Mu and delta opioid receptors inhibit the acrosome reaction by the modulation of calcium channels in human spermatozoa
2 Department of Physiology, Biocruces Bizkaia Health Research Institute, Bizkaia, Spain
Received: 16-May-2023, Manuscript No. PULJMAP-20-2445; Editor assigned: 21-May-2023, Pre QC No. PULJMAP-20-2445(PQ); Accepted Date: Jun 08, 2023; Reviewed: 04-May-2023 QC No. PULJMAP-20-2445; Revised: 02-Jun-2023, Manuscript No. PULJMAP-20-2445(R); Published: 28-Jun-2023
Citation: Urizar-Arenaza I, Munoa-Hoyos I, Gomez-Gimenez B, et al. Mu and delta opioid receptors inhibit the acrosome reaction by the modulation of calcium channels in human spermatozoa. J Reprod Biol Endocrino 2022;6(5):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Opioids exert their effect by binding to mu, delta and kappa opioid receptors in human spermatozoa. As it is known, the three receptors are involved in the regulation of sperm motility. Recently we have described the involvement of the kappa opioid receptor in the inhibition of the sperm motility and acrosome reaction by blocking the calcium channels and producing phosphorylation changes in sperm specific proteins. However, the role of mu and delta in the human sperm capacitation and acrosome reaction, as well the signaling pathways underlying them are largely understood.
Methods: 100 human seminal samples were obtained from Cruces university hospital. Samples were isolated and capacitated by swim up. Sperm cells were treated with morphine (selective Mu opioid receptor agonist) and DPDPE (selective delta-opioid receptor agonist) for 60 minutes. Moreover, both agonists were co-incubated for 60 minutes with different inhibitors of main proteins from the calcium and MAPK signaling pathways (U73122: Phospholipase C inhibitor, mibefradil: A calcium channel activator, NNC55-0395: Catsper specific inhibitor; IBMX: Phosphodiesterases inhibitor, BARK1: GRK-2 inhibitor). Immuno blotting studies were conducted to evaluate capacitation using the anti-phosphotyrosine antibody and the results were analyzed by densitometry. Acrosome reaction was measured by flow cytometer using the FITC-anti-CD46 antibody. The statistical analysis was performed using the SPSS Statistics 22 program.
Results: Morphine and DPDPE did not change the phosphotyrosine levels in human spermatozoa following 1 hour stimulation. However, both agonists inhibited the human sperm acrosome reaction blocking the calcium channels. Morphine and DPDPE were able to blunt the acrosome reaction induced by mibefradil (p<0.05). The inhibition of the catsper calcium channel and the phospholipase C by NNC55-0395 and U73122, respectively had effect on human sperm acrosome reaction, and the agonists had no statistically significant synergic effects.
Conclusion: The Mu and delta opioid receptors participate in the inhibition of acrosome reaction modulating the calcium channels in human spermatozoa. This supports the idea that opioid system could be used as a therapeutic target in the finding of new male contraceptives.
Keywords
Mu opioid receptor; Delta opioid receptor; Sperm capacitation; Acrosome reaction; Calcium channels
Abbreviations
MOR: Mu-Opioid Receptor; DOR: Delta-Opioid Receptor; KOR: KAPPAOpioid Receptor; GPCR: G Protein Coupled Receptors; MAPK: Mitogen Activated Protein Kinases; cAMP: Cyclic Adenosine Monophosphate; GRK: G-Protein Coupled Receptors Kinase
Introduction
The sperm cells that are produced during the spermatogenesis are immature and infertile, and are not able to fertilize an oocyte in vivo or in vitro. To acquire the fertilizing capacity, they must undergo many physiological and biochemical modifications that happen sequentially in the female reproductive tract. These processes such as the acquisition of sperm motility, capacitation, hyper activation and acrosome reaction are considered key functions in the control of sperm fertility. Sperm capacitation leads to biochemical changes which, among other, involve removal of cholesterol, reorganization of plasma membrane and permeability of calcium ions. This process is regulated by bicarbonate, which promotes the stimulation of the soluble adenylate cyclase and the subsequent production of Cyclic Adenosine Monophosphate (CAMP) and protein kinase activation. On the other hand, the acrosome reaction is a calcium dependent process which is essential for spermatozoa to fertilize an oocyte, as allows the membranes fusion by the release of the acrosomal contents. To date, several calcium channels have been described in human spermatozoa. In particular, catsper is a voltage dependent channel induced by progesterone and involved in hyperactive motility, acrosome reaction and chemo taxis.
