Neuroanatomical Variation in a Patient with Unilateral Motor Deficit
Received: 04-Jun-2023, Manuscript No. ijav-23-6529; Editor assigned: 05-Jun-2023, Pre QC No. ijav-23-6529 (PQ); Accepted Date: Jun 23, 2023; Reviewed: 19-Jun-2023 QC No. ijav-23-6529; Revised: 23-Jun-2023, Manuscript No. ijav-23-6529 (R); Published: 30-Jun-2023, DOI: 10.37532/1308-4038.16(6).271
Citation: Karl M. Neuroanatomical Variation in a Patient with Unilateral Motor Deficit. Int J Anat Var. 2023;16(6):317-318.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Neuroanatomy plays a crucial role in understanding the organization and function of the nervous system. This case report presents a unique instance of neuroanatomical variation observed in a patient with a unilateral motor deficit. The study aims to describe the atypical neural pathway identified through imaging techniques and highlight its potential implications for motor function. This case underscores the importance of individual variations in Neuroanatomy and their impact on clinical presentations, emphasizing the need for personalized approaches in diagnosis and treatment.
Keywords
Neuroanatomy; Case report; Motor deficit; Neural pathway; Imaging techniques; Individual variation
INTRODUCTION
Neuroanatomy provides valuable insights into the structural organization and connectivity of the nervous system. Variations in neuroanatomical structures have been widely documented and have been associated with diverse clinical presentations. This case report describes a patient with a unilateral motor deficit resulting from an atypical neural pathway [1]. The study aims to elucidate the neuroanatomical basis of the patient’s condition through advanced imaging techniques and explore the potential implications for motor function. Understanding such variations in Neuroanatomy can significantly contribute to the development of personalized diagnostic and therapeutic approaches, enhancing patient care [2-3].
CASE REPORT
A 42-year-old male presented with a three-month history of progressive weakness and impaired motor control in his left upper limb. Physical examination revealed muscle atrophy and weakness predominantly affecting the left forearm and hand. Neurological evaluation suggested a lesion in the corticospinal tract, commonly associated with contralateral motor deficits. However, magnetic resonance imaging (MRI) of the brain revealed an unexpected finding.
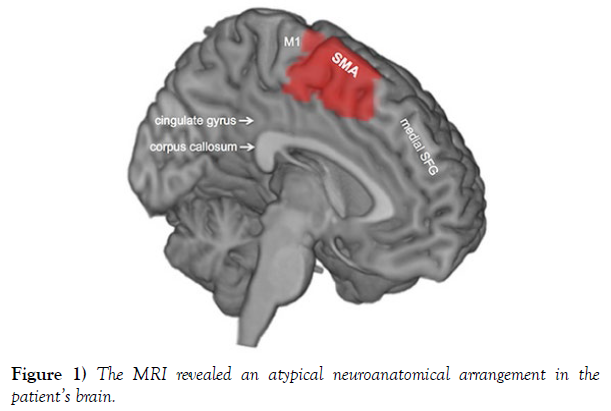
Imaging Findings: The MRI revealed an atypical neuroanatomical arrangement in the patient’s brain. While the right hemisphere demonstrated the expected corticospinal tract pathway originating from the primary motor cortex, the left hemisphere exhibited a distinct neural pathway originating from the supplementary motor area (SMA). This aberrant pathway descended through the corona radiata and internal capsule, bypassing the primary motor cortex, and ultimately innervated the contralateral motor neurons in the ventral horn of the spinal cord (Figure 1).
DISCUSSION
The observed neuroanatomical variation in this patient challenges the conventional understanding of motor control pathways [4]. Typically, the corticospinal tract originating from the primary motor cortex is responsible for voluntary motor control. However, in this case, the supplementary motor area, known for its involvement in motor planning and coordination, seemed to compensate for the absence of direct input from the primary motor cortex [5].
This unique neural pathway may account for the unilateral motor deficit observed in the patient. The atypical arrangement suggests a compensatory mechanism, where the SMA assumes a primary role in motor control, bypassing the primary motor cortex. Further investigations using functional imaging modalities, such as functional MRI or diffusion tensor imaging, could provide additional insights into the functional connectivity of this neural pathway [6-7].
Understanding the individual neuroanatomical variations is crucial for accurate diagnosis and appropriate treatment planning. In this case, a conventional diagnosis based solely on clinical presentation would have led to an incomplete understanding of the underlying pathology. Clinicians should consider such variations when encountering atypical clinical presentations to ensure optimal patient care [8-10].
CONCLUSION
This case report highlights the significance of neuroanatomical variations and their impact on clinical manifestations. The observed atypical neural pathway in a patient with a unilateral motor deficit challenges the traditional understanding of motor control. Accurate identification of such variations through advanced imaging techniques is essential for personalized diagnosis and treatment planning. Future research should focus on investigating the functional connectivity of these atypical pathways to improve our understanding of the complex neuroanatomical organization and its clinical implications. Incorporating individual neuroanatomical variations into clinical practice will enhance patient care and contribute to the development of tailored treatment strategies.
ACKNOWLEDGEMENT
None.
REFERENCES
- Xin W, Bofu L. Aortic Dissection with Rare Anatomical Aortic Arch Variation Depicted by Computed Tomography Angiography. Heart Surg Forum. 2021; 24(2): E407-E408.
- Foivos I, Jonathon K, Daryll B. Aberrant right subclavian artery - a rare congenital anatomical variation causing dysphagia lusoria. Vasa. 2021; 504(5):394-397.
- Schizas N, Patris V, Lama N. Arc of Buhler: A lifesaving anatomic variation. A case report. J Vasc Bras. 2012; 37(11):9-326.
- Penprapa SK, Brianna KR. Duplication of the inferior vena cava: evidence of a novel type IV. Folia Med Cracov. 2020; 28; 60(2):5-13.
- Laurent de K, Stefano M. Variability of repairable bicuspid aortic valve phenotypes: towards an anatomical and repair-oriented classification. Eur J Cardiothorac Surg. 2019; 37(11):9-828.
- Jun S, Zhang-Y, Chuan C. Postoperative neovascularization, cerebral hemodynamics, and clinical prognosis between combined and indirect bypass revascularization procedures in hemorrhagic moyamoya disease. Clin Neurol Neurosurg. 2021 Sep; 208:106869.
- Qi L, Xiaojie T, Yafang D. Evaluation of Carotid Plaque Rupture and Neovascularization by Contrast-Enhanced Ultrasound Imaging: an Exploratory Study Based on Histopathology. Transl Stroke Res. 2021 Feb; 12(1):49-56.
- Kuo-Shyang J, Shu-Sheng L, Chiung-FC. The Role of Endoglin in Hepatocellular Carcinoma. Int J Mol Sci. 2021 Mar 22;22(6):3208.
- Anri S, Masayoshi O, Shigeru H. Glomerular Neovascularization in Nondiabetic Renal Allograft Is Associated with Calcineurin Inhibitor Toxicity. Nephron. 2020; 144 Suppl 1:37-42.
- Mamikonyan VR, Pivin EA, Krakhmaleva DA. Mechanisms of corneal neovascularization and modern options for its suppression. Vestn Oftalmo. 2016; 132(4):81-87.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref