemical staining of EPS samples was also used to ascertain, during light microscopy observations, the presence of acidic groups in EPS residues.
Results and Conclusions: MPB assemblages developed in biofilms comprising both eukaryotic and prokaryotic members. Communities were dominated by diatom species, mainly of the genera Navicula and Nitzschia, associated with cyanobacteria and green algae in most samples. Microorganisms were distributed in patches of different thickness and embedded in a polymeric matrix, characterized by both neutral and acidic polysaccharidic residues. Spatial and temporal patterns in species composition and biomass were also observed, likely due to the habitat heterogeneity within each site. Sediment properties seemed to influence the diatom assemblages. The muddy nature of sediments at site C2 enabled the development of epipelic forms, such as Surirellales diatoms, known for their high motility through the sediment while sandy bottoms at site C1 and C3 supported the growth of episamnic species (i.e. among the genera Amphora, Cocconeis and Cymbella), that are able to adhere to the grains with stalks, tubes and/or apical pads. Cyanobacteria dominated the extremely variable (salinity, water temperature, light and water availability) site C2 over the other MPB members, likely due to their physiological versatility and capacity of producing protective exopolymers.
Keywords
Microphytobenthos; Phototrophic biofilms; EPS; Cyanobacteria; Coastal lagoon
Introduction
The Cabras lagoon (Sardinia, Italy, Mediterranean Sea) is a shallow transitional system renowned for its naturalistic (Ramsar Convention on Wetlands, Natura 2000 network for EU Habitat directive) and economic importance (e.g. artisanal fisheries). Anthropogenic pressure due to massive nutrient loading, reduction of freshwater input from upland, modifications of the inlets and other man-made interventions have reduced the water exchange with the adjacent Gulf of Oristano, leading to hypoxic and anoxic conditions in near-bottom waters, especially in summer. These dystrophic events caused major loss of the biological resources of the lagoon [1]. To assess the ecological quality of the lagoon, numerical models have been developed to predict the evolution of both hydrological and ecological variables within the lagoon system under different meteorological forcing [2]. In parallel, investigations of the physical and chemical characteristics of the sediments and the macrobenthic assemblages have shown a close link between the distribution of organic-C bounding fine sediments, benthic macroinvertebrates, and the water residence times computed from the model [1]. In this context, detailed analysis of sediment dynamics are particularly important because the partitioning and transport of fine sediments can strongly influence the redistribution and accumulation of large amounts of organic matter, and consequently the spatio-temporal distribution of benthic assemblages and the trophic status and functioning of the lagoon [1,3]. Recent investigations indeed demonstrated the effect of confinement, organic enrichment and saprobity levels on benthic community distribution in this shallow water body [4].
Primary producers are considered to be an important source of organic matter in the Cabras lagoon [3]. Nevertheless, studies on the presence, distribution and diversity of microphytobenthos (MPB) in the Cabras lagoon and more in general in these shallow lagoon systems are still lacking although the importance of the highly variable, biological component has been reported for different organic-enriched coastal systems [5,6]. MPB plays a role in modulating the exchange of nutrients between the sediments and the water column [7] and it is an important food source [8]. Benthic phototrophic primary producers may occur, at the sediment surface, in the form of biofilms where cyanobacteria and microalgae are embedded in a common matrix composed of exopolymeric substances (EPS) [9,10]. In the biofilms, filamentous cyanobacteria along with colonial and tube dwelling diatoms tend to entangle forming a network which also traps sediment particles, thereby producing tough and coherent structures that ultimately contribute to sediment stabilization [9].
In this study, a quali-quantitative approach based on the use of variety of descriptive and analytical techniques was used to provide the first insight into the spatio-temporal variations in taxonomic composition, biomass and exopolymeric substances of the microphytobenthic assemblages in relation to main environmental cues at three sampling sites of the Cabras lagoon, over one year.
Materials and Methods
Sampling
Three sites were selected along the salinity and saprobity gradients, based on past research carried out in the Cabras lagoon system [1,3,4,11-16]. Detailed site description has been already reported [4]. Briefly, the northern site, C1, with a mean depth of 1.5 m, is close to the main freshwater tributary (‘Mare e Foghe’ river) and characterized by sandy sediments and halophytic vegetation (Phragmites sp.) C2 site (mean depth: 0.2 m) is a confined area, connected to the main basin by a narrow inlet, closed during the warm season, located in the satellite ‘Sali e Pauli’ pond, it presents muddy sediments and halophytic vegetation (Salicornia sp.). The southern site, C3 (mean depth: 1.0 m) is close to the cannel connecting the lagoon to the Gulf of Oristano and presents muddy-sand sediments and abundant submerged vegetation, including Ruppia sp.
Field surveys were carried out in 2009-2010 period. A total of 72 sediment samples were taken on four sampling campaigns at the three study sites, in September 2009, March, July and September 2010. At each site and sampling occasion, water temperature, salinity, dissolved oxygen (DO) were measured, before and after sediment collection, using a salinometer WTW LF 197 and a portable oxymeter WTW Oxil 197. The mean value of the two measurements were then used for data analyses. Sediment samples were collected using a manual core tube (40 cm long and 5.5 cm in diameter) gently pushed into the sediments by hand, the core surface layers (3-5 mm) were then carefully extruded and sliced off. Six sample replicates at each site were randomly collected [6].