Over the last years, a wide variety of G Protein Coupled Receptors (GPCR) has been associated with the regulation of sperm motility, capacitation and acrosome reaction. Taking into account that the GPCRs are one of the best therapeutic targets in drug industry they might be potential candidates for sperm fertility management. Mu, Delta and Kappa Opioid Receptors (MOR, DOR and KOR, respectively) belong to the GPCR superfamily and are expressed in human spermatozoa. Recently we have described the involvement of the kappa opioid receptor in the inhibition of the sperm motility and acrosome reaction by blocking the calcium channels and producing phosphorylation changes in sperm specific proteins. Regarding to MOR and DOR, although there are involved in the regulation of sperm motility nowadays their function in sperm capacitation and acrosome reaction remain unknown. The present work describes the involvement of MOR and DOR in the inhibition of the acrosome reaction through the modulation of calcium channels as we have recently proved for KOR [1].
Materials and Methods
Spermatozoa isolation and treatments
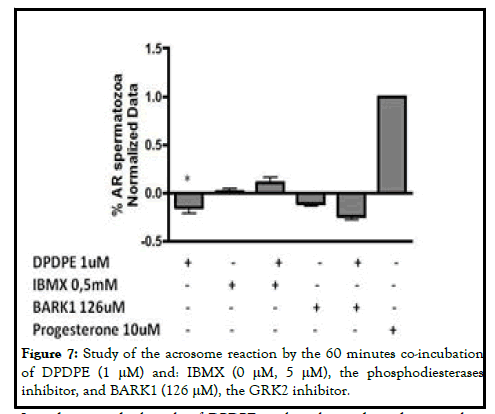
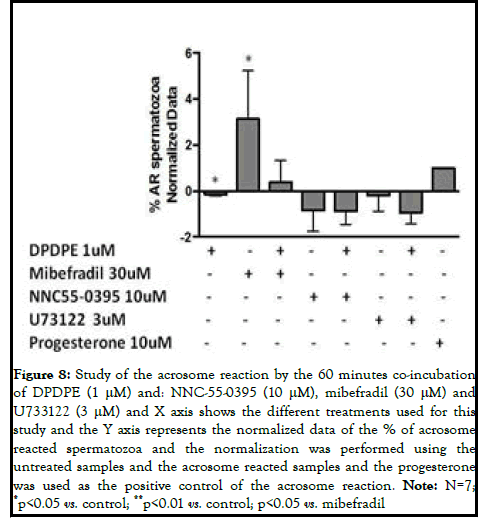
Freshly ejaculated semen was collected from patients undergoing routine semen analyses at the Cruces university hospital. The donors had normal sperm parameters according to World Health Organization (WHO) standards. Semen samples were obtained by masturbation after 3-4 days of sexual abstinence and immediately processed upon liquefaction (at 37°C for 30 minutes). Spermatozoa were capacitated by the swim up procedure and suspended in G-IVF (Vitrolife, Goteborg, Sweden) supplemented with 1% bovine serum albumin for 3 h at 37°C under 5% CO2. The spermatozoa were treated with 1 μM morphine and 1 μM DPDPE (sigma), the specific agonists of MOR and DOR respectively, for 60 minutes independently for functional analyses. The samples were co-incubated with morphine or DPDPE (Sigma-Aldrich) and the different activators and inhibitors of the different signaling pathways, such as: Calcium signaling pathway: U73122 (3 μM) (Sigma-Aldrich), a phospholipase C inhibitor; mibefradil (30 μM) (Tocris biosciences), a calcium channel activator and NNC55-0395 (10 μM) (Sigma-Aldrich), a catsper specific calcium channel inhibitor; MAPK signaling pathway: BARK1 (126 μM) (Calbiochem), the GRK-2 inhibitor and IBMX (0.5 mM) (Sigma-Aldrich), the phosphodiesterases inhibitor. The progesterone (10 μM) was used as an internal control for the acrosome reaction [2].
Flow cytometry
Acrosome reaction was evaluated using the anti-CD46 antibody by flow cytometry (FACScalibur flow cytometer; Becton, Dickinson, San Jose, CA, USA). For the staining of the cells the Fluorescein Iso-Tio-Cyanate (FITC) antihuman CD46 antibody (Biolegend, California, USA) (5 μl) was used for 60 minutes at room temperature as acrosome reaction molecular marker. As indicator of cell viability we used 0.1 μg/mL hoechst 33258 for 2 minutes. Fluorescence data from at least 10.000 alive sperm cells were analyzed and the green fluorescence belonging to spermatozoa was measured. Histograms were analyzed using the summit v4.3 software.
Western blotting
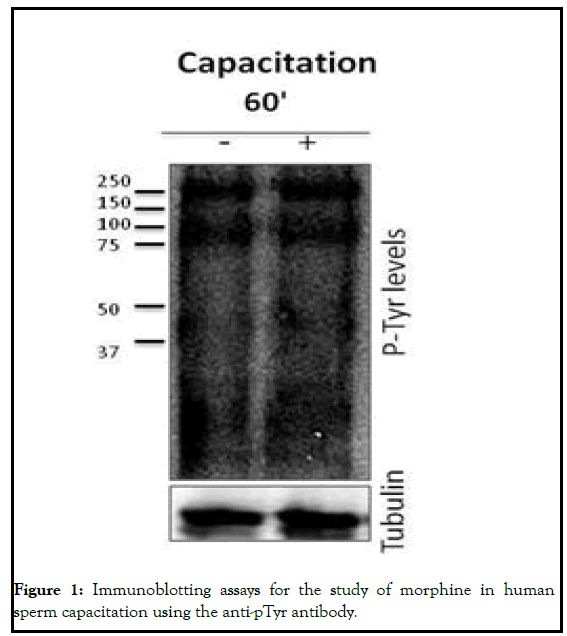
For western blotting analyses, whole cell extracts (500.000 cells) were diluted in 1x laemmly sample buffer containing dithiothreitol and boiled for 5 minutes. After loading samples onto gels and transferring proteins to Polyvinylidene Fluoride membranes (PVDF) (Amersham hybond, sigma), blots were blocked with blocking solution for 1 hour. Then membranes were incubated with a 1:1000 dilution of the monoclonal mouse 4G10 antiphosphotyrosine antibody (05-231, millipore) as a molecular marker of the human sperm capacitation. As a secondary antibody peroxidase conjugated donkey anti-mouse IgG antibody was used (1:2000, 1 hour) (Donkey antimouse IgG HRP, sc-2314; Santa Cruz biotechnology). Blots were revealed for peroxidase activity by enhanced chemoluminiscence (ChemiDoc XRS detector, Bio-Rad). Results were analyzed by semi-quantitative western blots densitometry analysis using image J (Image processing and analysis in java) software.
Statistical analysis
Acrosome reacted data were normalized as (Treatment control)/ (Progesterone-DMSO) × 100 and evaluated using students T-test and oneway ANOVA with post-hoc Bonferroni test. These procedures were undertaken using the IBM SPSS statistics program (version 22). Differences were considered significant at *p<0.05 and highly significant at **p<0.01. Data are expressed as mean ± SEM using the graph pad PRISM (version 6.0) (Graphpad company, California, USA) program.
Results
Role of MOR in human sperm physiology
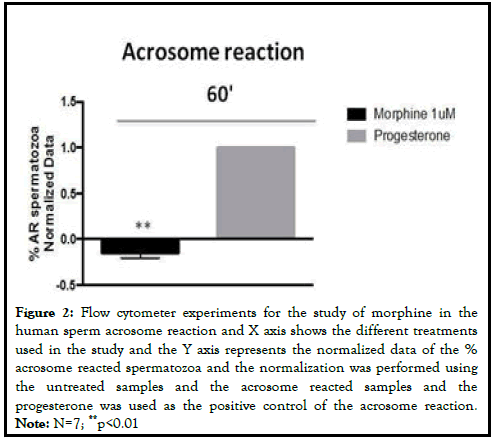
In order to analyze the role of MOR in human sperm fertility we first analyzed its involvement in human sperm capacitation. For that purpose, we treated human spermatozoa with 1 μM of Morphine (MOR agonist) for 60 minutes. Cell viability studies using Hoechst 33258 did not show toxicity after morphine addition. Taking into account that the phosphorylation of Tyrosine residues (pTyr) is one of the most important events that occur during the sperm capacitation we analyzed the effect of morphine on the total pTyr levels. The MOR agonists did not have an effect on the levels of tyrosine phosphorylated proteins at 60 minute. Next, to study the role of MOR human sperm acrosome reaction, we treated the samples with morphine and incubated them with the anti-CD46 antibody linked to Fluorescein Iso-Thio-Cyanate (FITC). CD46 is present in the inner acrosomal membrane and allows us to measure complete acrosome reacted spermatozoa. Morphine caused a decrease in the percentage of acrosome reacted sperm cells after at 60 minutes (p<0.01). The progesterone was used as the positive control of the acrosome reaction (Figure 1) [3].
