Multiple rare anatomical variants: Lessons from one neck
Received: 06-Dec-2019 Accepted Date: May 18, 2020; Published: 25-May-2020, DOI: 10.37532/1308-4038.20.13.1-3
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The thyroid gland and branches from the subclavian and external carotid arteries are individually subject to a fair degree of anatomical variation. Some of these variants are relatively common, occurring in 10-20% of individuals while others are extremely rare. Recognizing the possible occurrence of such variants is important in surgical procedures as a significant number of malpractice cases appear to result from failure to recognize common anatomical variants. Herein, we describe the presence of numerous anatomical variants involving the thyroid gland and cervical vasculature in the neck of a 76-year-old female cadaver. We found bilateral variants of the vertebral artery, multiple variants of the thyroid vasculature and a pyramidal lobe replete with skeletal muscle. The co-occurrence of these variants results in an extremely rare anatomical pattern in the neck.
Keywords
Artery; Thyroid; Vertebral; Anatomical variation
Introduction
While not explicitly stated, it is understood that the majority of gross anatomy textbooks and atlases demonstrate the most common or mode of anatomical patterns. There is however extensive literature documenting thousands of common anatomical variations [1]. While both frequently occurring and rare anatomical variants are of general interest to anatomists, many variants have clinical significance [2]. However, even the rarest anatomical variant can be clinically significant if not anticipated during a medical procedure. Furthermore, damage to variant blood vessels or nerves may put structures they support (muscles, organs) at risk of infarct or denervation.
The anterior triangle of the neck and the region deep to the sternocleidomastoid muscle are anatomically very complex and include a rich collection of neurovasculature, musculoskeletal elements and viscera [3]. The neck is also a common site of surgical intervention. The anterior neck is approached in carotid endarterectomy, anterior cervical discectomies and fusions, thyroidectomy, tracheostomy and cricothyrotomy. The area under the sternocleidomastoid is exposed in radical neck dissections for removal of diseased lymph nodes. Accordingly, a detailed understanding of the anatomy of these regions is essential for the surgeon.
Anatomical variations involving the thyroid gland are relatively common. The most frequent variant is the presence of a pyramidal lobe, which occurs in approximately 20% of individuals [4,5]. The arterial support of the thyroid gland is less variable. The most common arterial variant is the thyroid ima artery which is found in only 4-10% of subjects [1]. When present, the thyroid ima artery (as the name indicates) arises from below and proximal to the inferior thyroid artery, most commonly from the right brachiocephalic artery or arch of the aorta. The thyroid ima ascends along the anterior aspect of the trachea to supply the isthmus of the gland and anastomose with other thyroid vessels. When present, the thyroid ima is at risk during a tracheostomy [6]. Less frequently (<5% of subjects) the inferior thyroid artery is absent or there is variation in the origin or branching of thyroid arteries [1,7,8]. While there are numerous reports of these variations occurring individually, there appear to be no reports on their co-occurrence.
In this report, we describe the dissection and microscopic examination of a subject with numerous anatomical variants in their neck. Specifically, we identified variation in the structure, arterial support and venous drainage of the thyroid gland, ectopic parathyroid tissue and aberrant origins and course of the vertebral arteries. We provide estimates of the co-occurrence of these variations and clinical significance.
Case Report
During routine dissection of the anterior neck of a 76-year-old female (cause of death listed as cardiac arrest, respiratory failure and chronic obstructive pulmonary disease) at the Lake Erie College of Osteopathic Medicine, numerous anatomical variations were identified and studied.