Laboratory Analysis
Each sediment replicate was homogenized and subsamples were obtained to quantify photosynthetic pigments, water content (WC), organic matter (OM) and EPS. WC was computed after drying in a oven the sediment samples, 50°C for 24 h, and then expressed as percentage of the wet weight; OM content was measured by loss on ignition (LOI) at 500°C for 3 h from a subsample of 1 g and expressed as DW percentage. Chlorophyll a (Chl-a), used as an estimation of the MPB biomass, and pheopigment degradation products, were extracted from wet sediments (ca. 1 g) using 90 % acetone after 24 h of darkness at 4°C, samples were centrifuged (3000 rpm, 10 min) and the extracts spectrophotometrically analyzed. Pigment concentration values were obtained according to Lorenzen’s method [17] as described in [18], where the volume of water is substituted by grams, g, of sediment DW.
Two operationally defined EPS fractions were extracted [19] from lyophilized sediment samples incubated in 400 μL distilled water for 1 h at 30°C. The extract was centrifuged (5 min, 6000 g) and the water-extractable EPS fraction determined. This represents the EPS portion loosely bound to the sediments. The pellet was subsequently incubated with 500 μL 0.1 M Na2EDTA for 16 h at room temperature. The obtained extract was then centrifuged (5 min, 6000 g) and the EDTA-extractable EPS fraction assayed. This fraction is tightly bound to the sediments. Both fractions were then quantified by the phenol-sulphuric acid method, using glucose as reference [20]. The existence of associations between all the measured variables in this study was assayed by the Pearson product-moment correlation (significance levels p<0.05) by using the Sigma Stat (version 3.5) program.
Principal component analysis (PCA) was then used to determine the association between abiotic and biotic parameters. This was accomplished by diagonalization of the correlation matrix of the data. The eigenvalues of the PCs are a measure of their associated variance, and the participation of the original variables in the PCs is given by the loadings.
Microphytobenthos Observations
To assess the phototroph composition of MPB replicates, samples were suspended in filtered water. Aliquots were fixed in formaldehyde at 2% final concentration and stored at 4°C until observed. Observations were made using a light microscope equipped with differential interference contrast using 10, 40 and 100x objectives. MPB images were acquired using a digital camera Coolpix995 (Nikon Corp.).
EPS Analysis
Light microscopy observations of samples were also conducted after staining for 10 min with Alcian Blue (AB) 1%, in HCl 0.5 N (pH 0.5), specific for sulfated polysaccharidic residues in the EPS matrix or in 3% acetic acid (pH 2.5) specific for carboxylic moieties [21]. Fresh samples were stained with 100 μg mL−1 concanavalin A-Alexa Fluor 488- conjugate too, for 20 min at room temperature [22], to visualize glucopyranose and mannopyranose residues in the EPS. Stained samples were carefully washed three times to remove unbound dyes and observed using a confocal laser scanning microscope (CLSM) Olympus FV1000 using a 60x objective. Excitation was set at 488 and 635 nm, and two channels for detecting chlorophyll a (650–750 nm emission range) and the neutral monosaccharide groups, labeled by the fluorochrome (500–590 nm emission range) were used. Three-dimensional images were constructed from series of 2-D cross-sectional images (x-y plane) that were captured at 0.5-μm intervals along the z-axis using IMARIS 6.2.0 software (Bitplane AG).
Results and Discussion
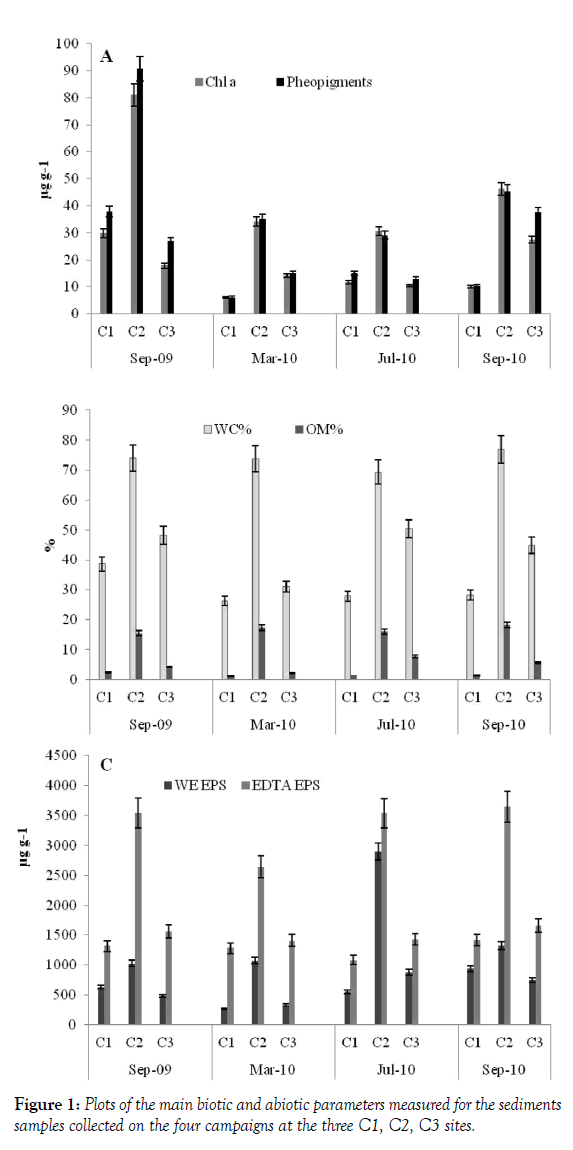
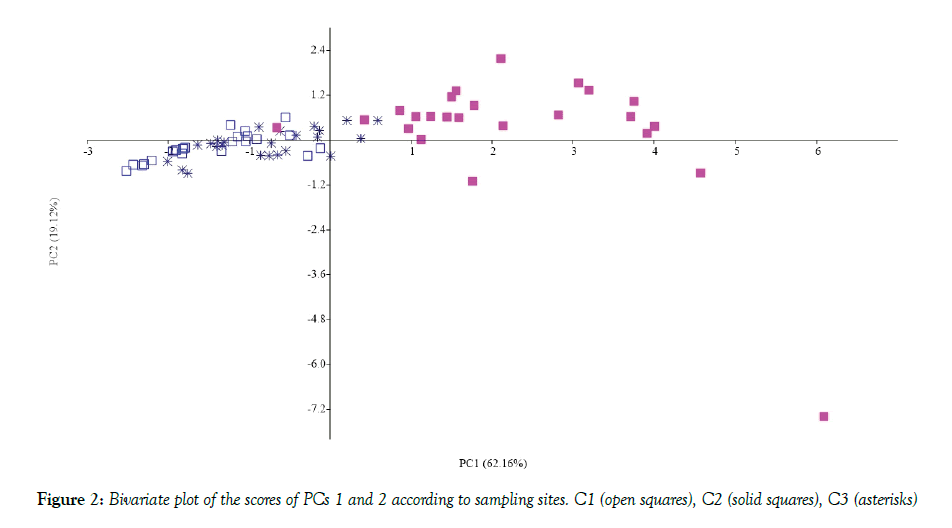