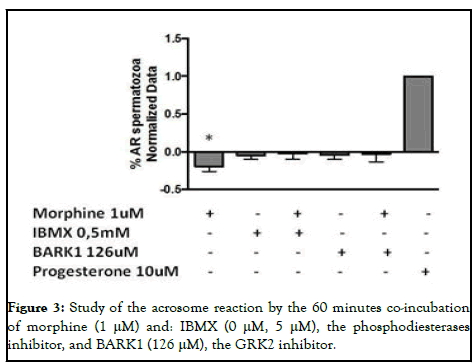
Once we described that morphine had an inhibitory effect in the human sperm acrosome reaction, we aimed to study the signaling pathways underlying MOR activation. For that, we treated the human sperm samples with the specific inhibitors of the phosphodiesterases and GRK-2 (IBMX and BARK1, respectively), involved in the internalization/desentization of the receptor and indirectly in MAPK-dependent signaling processes through β-arrestins. We did not observe any statistically significant changes by the coincubation of morphine with the specific inhibitors at 60 minutes (Figure 2).
Figure 2: Flow cytometer experiments for the study of morphine in the human sperm acrosome reaction and X axis shows the different treatments used in the study and the Y axis represents the normalized data of the % acrosome reacted spermatozoa and the normalization was performed using the untreated samples and the acrosome reacted samples and the progesterone was used as the positive control of the acrosome reaction. Note: N=7; **p<0.01
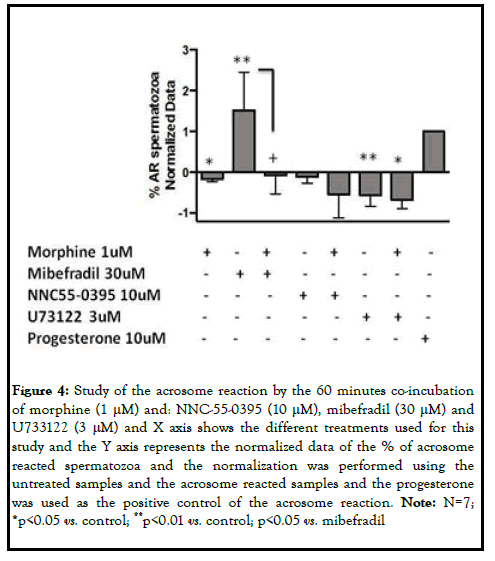
Taking into account that the acrosome reaction is mainly regulated by Ca2+ we decided to study more in deep the calcium signaling pathway underlying MOR. Morphine treated sperm cells were co-incubated with activators and inhibitors of key proteins in the calcium signaling. Mibefradil at high doses (30 μM) was used as a calcium channel activator, NNC55-0395 (10 μM) as the catsper sperm specific calcium channel selective inhibitor and U73122 (3 μM) as the phospholipase C inhibitor. The stimulation of spermatozoa with mibefradil gave rise to the stimulation of the acrosome exocytosis (p<0.01). The co-incubation of mibefradil and morphine yielded to the blunt of the acrosome reaction induced by the activator (p<0.05). On the other hand, although NNC55-0395 had an inhibitory effect on the acrosome reaction, which was accentuated after the co-incubation with morphine, these changes were not statistically significant. In the case of U73122, the phospholipase C inhibitor, the number of acrosome reacted spermatozoa decreased compared to the control samples (p<0.01). This effect was enhanced following the co-incubation with U73122 and MOR specific agonist (p<0.05). Considering these results, morphine inhibited the acrosome reaction at 60 minutes, blocking the calcium channels (Figures 3 and 4).
