Neuroprotective strategies of solanesol in mitochondrial impairment in experimentally induced Huntington disease
2 Department of Pharmacology, Rajendra Institute of Technology & Sciences, Haryana, India, Email: Medtox@gmail.com
3 Faculty of Medical Sciences, Department of Pharmaceutical Sciences, Guru Jambheshwar University of Science & Technology, India, Email: Medtox@gmail.com
Received: 21-Feb-2018 Accepted Date: Mar 12, 2018; Published: 25-Mar-2018
Citation: Mehan S, Rajput M, Dudi R, et al. Neuroprotective strategies of solanesol in mitochondrial impairment in experimentally induced Huntington disease. J Pharm Toxicol. 2018;1(1):3-7
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
HD is an autosomal dominant neurodegenerative disease characterized by functional incapacity, psychiatric impairment and memory dysfunction. Numerous pathological cascades are implicated in HD pathology, of which excitotoxicity and impaired cellular bio-energy are of major concern. Exploitation of these major pathological cascades shall prove to be efficient in slowing down the disease progression in HD. The existing treatment strategies include anti-psychotic drugs, NMDA receptor antagonists, caspase inhibitors, coenzyme Q10, transglutamine inhibitors, human single chain FV antibody, RNAi, stem cell therapy, ubiquilin and trophic factors. Despite of various advancements in HD research, no treatment strategy was found to be effective to cure or to combat all the symptoms associated with the disease. Hence currently, Solanesol is a phytochemical that can influence different pathological pathways and facilitate the amelioration of all symptoms can be considered to be potent in the management of HD Herein; an attempt was made to explore the neuroprotective protective strategies of Solanesol in mitochondrial impairment induced by 3-NP animal model of Huntington disease. This put forward the possibility of a novel neuroprotective therapy to ameliorate the major pathological features and to slow down the disease progression in HD.
Keywords
Huntington’s disease; Neuroinflammation; 3-Nitropropionic acid; Solanesol
New developments in the study of brain are among the most exciting frontiers of contemporary neuro-scientific research for the clinical or surgical practitioner. Increasing knowledge of neurocomplications and of their discrete localization in the various regions of brain, the uppermost region of the central nervous system permits new modes of pharmacological management of some major neurological disorders like Huntington’s disease and several others. Current concepts of the supplementary motor area, basal ganglia, hippocampus and cerebellum providing movements “Programs” serving volitional, goal-directed purposes to be turned on, monitored, and then switched off by sensory motor cortex – are more interesting and important. Knowledge of such basic physiological mechanisms may open the way for additional new uses of pharmacotherapeutic agents. During the past decades, negative influences on brain development were observed in laboratory animals and in humans. Such influences include malnutrition, infectious diseases, poisons and drugs and ionizing radiation. The extent of the disturbance can be judged as early as in the foetal phase of life, when data collected from the growth of damaged brains are compared with the normal values. The significance of knowledge pertaining to growth functions of the human brain and its various regions has achieved special recognition recently due to awareness of enhanced experimental vulnerability of the developing brain to injury such as toxins. Knowledge of normal development of different functional areas are required in order to determine whether all brain regions are equally susceptible and at which developing stage it is vulnerable to various modes of injury. This field of research seems promising but is still at its beginning.
Huntington’s Disease: An Overview
Huntington’s disease (HD) is a hereditary autosomal dominant disorder of central nervous system [1]. The underlying genetic defect involves abnormal expansion of the CAG triple repeats (>40) in axon 1 of huntingtin gene (htt) (HDCRG, 1993). The htt gene located near the telomere of the short arm of chromosome 4 (locus 4pl6.3) encodes for huntingtin {Htt) protein [2]. Although, mutation in htt gene was discovered more than 17 years ago, the role of Htt in pathophysiology of HD is still under investigation [3]. The most striking neuropathological hallmark of this disorder is the atrophy of the striatal region, that control movement, memory and emotions suggesting that striatal degeneration is an important aspect of HD pathophysiology [4]. In patients with HD, selective loss of medium spiny neurons has not only been observed in the caudate and putamen of the striatum of basal ganglia, but also in pyramidal neurons of the cerebral cortex and to lesser extent in hippocampal and sub-thalamus neurons [5]. In patients with severe HD, neuronal loss to an extent of 80% has been observed [6]. HD is characterized by chorea, seizures, involuntary movements, dystonia, cognitive decline, intellectual impairment and emotional disturbances [7]. HD usually occurs in mid 40s with some exceptional cases of early onset (2 years of age) and of late onset (in the mid 80s) is reported [8,9].