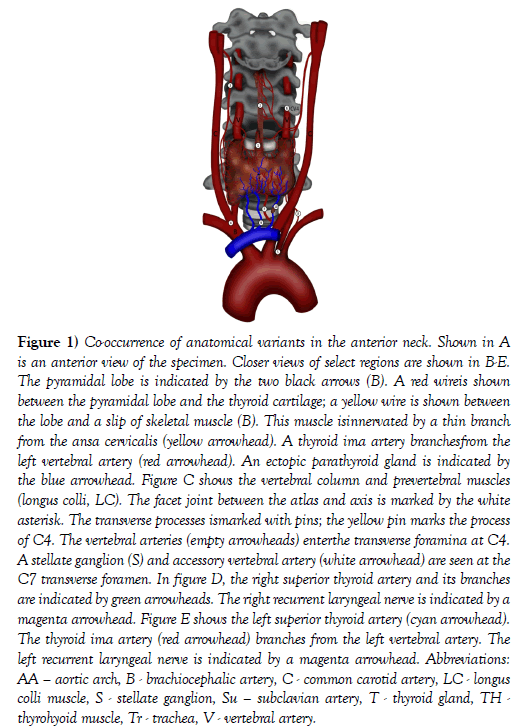
The thyroid gland appeared of normal size and location. A pyramidal lobe was identified arising from the thyroid isthmus. The pyramidal lobe was situated superficial to the cricoid and thyroid cartilages and coursed deep to the hyoid bone. The pyramidal lobe was supplied exclusively by branches from the left superior thyroid artery. The lobe perforated the deep surface of the hyoid bone and had a slip of skeletal muscle along its anterior surface (levatorglandulaethyroidae). A thin branch from the right ansa cervicalis was traced into the muscle. This subject had two different slips of a levatorglandulaethyroidae: hyopyramidalis and hyoglandularis muscles (Figure 1) [1].
Figure 1: Co-occurrence of anatomical variants in the anterior neck. Shown in A is an anterior view of the specimen. Closer views of select regions are shown in B-E. The pyramidal lobe is indicated by the two black arrows (B). A red wireis shown between the pyramidal lobe and the thyroid cartilage; a yellow wire is shown between the lobe and a slip of skeletal muscle (B). This muscle isinnervated by a thin branch from the ansa cervicalis (yellow arrowhead). A thyroid ima artery branchesfrom the left vertebral artery (red arrowhead). An ectopic parathyroid gland is indicated by the blue arrowhead. Figure C shows the vertebral column and prevertebral muscles (longus colli, LC). The facet joint between the atlas and axis is marked by the white asterisk. The transverse processes ismarked with pins; the yellow pin marks the process of C4. The vertebral arteries (empty arrowheads) enterthe transverse foramina at C4. A stellate ganglion (S) and accessory vertebral artery (white arrowhead) are seen at the C7 transverse foramen. In figure D, the right superior thyroid artery and its branches are indicated by green arrowheads. The right recurrent laryngeal nerve is indicated by a magenta arrowhead. Figure E shows the left superior thyroid artery (cyan arrowhead). The thyroid ima artery (red arrowhead) branches from the left vertebral artery. The left recurrent laryngeal nerve is indicated by a magenta arrowhead. Abbreviations: AA – aortic arch, B - brachiocephalic artery, C - common carotid artery, LC - longus colli muscle, S - stellate ganglion, Su – subclavian artery, T - thyroid gland, TH -thyrohyoid muscle, Tr - trachea, V - vertebral artery.
On the right side, the superior thyroid artery arose from the external carotid artery and split into two nearly equal diameter branches that together supplied the entire right side of the thyroid gland. The left superior thyroid artery was unremarkable. There was no inferior thyroid artery on either the right or left side and accordingly no artery traveling with either recurrent laryngeal nerve (Figure 1).
There were multiple variations of the vertebral arteries. On the right side of the neck, the vertebral artery arose from a trifurcation of the brachiocephalic trunk. On the left side, the vertebral artery arose from the aortic arch. Both vertebral arteries demonstrated mild tortuosities and ascended posterior to the lateral lobes of the thyroid gland along the anterior aspect of the cervical vertebral column entering the transverse foramina at the level of CV4.
About 6 cm from the origin of the left vertebral artery, a thyroid ima artery arose and was followed directly to the thyroid isthmus. On the left side, the vertebral vein was traced into the transverse foramen at CV7 along with a prominent vertebral nerve (plexus) and a small vertebral artery that arose from the costocervical trunk. Additionally, there were three inferior thyroid veins draining the gland that all joined the left brachiocephalic trunk (Figure 1).