In the Cabras lagoon, differences between sampling sites were evidenced by both abiotic and biotic variables, especially salinity and DO (Table 1). In fact, salinity increased along a north-south gradient from C1 to C3 and DO tended to increase progressively up to C3 site, on all sampling occasions, this being consistent with data obtained previously [4]. Water content, used as an indirect measure of particle size, was lower in the sandy (C1) and muddy-sand (C3) locations than in the muddy, C2, site (Figure 1) as expected. Consistently, the relationship between OM % and WC % showed an increase of organic matter from sandy to muddy substrata (Figure 1). Here, the OM values fall within the range typical of organically over-enriched, sheltered coastal environments [4]. Higher Chl-a and both EPS fraction values were also found at this site, demonstrating the important contribution of MPB (Chl-a being a proxy of its biomass) to the OM standing stock (The C/chl-a ratio = ca. 40, see [23]. EDTA-exatractable EPS showed a correlation with OM% and WC% values (Table 2). This correlation could be due to higher presence EPS-producing organisms in finer sediments or to the capability of these sediments of retaining the exopolymers [9]. Principal Component Analysis, carried out by a diagonalization of the correlation matrix (normalized varcovar) in order to avoid problems arising from different measurement scales and numerical ranges of the original variables, confirmed that sampling site typologies mostly contributed to cluster the MPB biomass data. The first principal component (PC1) explained 62.16% of the variance with the highest loading factors associated with OM % (0.44) and WC (0.42). The bivariate plot of the PC1 and PC2 (19.12% of the variance) scores, reported in Figure 2, showed that C2 data are clustered on the positive side of the axis, while the C1 and C3 samples are positioned at the negative side of the axis. Given the relatively high N:P values of the water (low aqueous TP, [24] at muddy sites the MPB) likely obtained phosphorus from the organic material present in the sediments.
Physico-chemical characteristics of water at the three sampling sites |
|||||
|---|---|---|---|---|---|
| SITES | Sep-09 | Mar-10 | Jul-10 | Sep-10 | |
| Temperature (°C) | C1 | 24.0 | 12.0 | 29.2 | 24.3 |
| C2 | 26.0 | 12.8 | 27.6 | 25.6 | |
| C3 | 26.0 | 13.8 | 32.4 | 26.9 | |
| DO (mgL-1) | C1 | 5.5 | 7.0 | 2.2 | 4.3 |
| C2 | 8.4 | 5.0 | 4.6 | 5.6 | |
| C3 | 9.7 | 9.3 | 14.2 | 9.7 | |
| Salinity (%) | C1 | 11.2 | 5.5 | 4.5 | 6.7 |
| C2 | 13.5 | 9.0 | 10.7 | 12.6 | |
| C3 | 34.0 | 10.2 | 24.4 | 29.1 | |
Table 1: Physico-chemical characteristics of water at the three sampling sites
| Chl a | WC | OM | WE-EPS | EDTA-EPS | |
|---|---|---|---|---|---|
| Chl a | CC: 0.763 p: 0.0039 | CC: 0.717 p:0.00865 | CC: 0.325 p: 0.303 | CC: 0.795 p: 0.00200 | |
| WC | CC: 0.763 p: 0.0039 | CC: 0.979 p<<0.05 | CC: 0.629 p: 0.0284 | CC: 0.913 p<<0.05 | |
| OM | CC: 0.717 p: 0.00985 | CC: 0.979 p<<0.05 | CC: 0.682 p: 0.0147 | CC: 0.936 p<<0.05 | |
| WE EPS | CC: 0.325 p: 0.303 | CC: 0.629 p: 0.0284 | CC: 0.682 p: 0.0147 | CC: 0.729 p: 0.00799 | |
| EDTA EPS | CC: 0.795 p: 0.00200 | CC: 0.913 p<<0.05 | CC: 0.936 p<<0.05 | CC: 0.729 p: 0.00799 |
Table 2: Pearson Correlation
Consistently, the habitat heterogeneity within each sampling site can explain the spatial variability of MPB species composition recorded at the three locations. MPB assemblages indeed grew at the sediment surface in the form of biofilms with a high degree of species diversity, comprising both eukaryotic and prokaryotic microorganisms embedded in an EPS matrix. Taxonomic assessment of MPB communities, over the study year, showed the dominance of diatoms (Table 3) with cyanobacteria and green algae present in most samples. Benthic diatoms included species of the genera Achnanthes, Amphora, Cocconeis, Cymatopleura, Fragilaria, Gyrosigma, Navicula, Nitzschia, and Surirella, with the majority of species belonging to the genera Navicula and Nitzschia.
