NGS genomic analysis of the TERT gene in acral-lentiginous (non-CSD) and superficial spreading (low-CSD) subtypes of melanoma: A critical review of possible flaws in genomic analysis
Received: 24-Apr-2023, Manuscript No. PULHHR-23-6380; Editor assigned: 27-Apr-2023, Pre QC No. PULHHR-23-6380(PQ); Reviewed: 11-May-2023 QC No. PULHHR-23-6380; Revised: 12-May-2023, Manuscript No. PULHHR-23-6380(R); Published: 09-Jun-2023, DOI: 10.37532/pulhhr.23.7(2).1-5
Citation: Denise B, Rodrigues M, Landman G. NGS genomic analysis of the TERT gene in acral-lentiginous (non-CSD) and superficial spreading (low-CSD) subtypes of melanoma: A critical review of possible flaws in genomic analysis. J Histol Histopathol Res 2023;7(2):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
NGS technology is nowadays widely known to be a major ally in cancer mutation research; however, analytical results and deep knowledge of its stages still represent a formidable challenge. In this research, a genomic analysis of the TERT gene in acral-lentiginous and superficial spreading melanomas by NGS were performed in order to detect and evaluate variant mutations, in addition, to discuss methods and resources that may ensure a more accurate quality control in the use of FFPE in NGS genetic investigation. In this study, false variants c.3327delG (p.Gly1109fs) and c. 2259delG (p.Gly753fs) were found, both can serve as a complicating factor during analysis. Furthermore, factors such as low DNA count and errors in the alignment of short readings should be taken into consideration and analyzed, as they might represent false variants. NGS data is prone to calls for artifact-generating variants; however, it may be filtered systematically without compromising sensitivity. As NGS platforms and its respective language become commonly used in molecular diagnosis, the further processes involved with the production of artifacts will be identified and researched, avoiding diagnostic errors in clinical practice.
Keywords
Genomic; Acral-lentiginous; Superficial spreading; Melanoma
Introduction
Currently, the use of genetic panels through NGS are frequently used for the detection of mutations in oncology. These NGS assays seek to isolate clinically relevant segments of the genome. The crude sequence of the readings are initially aligned with the human genome as a reference, and then variants are searched to identify minor incompatibilities in these alignments that may indicate the presence of mutations within the examined sample. The analysis and interpretation of variants should then be performed to validate the technique and to determine the clinical usefulness of each variant [1].
The use of paraffin samples (FFPE) in pathological diagnosis have been the only resource in pathology laboratories for many decades. However, it represents a challenge in molecular examinations, because the more sensitive the molecular method of detection used, the greater the interference of the pre-analytical steps in the analysis results. DNA samples extracted from FFPE tissues present structural and molecular characteristics that can interfere with laboratory techniques, and the NGS technique is especially sensitive to these characteristics [2].
Formalin preserves the tissue through its chemical interaction with proteins and nucleic acids, one of the occasional effects resulting from this chemical interaction is deamination. When adenine is delaminated, it forms hypoxanthine and goes to pair with cytosine instead of thymine. Thus, the deamination of the adenine causes the exchange of a pair of A-T bases for G-C instead. Three bases are subject to deamination, guanine, cytosine and adenine, but the most frequent reaction occurs in cytokines resulting in Uracil. This process results in the addition of an erroneous nucleotide in all amplification cycles, which may lead to the detection of a false mutation [3]. Melanoma is a fast-developing form of aggressive cancer with a worse prognosis in patients with metastatic disease. It may often develop resistance to drugs used during treatment.