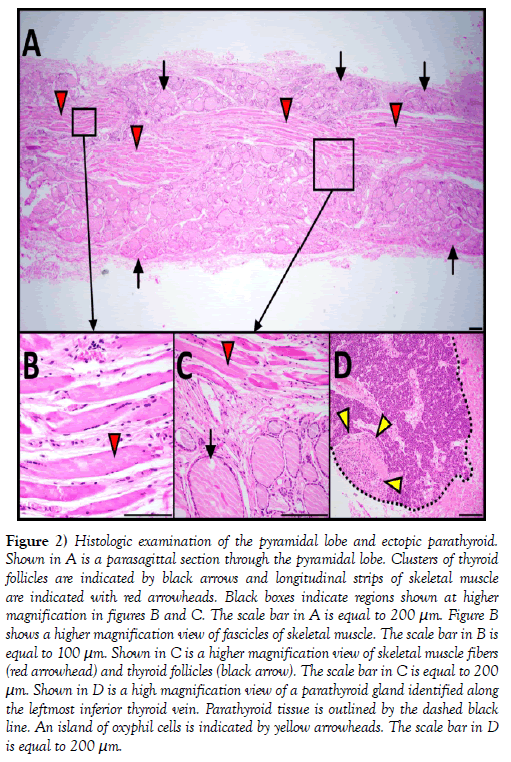
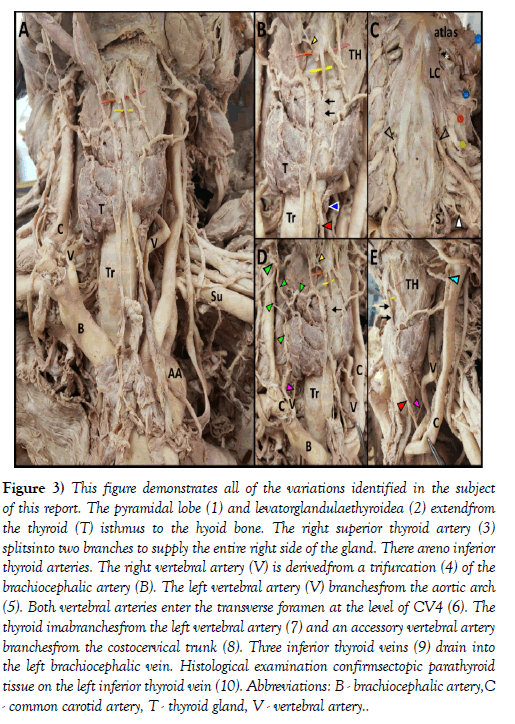
Histological examination of H&E stained, parasagittal sections of the pyramidal lobe revealed myriad thyroid follicles embedded in dense irregular connective tissue and multiple longitudinal bands of skeletal muscle (Figure 2). The anterior neck and mediastinum included many small round/ oval bodies. Microscopic examination revealed these to be either ectopic parathyroid tissue (Figures 1 and 2) or lymphatic tissue (on the aortic arch). A summary figure of the anatomical variations identified in this case report is provided in (Figure 3).
Figure 2: Histologic examination of the pyramidal lobe and ectopic parathyroid. Shown in A is a parasagittal section through the pyramidal lobe. Clusters of thyroid follicles are indicated by black arrows and longitudinal strips of skeletal muscle are indicated with red arrowheads. Black boxes indicate regions shown at higher magnification in figures B and C. The scale bar in A is equal to 200 μm. Figure B shows a higher magnification view of fascicles of skeletal muscle. The scale bar in B is equal to 100 μm. Shown in C is a higher magnification view of skeletal muscle fibers (red arrowhead) and thyroid follicles (black arrow). The scale bar in C is equal to 200 μm. Shown in D is a high magnification view of a parathyroid gland identified along the leftmost inferior thyroid vein. Parathyroid tissue is outlined by the dashed black line. An island of oxyphil cells is indicated by yellow arrowheads. The scale bar in D is equal to 200 μm.