Figure 4: Study of the acrosome reaction by the 60 minutes co-incubation of morphine (1 μM) and: NNC-55-0395 (10 μM), mibefradil (30 μM) and U733122 (3 μM) and X axis shows the different treatments used for this study and the Y axis represents the normalized data of the % of acrosome reacted spermatozoa and the normalization was performed using the untreated samples and the acrosome reacted samples and the progesterone was used as the positive control of the acrosome reaction. Note: N=7; *p<0.05 vs. control; **p<0.01 vs. control; p<0.05 vs. mibefradil
Role of DOR in human sperm physiology
Similarly to MOR, we studied the involvement of DOR in human sperm capacitation. In this case, we treated human spermatozoa with 1 μM of DPDPE (DOR agonist) for 60 minutes. Cell viability studies using Hoechst 33258 did not show toxicity after DPDPE addition (Figure 5) [4].
Then, we investigated the effect of DPDPE on the total pTyr levels. DPDPE had no effect on pTyr levels. On the other hand, we studied the role of DOR in human sperm acrosome reaction. DPDPE had an inhibitory effect on acrosome reaction (p<0.01). In order to analyze the signaling pathways underling this effect, we co-incubated DPDPE with IBMX and BARK1. As happened with morphine, we did not observe statistically significant changes following DPDPE and specific inhibitors co-incubations (Figure 6).
Figure 6: Flow cytometer experiments for the study of DPDPE in the human sperm acrosome reaction and X axis shows the different treatments used in the study and the Y axis represents the normalized data of the % acrosome reacted spermatozoa and the normalization was performed using the untreated samples and the acrosome reacted samples and the progesterone was used as the positive control of the acrosome reaction. Note: N=7; **p<0.01.
According to these results, the inhibition of the acrosome reaction by DPDPE is not dependent of phosphodiesterases and GRK-2, suggesting that the activation of the receptors do not involve the MAPK-dependent signaling pathway following 60 minutes stimulation (Figure 7).
In order to study the role of DPDPE in the calcium dependent signaling pathway, we measured the acrosome reacted sperm cells following the coincubation with the specific agonist and mibefradil, NNC55-0395 and U73122. In the case of Mibefradil, the DPDPE also blunted the effect of the calcium channel activator in the acrosome reaction, although it was not statistically significant. In regard to U73122, its inhibition on the acrosome reaction was accentuated but the effect was not statistically significant. Considering these results, DPDPE may inhibit the acrosome reaction at 60 minutes, by blocking the calcium channels (Figure 8) [5].
Figure 8: Study of the acrosome reaction by the 60 minutes co-incubation of DPDPE (1 μM) and: NNC-55-0395 (10 μM), mibefradil (30 μM) and U733122 (3 μM) and X axis shows the different treatments used for this study and the Y axis represents the normalized data of the % of acrosome reacted spermatozoa and the normalization was performed using the untreated samples and the acrosome reacted samples and the progesterone was used as the positive control of the acrosome reaction. Note: N=7; *p<0.05 vs. control; **p<0.01 vs. control; p<0.05 vs. mibefradil
Discussion
In 2006, we have reported for first time the presence of MOR, DOR and KOR receptors in human spermatozoa describing the involvement of MOR and DOR in sperm motility. However, to date the function of MOR and DOR in human sperm capacitation and acrosome reaction, as well as the molecular mechanisms underlying them has not been yet attributed [6].
To elucidate the effect of the treatment with the MOR and DOR specific ligands on the physiology of human spermatozoa, we analyzed the role of these receptors in sperm capacitation and acrosome reaction. For that we stimulated independently the human spermatozoa with morphine and DPDPE, the MOR and DOR respective agonists, for 60 minutes. As we reported to happen with KOR both morphine and DPDPE do not have any effect on human sperm capacitation at 60 minutes stimulation, as the ligands do not induce phosphotyrosine dependent changes. However, morphine and DPDPE have inhibitory effects in human sperm acrosome reaction. This result is consistent with the fact that U50488H, KOR specific agonist, also inhibits the acrosome reaction at 60 minutes suggesting that the three opioid receptors may have the same effect on sperm physiology at this time point [7-10].
Classically, the internalization of GPCRs begins with the phosphorylation of the receptor via the G-protein coupled Receptors Kinases (GRKs). Once the receptor is internalized and desensitized, the GRKs have the capacity to induce indirectly different signaling pathways like the MAPK through β- arrestins [11]. In order to study if the inhibition of the acrosome reaction induced by morphine and DPDPE involved the activation of MAPKdependent signaling, we used the GRK-2 (the most studied GRK) and phosphodiesterases inhibitors. Functional assays indicate that the inhibition of the acrosome reaction by morphine and DPDPE at 60 minutes is not dependent of GRK-2 or phosphodiesterases. Therefore, our results prove that the inhibition of acrosome reaction underlying MOR and DOR does not involve the activation of MAPK signaling pathway, as reported for KOR [12].