It is classified into different subtypes according to clinical and histological characteristics of primary tumors. Currently, it is well founded in the scientific literature that the Superficial Spreading Melanoma (SSM) subtype is associated with mutations in BRAF and/or NRAS genes while the acrallentiginous subtype has frequent association with mutations in the KIT11 gene [4]. In addition to the activation of these genes, another important step in the process of cellular immortality in melanomas is telomerase reactivation, for telomere maintenance. This means that some oncogenic mutations in the BRAF and/or NRAS genes, often related to the activation of the MAP-kinase pathway, activate the TERT gene promoter, which is responsible for encoding the telomerase catalytic subunit, reflecting a hyperactivation of this gene. Thus, the increase in telomerase activity is correlated with the hyper-expression of TERT and is also an important factor in the pathogenesis of several types of tumors [5].
The TERT gene (NCBI gene ID:7015) is responsible for the synthesis of the catalytic subunit with the function of telomerase reverse transcriptase, it is located on chromosome 5 (5p 15.33) and has 16 exons with a total size of 35 kb 18. Mutations in its coding region are rare, while somatic mutations in the promoter or regulatory region of TERT are the main causes in the increase of cancers involved with telomerase activity. Thus, the analysis of the coding region of this gene, due to its low mutation rate, allows a clear analysis of false variants and possible presence of background [6].
It is important to maximize efforts in the search for true variants present in the DNA of FFPE samples in order to guarantee a real genomic analysis. Therefore, this work carried out a genomic analysis of the TERT gene in acral-lentiginous and superficial spreading melanomas by NGS to detect and discuss the presence of variants in the two subtypes, additionally, this work discusses the possible ways to recognize and avoid backgrounds, guaranteeing a more precise quality control in the use of FFPE in NGS genetic research..
Literature Review
Project design
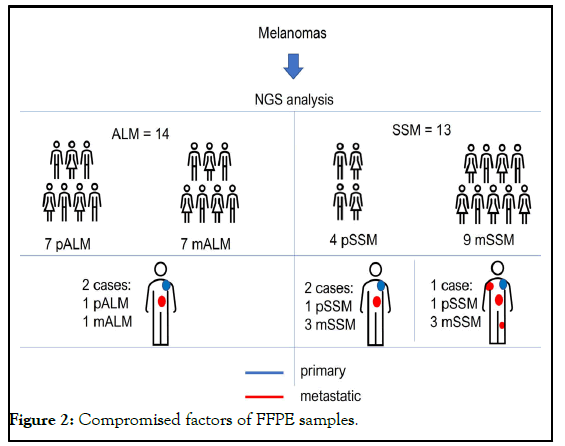
Two subtypes of melanomas were included in this work, the primary (pALM) and metastatic (mALM) Acral-Lentiginous subtype and the primary Superficial (pSSM) and metastatic (mSSM) subtype. 7 pALM and 7 mALM were studied, two of these tumors had paired samples (primary tumors and metastases from the same patient) and 13 cases of Superficial Spreading Melanoma (SSM), of which 4 were pSSM and 9 mSSM, two cases presented paired samples, and in one of the cases, 3 with metastases from the same patient were studied. All samples were submitted to NGS and the variables found were compared [7]. This study was approved by the institution´s research ethics committee, under the registered number CAAE: 42922515.5.0000.5505/CEP:241/2015.
FFPE samples were selected from patients diagnosed with primary and metastatic acral-lentiginous and superficial spreading melanoma between 1996 and 2014, from the department of pathology, hospital Sao Paulo, Escola Paulista de medicina, universidade federal de Sao Paulo [8].
Inclusion criteria: Samples from patients with (a) primary Acral- Lentiginous Melanoma (ALM) and (b) Superficial Spreading Melanoma (SSM) were included in the study, in addition to metastatic melanomas with confirmed diagnosis after review by a pathologist; 2-Paraffin blocks in good conditions and with a remaining tumor sample and 3 samples with a thickness that would make it possible to perform a 1 mm punch. Samples from patients with more than one primary cutaneous melanoma or other malignancies, except basal cell carcinoma, were excluded [9].
A 1 mm punch was taken from the tumor region of each sample and placed in microtubes with deparaffinization solution (Qiagen) and subjected to DNA extraction, according to the protocol specified in the QIAamp DNA FFPE Tissue Kit (Qiagen). At each puncture, the needle was sanitized with a hypochlorite and alcohol solution to avoid contamination between samples [10].