Mitochondrial Dysfunction & Hd
Mitochondrial dysfunctions are suggested to be involved in HD pathogenesis (3). The nature and cause of mitochondrial deficits in HD is multifactorial, involving direct interaction of mutant Htt (mHtt) with mitochondria and indirect effects via transcriptional dysregulation accompanied by impaired trafficking, which compromise mitochondrial bioenergetics and dynamics (10). An aberrant association between mHtt and elements of the cellular metabolic machinery has been reported, demonstrating interaction between mHtt and mitochondria through the expanded CAG repeats [11]. The mHtt is reported to affect mitochondrial calcium buffering that, leads to mitochondrial dysfunctions and increase in free radical generation [12]. Whether excessive free radical generation results in mitochondrial dysfunction or mitochondrial alterations cause an increase in reactive oxygen species (ROS) is not yet clear. It is certain, however, that these pathologic processes are intertwined and each likely exacerbates the other [13]. Evidence suggests that HD may be associated with impaired energy metabolism. Inhibition of enzymes present in the respiratory chain can lead to an increase in electron leakage from the mitochondria, resulting in ROS production [14]. Activity of mitochondrial complexes I, II, III, and IV including aconitase enzyme were found to be reduced in the caudate and putamen of HD brains (Figure 1) [15]. In patients with HD, a reduction in striatal glucose use precedes tissue loss [16]. Lactate has also been shown to be elevated in the basal ganglion of patients with HD, indicating that neuronal death in HD may arise from the defect in energy metabolism [17].
Experimental Animal Model For Hd
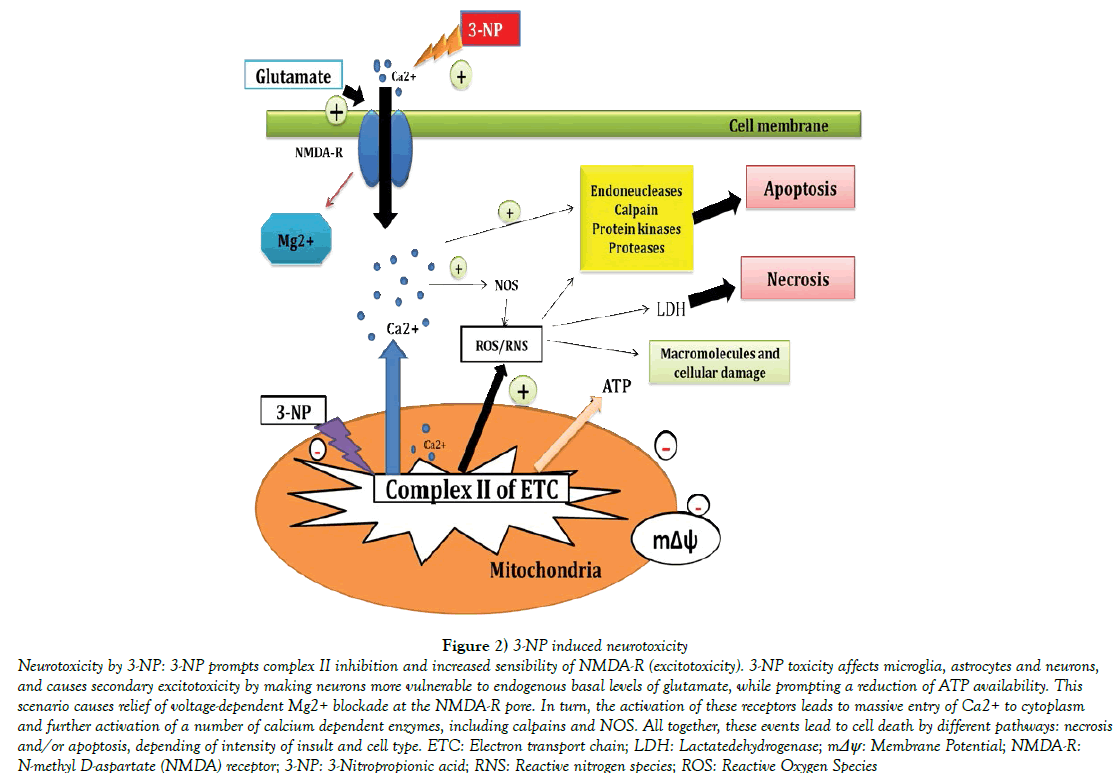
The hypothesis that mitochondrial dysfunctions contribute to the pathogenesis of HD was first tested pharmacologically by using 3-nitropropionic acid (3-NP), an irreversible inhibitor of succinate dehydrogenase [18]. (3-NP induced HD model replicates most of the clinical and pathophysiological hallmarks of HD, such as spontaneous choreiform and dystonic movements, cognitive deficits, including progressive degeneration of striatal tissue [19]. One of the mechanisms following 3-NP administration is the development of mitochondrial dysfunctions in HD animals. This bio-energetic defect involves three interacting processes such as: energy impairment, oxidative stress and excitotoxicity [20]. 