| C1 | C2 | C3 | |
|---|---|---|---|
| DIATOMS | |||
| Achnanthes brevipes | + | + | |
| Amphora ovalis | + | ||
| Amphora sp. | + | ||
| Cocconeis pellucida | + | ||
| Coscinodiscus sp. | + | ||
| Cymatopleura sp. Cymbella sp. |
+ | ||
| Diadesmis sp. | + | ||
| Diploneis ovalis | + | ||
| Diploneis sp. | + | + | |
| Entomoneis sp. | + | ||
| Fragilaria sp. | + | + | |
| Gyrosigma acuminatum | + | + | |
| Melosira moniliformis | + | + | |
| Melosira sp. | + | ||
| Navicula gregaria | + | ||
| Navicula sp. 1 | + | + | |
| Navicula sp. 2 | + | ||
| Nitzschia sp. 1 | + | + | |
| Nitzschia sp. 2 | + | ||
| Nitzschia sp. 3 | + | + | |
| Surirella sp. | + | ||
| Synedra sp. | + | ||
| CYANOBACTERIA | |||
| Anabaena augstumalis | + | ||
| Calothrix sp. | + | ||
| Coccal cyanobacteria | + | ||
| Geitlerinema sp | + | + | |
| Microcoleus sp. | + | + | |
| Nostoc sp. | + | ||
| Oscillatoria sp.1 | + | + | |
| Oscillatoria sp.2 | + | ||
| Trichormus variabilis | + |
Table 3: Species richness of microphythobenthic taxa at the three sampling sites
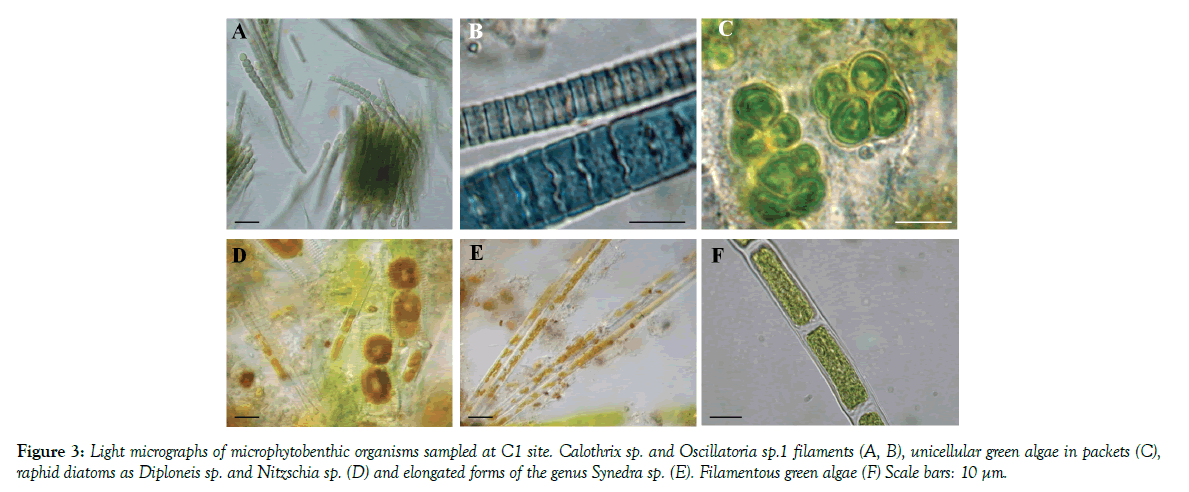
MPB assemblage at site C1 (Figure 3) was characterized by the dominance of one centric diatom that forms chains, Melosira moniliformis, along with raphids as Diploneis sp., a sigmoid species of Nitzschia and the naviculoid morphotype, with plastids along the girdle, regarded as Navicula sp. 1. Other diatoms frequently recorded were two dorsiventral taxa, attributable to the genus Amphora, and two morphotypes of Navicula. Rare species were one elongated araphid taxon attributed to Synedra sp. and two nitzschioid forms. Filamentous green algae were present in March and July 2010 only, while unicellular green algae, occurring in packets surrounded by mucilaginous capsules, only in September 2009. Filamentous, non-heterocytous cyanobacteria belonging to the genera Oscillatoria, two distinct morphotypes, and Phormidium were present in September 2009 along with the heterocytous species Calothrix sp.
Figure 3: Light micrographs of microphytobenthic organisms sampled at C1 site. Calothrix sp. and Oscillatoria sp.1 filaments (A, B), unicellular green algae in packets (C), raphid diatoms as Diploneis sp. and Nitzschia sp (D) and elongated forms of the genus Synedra sp. (E). Filamentous green algae (F) Scale bars: 10 μm.