DNA fragmentation quantification and analysis
All samples were quantified using the Qubit® fluorometer equipment and the Qubit® dsDNA HS assay kit (Thermofisher scientific®) to control DNA extraction and subsequent library preparations. Only samples that had more than 1 ng/μl of DNA were used for sequencing [11].
Next Generation Sequencing (NGS)
After DNA extraction, samples of primary and metastatic melanomas were analyzed using the Next Generation Sequencing (NGS) technique using the chip technology of the ion torrent equipment (Life Technologies). All samples were sequenced with final coverage in the target regions of 1000 times. The NGS technique was performed according to the manufacturer's specifications and protocols (Thermofisher scientific) [12].
DNA target regions were amplified with the Ion AmpliSeq™ 2.0 library kit, with subsequent digestion of amplicons with FuPa reagent and ligation of adapters and barcodes different for each sample with Switch and DNA Ligase solution. The library underwent the first bead purification using the Agencourt reagent AMPure XP and subsequent amplifications with 8 cycles, as the samples were paraffinized. A second amplification of the purified library was performed by binding the amplicons to the magnetic beads, in an attempt to improve the quality of the sample for sequencing. With the library ready, it was followed by quantification by PCR in real time with the Ion library reagent. TaqMan quantity assay™ and normalization by calculating the average concentration of Ion Ampliseq library™ multiplied by the concentration determined in the PCR by 100. Even with the improvements in the library preparation steps, 19 samples were eliminated either due to construction failure, low quantification or small fragment profile [13].
Template preparation and chip loading was done in Ion Chef™ with the library diluted to 25 pM. With this equipment, an emulsion PCR is performed to amplify fragments that have the adapter around a sphere. This was followed by an enrichment step, in which the beads without amplified fragments were removed. The loaded chips were placed in the Ion S5™ equipment for sample sequencing [14].
Variant analysis-bioinformatics
The sequences generated by NGS were transformed into BAM format files of the samples that had 20x coverage above 75%. Data were analyzed in Varstation® software, the pipeline was developed with two variant calls TVC5 and freebayes. The annotation of variants used was generated by the same software that generated the files analyzed in the Excel® version [15].
Interpretation of variants
To determine the impact of variants on cancer, through variant annotation, some aspects were analyzed such as Allelic Frequency Variation (AVF), population databases such as the database of Single Nucleotide Polymorphisms (dbSNP) and disease reporting, such as the Catalog of Somatic Mutations in Cancer (COSMIC). The classification according to the Association for Molecular Pathology (AMP) was also analyzed, which categorizes changes according to clinical significance: benign, in which the allele frequency is significant in population databases with no evidence of association with cancer; unknown, allele frequency has not been described in population or cancer databases; and strong when the change has Food and Drug approved therapy Administration (FDA) and has an association with cancer.
The impact of the single nucleotide mutations effects obtained in the melanomas of the study was made by the Consensus Deleterious score on single nucleotide variants (CONDEL), based on the weighted average of five predictive computational tools SIFT, Polyphen, FATHMM and mutation assessor. In general, these tools are based on the degree of conservation of the altered amino acid in the different regions of the protein, classifying mutations as probably neutral or deleterious.
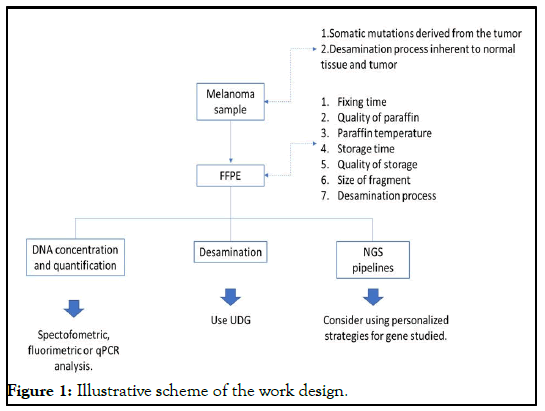
For mutation selection four criteria were used, following the parameters, (1) non-synonymous mutations, (2) exonic regions, (3) non-splicing regions and (4) presence of variant fraction above 20% (Figure 1).