3-NP administration results in ATP depletion, which impairs intracellular calcium buffering thereby leading to production of damaging ROS [21]. The most precise simulation of HD by 3-NP is produced when 3-NP is systemically and chronically injected in the animals [22]. Specific motor abnormalities and cognitive deficits, including working memory deficits can also be easily replicated in animals treated with 3-NP [23]. Other behavioral abnormalities in the 3-NP treated animals include bradykinesia, hypo activity followed by hyperactivity. Such specific behavioral changes arise from the selective striatal lesions produced by 3-NP administration (Figure 2) [24]. The therapies currently available to HD patients are aimed at symptomatic management rather than disease cure. These include SSRIs and atypical antipsychotics for psychiatric disturbances and Tetrabenazine for chorea {the first drug to be approved by the FDA specifically for the treatment of HD) [25]. However, different laboratory reports suggest the possible involvement of excitotoxic, neuroinflammatory, oxidative damage, neurochemical disturbance, mitochondrial dysfunction in its pathogenesis [26-29]. At present, N-methyl-Daspartate (NMDA) receptor antagonist [30], gamma amino butyric acid (GABA) modulators, dopamine blockers [31], cannabinoid agonists [32], energy production boosters, creatine [33] are being tried with a hope to treat and manage this disease symptomatically. Though HD has a single genetic cause, it has a very complex pathology with detrimental effects on a wide variety of cellular processes. As a result, a wide variety of therapies has been aimed at downstream events in both preclinical and clinical trials [34,35].
Target Drug: Solanesol
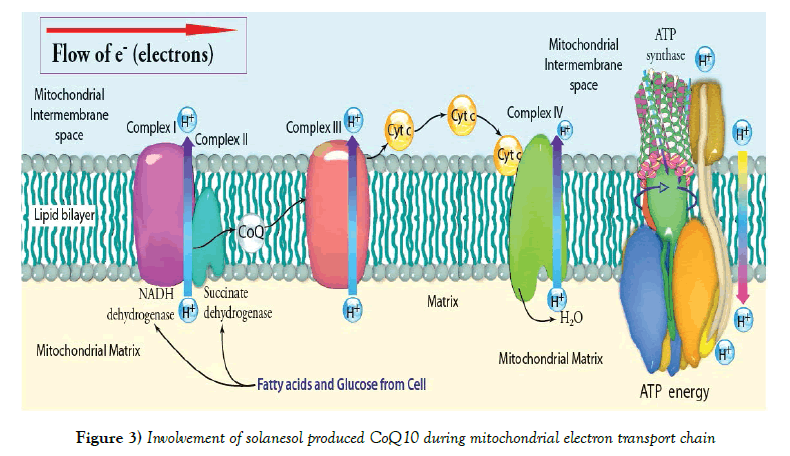
Several metabolic modifiers have been tried to ameliorate mitochondrial dysfunctions and oxidative stress in HD, but have been found to be beneficial to a limited extent [36,37]. Therefore, improving mitochondrial functions has now become a prime focus to combat neurodegeneration in HD. Mitochondrial cofactors, particularly Co-enzyme Q10, also known as ubiquinone (is a vitamin-like substance used by the human body to help produce ATP in the electron transport chain and is found throughout the body) [38-40] isolated from Solanesol obtained from Solanaceous plants, especially tobacco (Nicotiana tabacum L.) in combination have been shown to effectively ameliorate mitochondrial dysfunctions, reduce oxidative damage to neurons and improve behavioral functions in animals and increase ATP production, thus helps to maintain efficient mitochondrial functions (Figure 3). Solanesol is commonly found in plant leaves and is one of the ingredients present in tobacco, potato leaf and mulberry leaves [41]. Tobacco especially has up to 0.85-3.75% of Solanesol. It is also found widely distributed in higher plants of Solanaceae family like Solatium melongena, Solatium lycopersicum, and Capsicum annum. These contain Solanesol to an extent of 0.30 to 0.40%, while Datura stramonium, Solanum nigrum, Nicandra physaloides, Cestrum nocturnum and Solanum xantho carpum contain Solanesol to an extent of 0.05 to 0.25%. Solanesol (C45H74O) is a tri-sesquiterpenoid