Taking into account that the acrosome reaction is mainly regulated by calcium and that KOR is involved in the inhibition of sperm motility and acrosome reaction by the blocking of calcium channels and phosphorylation changes in sperm specific proteins we decided to study if MOR and had the same molecular mechanisms [13,14]. Morphine and DPDPE were able to decrease the effect produced by mibefradil in the acrosome reaction, suggesting that MOR and DOR may inhibit of the acrosome reaction by the blocking of calcium channels in human spermatozoa. This result is consistent with the fact that classically, the agonist stimulation of the opioid receptors has been tightly associated with modulation of the calcium and potassium channels as well as with the inhibition of the cAMP production [15,16].
Conclusion
The present study provides evidence that MOR and DOR are involved in the regulation of sperm physiology through the modulation of calcium channels, as happens with KOR. Although further studies are needed, this finding suggests that a better comprehension of the opioid system in human spermatozoa could contribute in the development of useful biochemical tools for the diagnosis and treatment of male infertility. Moreover, it could also represent an innovative opportunity for reproductive management, for either enhancing the probability of fertilization or reducing it through the development of novel target contraceptives.
Ethics Approval and Consent to Participate
To perform this study we previously obtained the ethical approval from the ethics committee of the university of the Basque country (CEISHUPV/ EHU (M10/2016/254)). Written informed consent for the analysis of data was obtained from patients undergoing routine semen analyses at the center.
Consent for Publication
Not applicable.
Availability of Data and Materials
The primary data for this study is available from the authors on direct request.
Competing Interests
The authors declare that they have no competing interests.
Funding
Basque government and university of the Basque country (UPV/EHU). IUA is supported by a fellowship from the university of the Basque country (UPV/EHU). IM-H is supported by a fellowship from the Basque government.
Authors Contributions
NS designed the study; IU-A, IM-H and MG performed the experiments and analyzed the data, IU-A and NS wrote the paper; and RM revised the paper. All authors read and approved the final manuscript.
Acknowledgement
Not applicable.
References
- Visconti PE, Westbrook VA, Chertihin O, et al. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53(1-2):133-50.
- Hess KC, Jones BH, Marquez B, et al. The soluble adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9(2):249-59.
- Florman HM, Jungnickel MK, Sutton KA. Regulating the acrosome reaction. Int J Dev Biol. 2004;52(5-6):503-10.
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387-91.
- Strunker T, Goodwin N, Brenker C, et al. The catsper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471(7338):382-6.
- Kohn FM, Dammshauser I, Neukamm C, et al. Ultrastructural localization of angiotensin-converting enzyme in ejaculated human spermatozoa. Hum Reprod. 1998;13(3):604-10.
- Jimenez-Trejo F, Tapia-Rodriguez M, Cerbon M, et al. Evidence of 5-HT components in human sperm: Implications for protein tyrosine phosphorylation and the physiology of motility. Reproduction. 2012;144(6):677.
- Rossato M, Ion Popa F, Ferigo M, et al. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocr. 2005;90(2):984-91.
- Nambi P, Aiyar N. G protein-coupled receptors in drug discovery. Assay Drug Dev Technol. 2003;1(2):305-10.
- Agirregoitia E, Valdivia A, Carracedo A, et al. Expression and localization of δ-, κ-, and μ-opioid receptors in human spermatozoa and implications for sperm motility. J Clin Endocr Met. 2006;91(12):4969-75.
- Urizar-Arenaza I, Osinalde N, Akimov V, et al. Phosphoproteomic and functional analyses reveal sperm-specific protein changes downstream of kappa opioid receptor in human spermatozoa. Mol Cellular Proteom. 2019;18:118-31.
- Leclerc P, De Lamirande E, Gagnon C. Regulation of protein tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22(4):643-56.
- Naz RK, Rajesh PB. Role of tyrosine phosphorylation in sperm capacitation/acrosome reaction. Reprod Biol Endocrinol. 2004;2(1):1-2.
- Roldan ER, Shi QX. Sperm phospholipases and acrosomal exocytosis. Front Biosci. 2007;12(1):89-104.
- Penela P, Murga C, Ribas C, et al. The complex G protein‐coupled Receptor Kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160(4):821-32.
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor dependent signaling and behavior. J American Soc Anesthesiologists. 2011;115(6):1363-81.