Figure 3: This figure demonstrates all of the variations identified in the subject of this report. The pyramidal lobe (1) and levatorglandulaethyroidea (2) extendfrom the thyroid (T) isthmus to the hyoid bone. The right superior thyroid artery (3) splitsinto two branches to supply the entire right side of the gland. There areno inferior thyroid arteries. The right vertebral artery (V) is derivedfrom a trifurcation (4) of the brachiocephalic artery (B). The left vertebral artery (V) branchesfrom the aortic arch (5). Both vertebral arteries enter the transverse foramen at the level of CV4 (6). The thyroid imabranchesfrom the left vertebral artery (7) and an accessory vertebral artery branchesfrom the costocervical trunk (8). Three inferior thyroid veins (9) drain into the left brachiocephalic vein. Histological examination confirmsectopic parathyroid tissue on the left inferior thyroid vein (10). Abbreviations: B - brachiocephalic artery,C - common carotid artery, T - thyroid gland, V - vertebral artery.
Discussion
In our dissection, we encountered a number of anatomical variations. Some of these appear many times in the literature while we were unable to find any reports for others. Based on the known incidence of most of the vascular variations found in this subject, the simultaneous occurrence of these variations appearsto comprise a unique anatomical pattern. Specifically, the thyroid ima artery is present in only 4-10% of subjects [1]. The literature indicates that the left vertebral artery arises from the aortic arch in only ~3% of people [9] and the right vertebral arises from a trifucated brachiocephalic artery in only ~1% of subjects [10]. Of particular note, our survey of the literature failed to find any reports of the thyroid ima artery arising from a left vertebral artery that branched from the aortic arch. While the vertebral arteries most commonly enter the transverse foramen at CV6 [3], they enter this foramen as high as CV4 in only 1% of subjects [11]. The subject of this case study had bilateral vertebral arteries entering the transverse foramen at CV4. It is important to note that the vertebral arteries and their variants are at particular risk of iatrogenic injury during anterior cervical discectomies and fusions [10]. Further, we propose that the large caliber and tortuosity of the vertebral arteries in this subject would put these vessels in further danger during procedures involving the cervical spine. The subject in this case report did not have an inferior thyroid artery on either side of their neck. The literature indicates that the inferior thyroid artery is unilaterally absent in only about 3% of subjects [8]. We rationalize that bilateral absence of the inferior thyroid artery would occur in less than 1% of subjects.
A midline pyramidal lobe is found in only about 6% of individuals [12,13]. A detailed investigation of pyramidal lobes indicate that about 25% of pyramidal lobes include some degree of skeletal muscle (i.e. levatorglandulaethyroidae [14,15]). Together, these figures suggest that only about 1.4% of people have a midline pyramidal lobe with some version of levatorglandulaethyroidae. While slips of muscle have been reported along pyramidal lobes and thyroglossal ducts, this specimen included numerous fascicles of skeletal muscle throughout the thickness of the pyramidal lobe. We interpret the observation of a branch from the ansa cervicalis penetrating the pyramidal lobe to be consistent with the muscle deriving from the hypomere of cervical somites. However, other authors found this muscle to be innervated by branches from the external laryngeal nerve, which would be consistent with the muscle deriving from the fourth pharyngeal arch [16]. It seems unlikely that the same muscle, based on location and attachments, would arise from variable sources in different individuals. Rather, we propose that different slips of the levatorglandulaethyroidae may have different origins and that the hyopyramidalis is derived from cervical somites. Hegazy [17] stated that both levatorglandulaethyroidae and the pyramidal lobe of the thyroid are anatomical variations caused by incomplete degeneration of the embryonic thyroglossal duct.