alcohol which was first isolated from tobacco. Solanesol is widely distributed in plants of Solanaceae family. Nicotiana genus (tabacum) is the richest source of Solanesol, other members of the family containing Solanesol include pepper plants, potato plants, tomato plants and egg plants. Some of the chemical compounds related to Solanesol are Solanesyl acetone, Solanesyl bromide, Phytol, Solanofuran, Solanesol Acetate, Solanesol 3, 5-Dinitrobenzoate, Solanesol 3-Nitrophthalate, Solanesol p-Phenylbenzoate1, 4etc. Solanesol is considered to be a good source of a large number of bioactive substances and is the starting material for many high value bio-chemicals, including Co-enzyme Q10 and vitamin-K analogues. Solanesol is also a potentiating agent in many medicines [42]. Solanesol is medically important noncyclic terpene alcohol that is synthesized by the condensation of nine isoprene units. The molecule is a precursor of ubiquinone, also known as coenzyme Q10, vitamin K2, and anticancer drugs, includingN-solanesyl-N,N0- is (3,4-dimethoxybenzyl) ethylenediamine (SDB) [43-45]. Coenzyme Q10 possesses both antioxidant and anti-aging properties and is reported to strengthen the body’s immune system and cardiovascular function, to improve brain health, and to moderate blood lipids. As a result, coenzyme Q10 has potential usefulness in the treatment of migraines, neurodegenerative diseases, hypertension, and cardiovascular diseases [46,47], and it is also being used as a dietary supplement by patients with type-2 diabetes [48]. Meanwhile, vitamin K2 promotes bone formation and mineralization, inhibits bone resorption, prevents and mitigates osteoporosis, promotes blood coagulation, and improves arterial stiffness [49]; and SDB, mediated by P-proteins, is used in the treatment of several types of drug resistance in tumors and plays a synergistic role with certain antitumor drugs [50,51]. Recently, Yao et al. [52] reported that Solanesol can protect human hepatic L02 cells from ethanolinduced oxidative injury via up regulation of HO-1 and Hsp70 expression. Thus, the medical benefits of Solanesol and its derivatives are well established. The molecule was first isolated from tobacco (Nicotiana tabacum L.) in 1956 and has subsequently been reported to occur in other solanaceous plants, including tomatoes, potatoes, eggplants, and peppers [53,54], in which it occurs in both free and ester-bound states [55,56]. Additionally, Solanesol participates in cellular energy production and in repair of damaged neurons [57-61]. On the basis of previous studies, supplementation with Solanesol can be effective in cognitive and motor performance tests and this supplementation could ameliorate age-associated mitochondrial functional changes and mitochondria associated structural damage and oxidative stress in animals [62-65].