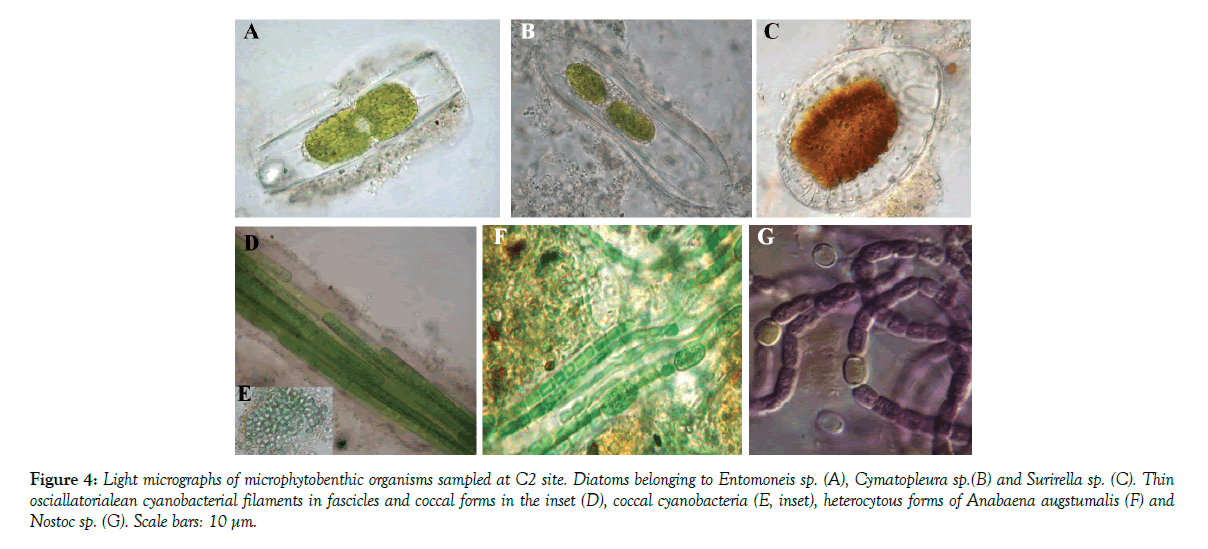
At site C2 (Figure 4), MPB were dominated by epipelic Surirellales diatoms of the genera Entomoneis, characterized by arched and torsioned morphology, and Cymatopleura distinctedly ondulated in valve face. Cyanobacteria were also observed in all the six replicates collected at C2 site, where they showed the highest species richness, with at least two different heterocytous species (Anabaena augstumalis and Nostoc sp.), one thin oscillatorialean (<3 µm in diameter) and one Phormidium morphotype. Chroococcal, unicellular, cyanobacteria along with numerous unicellular, flagellated chlorophytes, and diatoms belonging to the genus Surirella characterized the MPB population in September 2009 and 2010.
Figure 4: Light micrographs of microphytobenthic organisms sampled at C2 site. Diatoms belonging to Entomoneis sp. (A), Cymatopleura sp.(B) and Surirella sp. (C). Thin osciallatorialean cyanobacterial filaments in fascicles and coccal forms in the inset (D), coccal cyanobacteria (E, inset), heterocytous forms of Anabaena augstumalis. (F) and Nostoc sp (G). Scale bars: 10 μm.
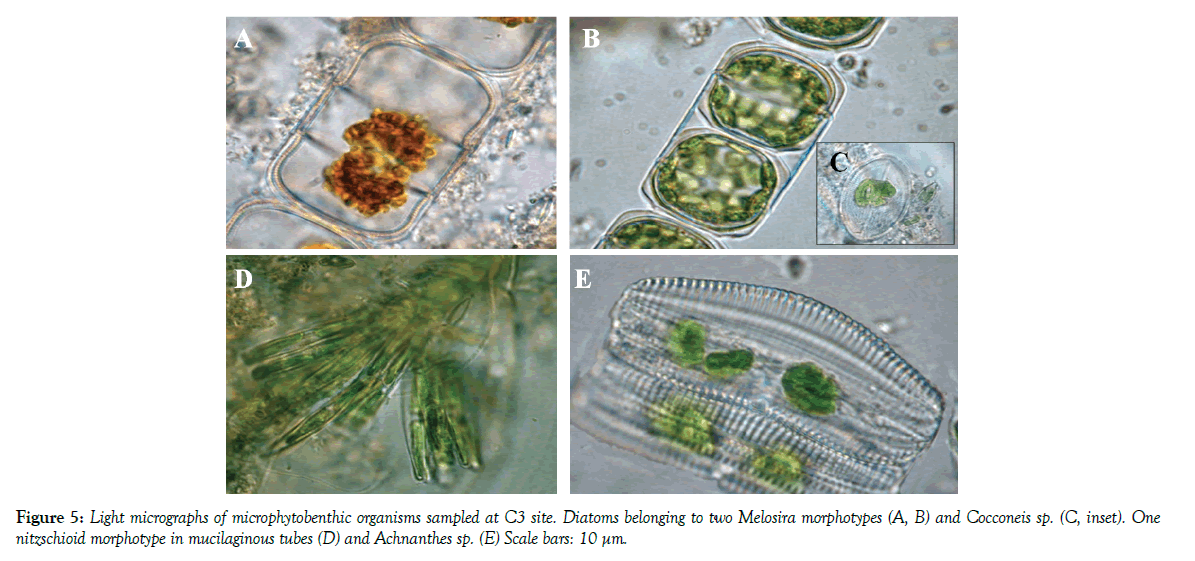
At site C3 (Figure 5), MPB presented the highest diatom richness. Two Melosira morphotypes and Cocconeis sp. were dominant in all samples. Two nitzschioid morphotypes in mucilaginous tubes were also always present, while Achnanthes sp. was observed only in March 2010. Other diatoms frequently recorded were two Amphora species, two Navicula and three Nitzschia morphotypes.