Conclusion
Our search of the literature was unable to uncover a percentage occurrence for an accessory vertebral artery derived from the costocervical trunk or thyroid ima arising from the left vertebral artery. Nonetheless, based on the literature and figures we have provided above, we conservatively estimate the co-occurrence of all of the variations found in this subject to be approximately 1 in 40 trillion. While extremely rare, it is important to document such unique cases in the literature. Because of the numerous vascular variants in this subject, we believe these issues arise from abnormal vascular signaling and growth during the embryonic period. We believe that this subject illustrates two important lessons in anatomical variants. First, even when there are significant developmental variations in vascular patterns, the body still manages to develop adequate vascular support of tissue and organs. Second, when one anatomical variant is present, the individual is more likely to have additional anatomical variations.
Based on our observations, we conclude that the presence of a single anatomical variant should raise suspicion for additional variants and further, that multiple variations may result in unique structural patterns leading to increased susceptibility and iatrogenic injuries.
Acknowledgements
The authors would like to thank the donor and her family for their generous contribution to medical education.
REFERENCES
- Bergman RA, Tubbs RS, Shoja MM, et al. Bergman’s comprehensive encyclopedia of human anatomic variation. Hoboken, John Wiley & Sons. 2016.
- Sanudo J, Vázquez R, Puerta J. Meaning and clinical interest of the anatomical variations in the 21st century. Eur J Anat. 2003;7:1-3.
- Drake RL, Vogl AW, Mitchell AWM. Gray’s Anatomy for Students. 3rd Ed., Philadelphia, Elsevier. 2015; 89-90.
- Cengiz A, Sakı H, Yürekli Y. Scintigraphic evaluation of thyroid pyramidal lobe. Mol Imaging Radionucl Ther. 2013;22:32-5.
- Sinos G, Sakorafas GH. Pyramidal Lobe of the Thyroid: Anatomical Considerations of Importance in Thyroid Cancer Surgery. Oncol Res Treat. 2015;38:309-10.
- Yilmaz E, Celik HH, Durgun B, et al. Arteria thyroideaima arising from the brachiocephalic trunk with bilateral absence of inferior thyroid arteries: a case report. Surg Radiol Anat. 1993;15:197-9.
- Sirasanagandla SR, Nayak SB, Pamidi N. Paramedian Ectopic Thyroid Gland and Unusual Origin of Superior Thyroid Artery-A Case Report and Review of Literature. Erciyes Med J. 2017;39:37-9.
- Lippert H, Pabst R. Arterial Variations in Man. Classification and Frequency. Munich: J. F. Bergmann‐Verlag. 1985.
- Tardieu G, Edwards B, Alonso F, et al. Aortic arch origin of the left vertebral artery. Clini Anat. 2017;30:810-6.
- Lazaridis N, Piagkou M, Loukas M, et al. A systematic classification of the vertebral artery variable origin: clinical and surgical implications. Surg Radiol Anat. 2018;40:779-97.
- Bruneau M, Cornelius JF, Marneffe V, et al. Anatomical variations of the V2 segment of the vertebral artery. Operative Neurosurgery. 2006;59:20-24.
- Cengiz A, Saki H, Yurekli Y. Scintigraphic evaluation of thyroid pyramidal lobe. Mol Imaging Radionucl Ther. 2013;22:32-35.
- Sinos G, Sakorafas GH. Pyramidal Lobe of the Thyroid: Anatomical Considerations of Importance in Thyroid Cancer Surgery. Oncol Res Treat. 2015;38:309-10.
- Loukas M, Merbs W, Tubbs RS, et al. Levatorglandulaethyroideae muscle with three slips. ANAT SCI INT. 2008;83:273.
- Prakash Rajini T, Ramachandran A, Basavraj-Savalji G, et al. Variations in the anatomy of the thyroid gland: clinical implications of a cadaver study. ANAT SCI INT. 2012;87:45.
- Chaudhary P, Singh Z, Khullar M, et al. Levatorglandulaethyroideae, a fibromusculoglandular band with absence of pyramidal lobe and its innervation: a case report. J Clin Diagn Res. 2013;7:1421-4.
- Hegazy A. Clinical embryology for medical students and postgraduate doctors. LAP, Berlin. 2014.