Conclusion And Future Perspectives
In recent years, many studies have focused on the fate and potential of neural progenitors in vertebrates while much progress has been made, many questions remain about the mechanism which lead to neural diversity, in terms of both the regionalization of the nervous system and specification of cell fates within those regions. There is no cure or effective treatment for HD till date. It is currently being treated symptomatically and drugs are used to reduce the severity of its symptoms. For many of these treatments, comprehensive clinical trials to confirm their effectiveness in treating HD symptoms is in various phase of clinical trial so far. As the disease progresses and a person’s ability to tend to their own needs reduce, carefully managed multidisciplinary care giving becomes increasingly necessary. The research work to be reported by our research team to study the role of neuroinflammation and excitotoxicity in the pathogenesis of Huntington’s disease and further to explore the potential targets for the development of newer therapeutics for the management of HD and related problems.
Acknowledgement
The authors express their gratitude to Chairman, Mr. Parveen Garg and Director, Dr. G.D. Gupta, ISF College of Pharmacy, Moga (Punjab), India for their great vision and support. Authors are really thankful to Administrator, Dr. Sanjeev Kalra, Rajendra Institute of Technology & Sciences, Sirsa, Haryana, India for valuable support and encouragement.
REFERENCES
- Zheng Z, Diamond MI. Huntington disease and the huntingtin protein. Prog Mol Biol Transl Sci. 2012;107:189-214.
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787-95.
- Maroof DA, Gross AL, Brandt J. Modeling longitudinal change in motor and cognitive processing speed in presymptomatic Huntington's disease. J Clin Exp Neuropsychol. 2011;33:901-9.
- Vonsattel JP, Myers, RH, Stevens TJ, et al. Neuropathological classification of Huntington's disease. J Neuropathol Exp Neurol. 1985;44:559-77.
- Ross CA. Huntington's disease: New paths to pathogenesis. Cell. 2004;118:4-7.
- Montoya A, Price BH, Menear M, et al. Brain imaging and cognitive dysfunctions in Huntington's disease. J Psychiatry Neurosci. 2006;31:21-9.
- Vis JC, Verbeek M, M De Waal, et al. 3-Nitropropionic acid induces a spectrum of Huntington's disease-like neuropathology in rat striatum. Neuropathol Appl Neurobiol. 1999;25:513-21.
- Finkbeiner S. Huntington's disease. Cold Spring Harb Perspect Biol. 2011;3.
- Oliveira JM. Nature and cause of mitochondrial dysfunction in Huntington's disease: focusing on huntingtin and the striatum. J Neurochem. 2010;114:1-12.
- Panov AV, Burke JR, Strittmatter WJ, et al. In vitro effects of polyglutamine tracts on Ca2+-dependent depolarization of rat and human mitochondria: relevance to Huntington's disease. Arch Biochem Biophys. 2003;410:1-6.
- Schulz JB, Beal MF. Mitochondrial dysfunction in movement disorders. Curr Opin Neurol. 1994;7:333-9.
- Jin YN, Johnson GV. The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease. J Bioenerg Biomembr. 2010;42:199-205.
- Pandey M, Mohanakumar KP, Usha R. Mitochondrial functional alterations in relation to pathophysiology of Huntington's disease. J Bioenerg Biomembr. 2010;42:217-26.
- Tabrizi SJ, Cleeter MW, Xuereb J, et al. Biochemical abnormalities and excitotoxicity in Huntington's disease brain. Ann Neurol. 1999;45:25-32.
- Aziz NA, Pijl H, Frolich M, et al. Systemic energy homeostasis in Huntington's disease patients. J Neurol Neurosurg Psychiatry. 2010;81:1233-37.
- Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington's disease: correlations with CAG repeat numbers. Neurology. 1998;50:1357-65.
- Brouillet E, Guyot MC, Mittoux V, et al. Partial inhibition of brain succinate dehydrogenase by 3-nitropropionic acid is sufficient to initiate striatal degeneration in rat. J Neurochem. 1998;70:794-805.