The different sediment characteristics likely influenced the diverse diatom assemblages found in the study sites. Cohesive sediments generally enable the development of epipelic forms able to move through the sediment while sand commonly supports the growth of episamnic species, that adhere to the grains with stalks, tubes and apical pads [25]. The occurrence of morphotypes of Nitzschia in mucilaginous tubes, as well as the presence of episamnic species among the genera Amphora, Cocconeis and Cymbella at sites C1 and C3 was probably due to the sandy characteristics of the sediments. On the contrary, the prevalence of the epipelic Surirellales diatoms at site C2, was likely attributable to the cohesive nature of the sediments characterized by a high mud content.
The observed highest cyanobacterial species richness found at site C2 represent a novel finding. In fact generally, fine sediments are characterized by very low light penetration in combination with high rate of sedimentation and these conditions should make phototaxis impossible, thus preventing cyanobacteria from migrating to the sediment surface. Moreover, cyanobacteria are generally adapted to low nutrient conditions and show low growth rates, therefore the high amounts of nutrients, characteristic of fine silt and muddy sediments, allow cyanobacteria to be outcompeted by other fast growing microorganisms [9]. In any case, the presence of a high number of cyanobacterial species could be explained by the overall extremely peculiar environmental condition of site C2. In particular, this site is characterized by variable salinity, from oligo- to mesohaline values, stagnant and nutrient-enriched water conditions and large day-night fluctuations in DO concentrations, transient to anoxia [4,11]. It is known that cyanobacteria are well adapted to extreme conditions such as salinity, water temperature, light and water availability and this could confer them an advantage over other phototrophs to thrive in C2 sediment [9].
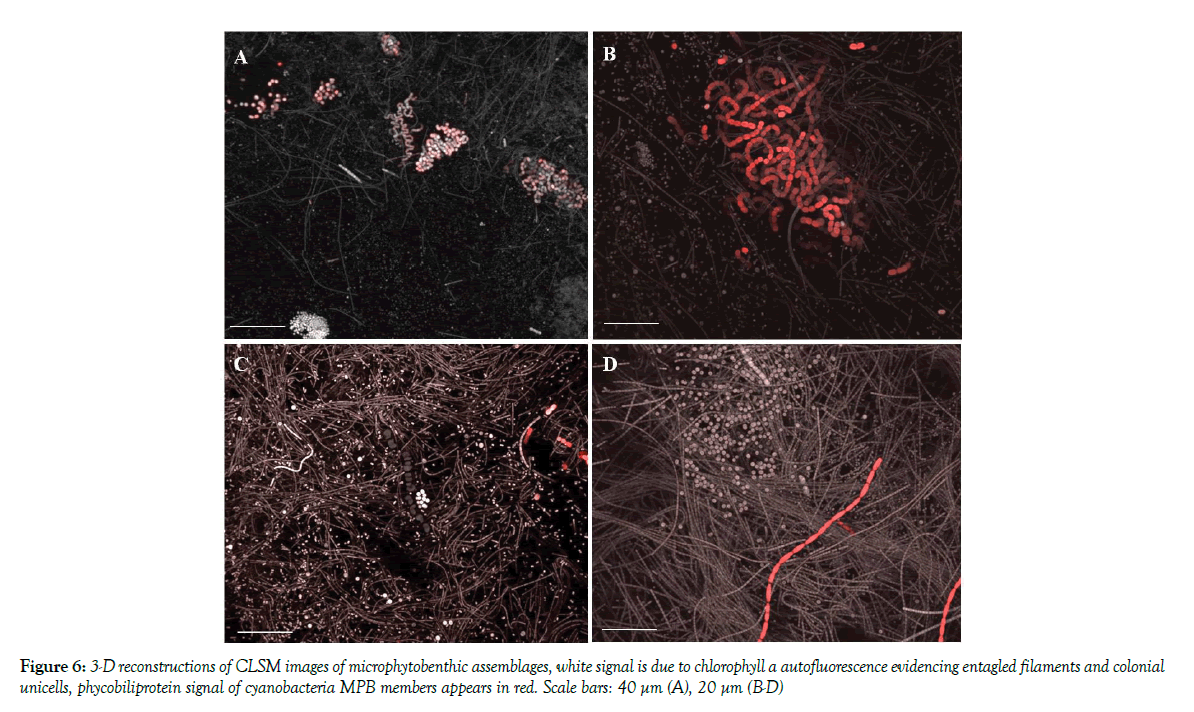
CLSM examination revealed the presence of biofilm assemblages in the sediment samples with microorganism distributed in patches of different thickness commonly presenting voids in their inner structure. 3-D reconstructions of biofilm communities (Figure 6) revealed that C2 sediments were inhabited by highly complex and multi-stratified MPB communities, with microcolonies of coccal cyanobacteria closely associated with the cyanobacterial filaments as expected from taxon richness data. All microorganisms lay immersed in the polymeric matrix. Fluorochrome labeling also revealed the presence of neutral sugar residues in the fine envelopes of most oscillatorialean and nostocacean cyanobacteria and also spread within the biofilm matrix. Acidic residues (carboxylic and sulphated groups) in the biofilm EPS were also localized in the sheaths of heterocytous and non-heterocytous cyanobacteria as well as in the EPS released in the sediments, as indicated by the reaction with Alcian Blue staining. sediments of retaining the exopolymers [9]. Principal Component Analysis, carried out by a diagonalization of the correlation matrix (normalized varcovar) in order to avoid problems arising from different measurement scales and numerical ranges of the original variables, confirmed that sampling site typologies mostly contributed to cluster the MPB biomass data. The first principal component (PC1) explained 62.16% of the variance with the highest loading factors associated with OM % (0.44) and WC (0.42). The bivariate plot of the PC1 and PC2 (19.12% of the variance) scores, reported in Figure 2, showed that C2 data are clustered on the positive side of the axis, while the C1 and C3 samples are positioned at the negative side of the axis. Given the relatively high N:P values of the water (low aqueous TP, [24] at muddy sites the MPB) likely obtained phosphorus from the organic material present in the sediments.