- Tasset I, Medina FJ, Jimena I, et al. Neuroprotective effects of extremely low-frequency electromagnetic fields on a Huntington's disease rat model: effects on neurotrophic factors and neuronal density. Neuroscience. 2012;209:54-63.
- Damiano M, Galvan L, Deglon N, et al. Mitochondria in Huntington's disease. Biochim Biophys Acta. 2010;1802:52-61.
- Panov AV, Gutekunst CA, Leavitt BR, et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731-6.
- Colle D, Santos DB, Hartwig JM, et al. Succinobucol versus probucol: higher efficiency of succinobucol in mitigating 3-NPinduced brain mitochondrial dysfunction and oxidative stress in vitro. Mitochondrion. 2013;13:125-33.
- Dautry C, Conde F, Brouillet E, et al. Serial 1H-NMR spectroscopy study of metabolic impairment in primates chronically treated with the succinate dehydrogenase inhibitor 3-nitropropionic acid. Neurobiol Dis.1999;6:259-68.
- Akopian G, Crawford C, Petzinger G, et al. Brief mitochondrial inhibition causes lasting changes in motor behavior and corticostriatal synaptic physiology in the Fischer 344 rat. Neuroscience. 2012;215:149-59.
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: A randomized controlled trial. Neurology. 2006;6:366-72.
- Tunez I, Montilla P, Del Carmen Munoz M, et al. Protective effect of nicotine on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease, European Journal of Pharmacology. 2004a;504:169-175.
- Tunez I, Montilla P, Del Carmen Munoz M, et al. Protective effect of melatonin on 3-nitropropionic acid-induced oxidative stress in synaptosomes in an animal model of Huntington's disease. J Pineal Res. 2004 ;37:252-56.
- Kumar A, Seghal N, Padi SV, et al. Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicine-induced dysfunction and oxidative stress in rats. Eur J Pharmacol. 2006;551:58-66.
- Kumar P, Padi SS, Naidu PS, et al. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485-92.
- Beister A, Kraus P, Kuhn W, et al. The N-methyl-Daspartate antagonist memantine retards progression of Huntington's disease. J Neural Transm Suppl. 2004;68:117-22.
- Hannan AJ. Huntington's disease: Which drugs might help patients? IDrugs. 2004;7:351-8.
- Maccarrone M, Battista N, Centonze D. The endocannabinoid pathway in Huntington's disease: a comparison with other neurodegenerative diseases. Prog Neurobiol. 2007;81:349-79.
- Bender A, Auer DP, Merl T, et al. Creatine supplementation lowers brain glutamate levels in Huntington's disease. J Neurol. 2005;252:36-41.
- Sah DW, Aronin N. Oligonucleotide therapeutic approaches for Huntington disease. J Clin Invest. 2011;121:500-7.
- Munoz-Sanjuan I, Bates GP. The importance of integrating basic and clinical research toward the development of new therapies for Huntington disease. J Clin Invest. 2011;121:476483.
- Virmani A, Gaetani F, Binienda Z. Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: metabolic inhibitors, MPTP, and methamphetamine. Ann N Y Acad Sci. 2005;1053:183-91.
- Hersch SM, Rosas HD. Neuroprotection for Huntington's disease: Ready, set, slow. Neurotherapeutics 2008;5:226-36.
- Beyer, R. E. An analysis of the role of coenzyme Q in free radical generation and as an antioxidant. Biochem. Cell Biol. 1992;70:390-403.
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;127:195-204.
- Do TQ, Schultz JR, Clarke CF. Enhanced sensitivity of ubiquinone-deficient mutants of Saccharomyces cerevisiae to products of autoxidized polyunsaturated fatty acids. Proc Natl Acad Sci. 1996;93:7534-9.
- Shefali S, Raj K, Prathiba K. Novel hybrid natural products derived from Solanesol as wound healing agents. Ind J Chem. 2009;48b:237-47.
- Yu X, Wang S, Chen F. Solid phase synthesis of Solanesol. J Comb Chem. 2008;10:605-10.
- Campbell R, Freitag S, Bryan GJ et al. Environmental and genetic factors associated with solanesol accumulation in potato leaves. Front Plant Sci. 2016;7:1263.
- Parmar SS, Jaiwal A, Dhankher OP, et al. Coenzyme Q10 production in plants: Current status and future prospects. Crit Rev Biotechnol. 2015;35:152-64.
- Yan N, Liu Y, Gong D, et al. Solanesol: A review of its resources, derivatives, bioactivities, medicinal applications, and biosynthesis. Phytochem Rev. 2015;14:403-17.
- Bentinger M, Tekle M, Dallner G. Coenzyme Q-Biosynthesis and functions. Biochem. Biophys Res Commun. 2010;396:74-9.
- Sarmiento A, Diaz-Castro J, Pulido-Moran M, et al. Coenzyme Q10 supplementation and exercise in healthy humans: A systematic review. Curr Drug Metab. 2016;17:345-58.
- Mezawa M, Takemoto M, Onishi S, et al. The reduced form of coenzyme Q10 improves glycemic control in patients with type 2 diabetes: An open label pilot study. Bio Factors. 2012;38:416-21.
- Hamidi MS, Gajic-Veljanoski O, Cheung AM. Vitamin K and bone health. J Clin Densitom. 2013;16:409-13.
- Enokida H, Gotanda T, Oku S, et al. Reversal of P-glycoprotein-mediated paclitaxel resistance by new synthetic isoprenoids in human bladder cancer cell line. Jpn J Cancer Res. 2002;93:1037-46.
- Sidorova TA, Nigmatov AG, Kakpakova ES, et al. Effects of isoprenoid analogues of SDB-ethylenediamine on multidrug resistant tumor cells alone and in combination with chemotherapeutic drugs. J Med Chem. 2002;45:5330-39.
- Yao X, Bai Q, Yan D, et al. Solanesol protects human hepatic L02 cells from ethanol-induced oxidative injury via upregulation of HO-1 and Hsp70. Toxicol In Vitro. 2015:29: 600-8.
- Roe SJ, Oldfield MF, Geach N, et al. A convergent stereocontrolled synthesis of (3-14C) solanesol. J Label Compd Radiopharm. 2013;56:485-91.
- Taylor MA, Fraser PD. Solanesol: Added value from Solanaceous waste. Phytochemistry. 2011;72:1323-27.
- Zhou HY, Liu CZ. Rapid determination of solanesol in tobacco by high performance liquid chromatography with evaporative light scattering detection following microwave-assisted extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;835:119-22.
- Fukusaki E, Takeno S, Bamba T, et al. Biosynthetic pathway for the C45 polyprenol, solanesol, in tobacco. Biosci Biotechnol Biochem. 2004;68:1988-90.
- Noack H, Kube U, Augustin W. Relations between tocopherol depletion and coenzyme Q during lipid peroxidation in rat liver mitochondria. Free Radical Res. 1994;20:375-86.
- Forsmark-Andree P, Lee CP, Dallner G, et al. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radical Biol Med. 1997;22:391-400.
- Abe K, Fujimura H, Nishikawa Y, et al. Marked reduction in CSF lactate and pyruvate levels after CoQ therapy in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Acta Neurol Scand. 1991;83:356-9.
- Bresolin N, Bet L, Binda A, et al. Clinical and biochemical correlations in mitochondrial myopathies treated with coenzyme Q10. Neurology 1988;38:892-9.
- Ihara Y, Namba R, Kuroda S, et al. Mitochondrial encephalomyopathy (MELAS): pathological study and successful therapy with coenzyme Q10 and idebenone. J Neurol Sci. 1989;90:263-71.
- Nishikawa Y, Takahashi M, Yorifuji S, et al. Long-term coenzyme Q10 therapy for a mitochondrial encephalomyopathy with cytochrome c oxidase deficiency: a 31P NMR study. Neurology. 1989;39:399-403.
- Shoffner JM, Lott MT, Voljavec AS, et al. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc Natl Acad Sci. 1989;86:7952-56.
- Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357-66.
- Beal MF. Mitochondrial gene mutations and human diseases: a prolegomenon. Ann Neurol. 1992;31:119-130.
- Matthews RT, Yang L, Browne S, et al. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuro-protective effects. Proc Natl Acad Sci. 1998;95:8892-7.