Consistently, the habitat heterogeneity within each sampling site can explain the spatial variability of MPB species composition recorded at the three locations. MPB assemblages indeed grew at the sediment surface in the form of biofilms with a high degree of species diversity, comprising both eukaryotic and prokaryotic microorganisms embedded in an EPS matrix. Taxonomic assessment of MPB communities, over the study year, showed the dominance of diatoms (Table 3) with cyanobacteria and green algae present in most samples. Benthic diatoms included species of the genera Achnanthes, Amphora, Cocconeis, Cymatopleura, Fragilaria, Gyrosigma, Navicula, Nitzschia, and Surirella, with the majority of species belonging to the genera Navicula and Nitzschia.
MPB assemblage at site C1 (Figure 3) was characterized by the dominance of one centric diatom that forms chains, Melosira moniliformis, along with raphids as Diploneis sp., a sigmoid species of Nitzschia and the naviculoid morphotype, with plastids along the girdle, regarded as Navicula sp.1. Other diatoms frequently recorded were two dorsiventral taxa, attributable to the genus Amphora, and two morphotypes of Navicula. Rare species were one elongated araphid taxon attributed to Synedra sp. and two nitzschioid forms. Filamentous green algae were present in March and July 2010 only, while unicellular green algae, occurring in packets surrounded by mucilaginous capsules, only in September 2009. Filamentous, non-heterocytous cyanobacteria belonging to the genera Oscillatoria, two distinct morphotypes, and Phormidium were present in September 2009 along with the heterocytous species Calothrix sp.
At site C2 (Figure 4), MPB were dominated by epipelic Surirellales diatoms of the genera Entomoneis, characterized by arched and torsioned morphology, and Cymatopleura distinctedly ondulated in valve face. Cyanobacteria were also observed in all the six replicates collected at C2 site, where they showed the highest species richness, with at least two different heterocytous species (Anabaena augstumalis and Nostoc sp.), one thin oscillatorialean (<3 µm in diameter) and one Phormidium morphotype. Chroococcal, unicellular, cyanobacteria along with numerous unicellular, flagellated chlorophytes, and diatoms belonging to the genus Surirella characterized the MPB population in September 2009 and 2010.
At site C3 (Figure 5), MPB presented the highest diatom richness. Two Melosira morphotypes and Cocconeis sp. were dominant in all samples. Two nitzschioid morphotypes in mucilaginous tubes were also always present, while Achnanthes sp. was observed only in March 2010. Other diatoms frequently recorded were two Amphora species, two Navicula and three Nitzschia morphotypes.
The different sediment characteristics likely influenced the diverse diatom assemblages found in the study sites. Cohesive sediments generally enable the development of epipelic forms able to move through the sediment while sand commonly supports the growth of episamnic species, that adhere to the grains with stalks, tubes and apical pads [25]. The occurrence of morphotypes of Nitzschia in mucilaginous tubes, as well as the presence of episamnic species among the genera Amphora, Cocconeis and Cymbella at sites C1 and C3 was probably due to the sandy characteristics of the sediments. On the contrary, the prevalence of the epipelic Surirellales diatoms at site C2, was likely attributable to the cohesive nature of the sediments characterized by a high mud content.
The observed highest cyanobacterial species richness found at site C2 represent a novel finding. In fact generally, fine sediments are characterized by very low light penetration in combination with high rate of sedimentation and these conditions should make phototaxis impossible, thus preventing cyanobacteria from migrating to the sediment surface. Moreover, cyanobacteria are generally adapted to low nutrient conditions and show low growth rates, therefore the high amounts of nutrients, characteristic of fine silt and muddy sediments, allow cyanobacteria to be outcompeted by other fast growing microorganisms [9]. In any case, the presence of a high number of cyanobacterial species could be explained by the overall extremely peculiar environmental condition of site C2. In particular, this site is characterized by variable salinity, from oligo- to mesohaline values, stagnant and nutrient-enriched water conditions and large day-night fluctuations in DO concentrations, transient to anoxia [4,11]. It is known that cyanobacteria are well adapted to extreme conditions such as salinity, water temperature, light and water availability and this could confer them an advantage over other phototrophs to thrive in C2 sediment [9].
CLSM examination revealed the presence of biofilm assemblages in the sediment samples with microorganism distributed in patches of different thickness commonly presenting voids in their inner structure. 3-D reconstructions of biofilm communities (Figure 7) revealed that C2 sediments were inhabited by highly complex and multi-stratified MPB communities, with microcolonies of coccal cyanobacteria closely associated with the cyanobacterial filaments as expected from taxon richness data. All microorganisms laid immersed in the polymeric matrix. Fluorochrome labeling also revealed the presence of neutral sugar residues in the fine envelopes of most oscillatorialean and nostocacean cyanobacteria and also spread within the biofilm matrix. Acidic residues (carboxylic and sulphated groups) in the biofilm EPS were also localized in the sheaths of heterocytous and non-heterocytous cyanobacteria as well as in the EPS released in the sediments, as indicated by the reaction with Alcian Blue staining.
Figure 7: Alcian Blue reaction of acidic and sulfated residues in the sheaths, capsules of MPB cyanobacteria and EPS matrix (A-E). CLSM rendering after Acridine Orange EPS labelling of sediments and Surirella sp. (F) and of Phormidium sp. sheath (H), while in (G) Surirella cell wall surface exopolysaccharides were reacted with Con-A Alexafuor 488 Scale bars: 10 µm except (F, G) 40 µm.
Acknowledgements
The authors would like to thank Andrea Satta, Dr Andrea Camedda and Dr Giovanni Fenzi, CNR-IAMC Oristano, for technical and field assistance. The authors acknowledge Dr Elena Romano from the Centre of Advanced Microscopy (CAM), Department of Biology, University of Rome “Tor Vergata,” for her assistance in the use of the facility.
REFERENCES
- Magni P, Como S, Cucco A, et al. A multidisciplinary and ecosystemic approach in the Oristano lagoon-gulf system (Sardinia, Italy) as a tool in management plans. Trans Waters Bull. 2008a;2(2):41-62.
- Magni P, Micheletti S, Casu D, et al. Relationship between chemical characteristics of sediments and macrofaunal communities in the Cabras lagoon (Western Mediterranean, Italy). Hydrobiologia. 2005;550(1):105-19.
- Magni P, De Falco G, Como S, et al. Distribution and ecological relevance of fine sediments in organic enriched lagoons: the case study of the Cabras lagoon (Sardinia, Italy). Mar Poll Bull. 2008b;56(3):549-64.
- Foti A, Fenzi GA, Di Pippo F, et al. Testing the saprobity hypothesis in a Mediterranean lagoon: Effects of confinement and organic enrichment on benthic communities. Mar Environm Res. 2014;99:85-94.
- Blasutto O, Cibic T, De Vittor C, et al. Microphytobenthic Primary Production and Sedimentary Carbohydrates Along Salinity Gradients in the Lagoons of Grado and Marano (Northern Adriatic Sea). Hydrobiologia. 2005;550(1):47-55.
- Brito A, Newton A, Tett P, et al. Seasonal, spatial and vertical variability of microphytobenthos in a shallow lagoon: Ria Formosa (Portugal). Estuar Coast Shelf Sci. 2009;83(1):67-76.
- MacIntyre HL, Geider RJ, Miller DC. Microphytobenthos: the ecological role of the ‘‘secret garden’’ of unvegetated, shallow-water marine habitats. I. distribution, abundance and primary production. Estuaries. 1996;19(2):186-201.
- Montagna PA, Blanchard GF, Dinet A. Effect of production and biomass of intertidal microphytobenthos on meiofaunal grazing rates. J Exp Mar Biol Ecol. 1995;185(2):149-65.
- Stal LJ. Microphytobenthos as a biogeomorphological force in intertidal sediment stabilization. Ecol Engin. 2010;36(2):236-45.
- Stal LJ, van Gemerden H, Krumbein WE. Structure and development of a benthic marine microbial mat. FEMS Microbiol Ecol. 1985;31(2):111-25.
- Bartoli M, Longhi D, Nizzoli D, et al. Short-term effects of hypoxia and bioturbation on solute fluxes, denitrification and buffering capacity in a shallow dystrophic pond. J Exp Mar Biol Ecol. 2009;381:39-47.
- Como S, Magni P, Casu D, et al. Sediment characteristics and macrofaunal distribution along a human-modified inlet in the Gulf of Oristano (Sardinia, Italy). Mar Poll Bull 2007;54(6):733-744.
- Como S, Magni P. Temporal changes of a macrobenthic assemblage in harsh lagoon sediments. Estuar Coast Shelf Sci. 2009;83:638-46.
- De Falco G, Magni P, Teräsvuori LMH, et al. Sediment grain-size and organic carbon distribution in the Cabras lagoon (Sardinia, west Mediterranean). Chem Ecol. 2004;20(1):367-77.
- Magni P, Micheletti S, Casu D, et al. Macrofaunal community structure and distribution in a muddy coastal lagoon. Chem Ecol. 2004;20(1):397-407.
- Magni P, Micheletti S, Casu D, et al. Relationship between chemical characteristics of sediments and macrofaunal communities in the Cabras lagoon (Western Mediterranean, Italy). Hydrobiologia. 2005;550:105-19.
- Lorenzen CJ. Determination of chlorophyll and pheopigments: spectrophotometric equations. Limnol Oceanogr. 1967;12:343-46.
- Parson T, Maita T, Lalli M. A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press. 1984.
- de Brouwer JFC, Stal LJ. Short-term dynamics in microphytobenthos distribution and associated extracellular carbohydrates in surface sediments of an intertidal mudflat. Mar Ecol Prog Ser. 2001;218:33–44.
- Dubois M, Gilles KA, Hamilton JK, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;38:350–56.
- Bellezza S, De Philippis R, Paradossi G, et al. Exopolysaccharides of two cyanobacterial strains from Roman hypogea. Geomicrobiol J. 2006;23:301–10.
- Zippel B, Neu TR. Growth and structure of phototrophic biofilms under controlled light conditions. Water Sci Technol. 2005;52:203–09.
- Montani S, Magni P, Abe N. Seasonal and interannual patterns of intertidal microphytobenthos in combination with laboratory and areal production estimates. Mar Ecol Progr Ser. 2003;249:79-91.
- Di Pippo F, Ellwood NTW, Magni P, et al. Spatiotemporal variability of microphytobenthos in the coastal lagoon of Cabras (Sardinia, Italy). 2011. SEDNET Congress.
- Miles A, Sundbck K. Diel variation in microphytobenthic productivity in areas of different tidal amplitude. Mar Ecol Prog Series. 2000;205:11-22.