Acral-lentiginous melanoma subtype
7 primary ALM (pALM) and 7 metastases (mALM) were studied. Only the pALM 9AP sample showed no variants of any type in the TERT gene. A total of 15 different variants were found (with the exception of c.3327delG), and the number of variants per sample ranged from 0-9. Unexpectedly, the c.3327delG variant with protein alteration (p.Gly1109fs), was the only one to present pathogenic potential indicated by AMP, was present in 85% of the samples, and of the 9 studied samples of pALM, two presented only this variant. In addition, it always presented a very high read depth compared to the others. Therefore, the set of these findings made us question their veracity.
All mALM samples showed at least one type of variant. A total of 10 different variants were found, with this number ranging from 1-11 per sample. Furthermore, again the c.3327G>A variant (p.Gly1109fs-frameshift deletion) was present in 85% of the researched samples. Only one sample (3MA2) of mALM had more than one variable classified by the AMP as probably pathogenic.
Analysis of the three paired samples of ALM showed that only 1 primary tumor did not show mutations in the TERT gene. The number of variants found ranged from 0-3 and the variant c.3327G>A (p.Gly1109fs) was absent in only one pALM sample. In Acral-Lentiginous Melanoma, mutations in the TERT gene had a VAF lower than one, that is, all mutations found were considered heterogeneous.
Superficial spreading melanoma
A total of 13 cases of Superficial Spreading Melanoma (SSM) were studied, of which 4 were primary SSM (pSSM) and 9 metastatic (mSSM). Two cases presented paired samples, that is, pSSM and mSSM from the same patient and in one of the cases, 3 mSSM from the same patient were studied. Of the four pSSM samples, only one did not show the c.3327delG variant. The number of variants found per sample, according to the criteria described. All mSSM samples presented the variant and the specific variant c. 3327delG. The number of variants found per sample, according to the criteria described, ranged.
The analysis of the paired cases showed different realities in the two cases. Sample 5 had one primary tumor (5PE) and three metastatic (5ME3, 5ME4 and 5ME5). Only one of the metastatic (5ME4) presented an uncertain variant. While in the other paired sample, both the primary (8PE) and the metastatic (8ME1) tumors showed a wide variety of variants.
Variant c.3327delG
According to the AMP Classification, the variant c.3327G>A (p.Gly1109fs) would have a deleterious character in the genomic stability of the gene, and could be associated with the pathogenesis of melanoma, especially when associated with mutations of other genes. It has been previously described in hepatocellular carcinoma. However, in our samples it was found in 85% of all melanomas studied.
When we excluded the c.3327delG variant from the analysis, we found a significant reduction in the rate of variants classified as pathogenic or probably pathogenic.
In a parallel study, we sequenced other genes (data not shown) associated with melanoma pathogenesis, such as KIT, BRAF, MAP2K1 and NRAS in the same panel developed for this genomic analysis of TERT, where the extraction parameters, genomic library formation and NGS were the same. With this, it was also possible to verify in this group of genes the existence of other variants that were frequently repeated in the studied samples, as occurred with the variant, c.3327G>A (p.Gly1109fs), and which were classified as “probably pathological” by the AMP. Thus, the high frequency of unknown variants in many different samples and in different genes drew our attention to their veracity.
Discussion
This study used samples of UV-dependent (Superficial spreading) and non- UV-dependent (Acral-Lentiginous) melanomas to investigate the presence of variants in the coding region of the TERT gene.
Tumors of the SSM subtype had a greater number of variants per sample in relation to the ALM subtype, which suggests that the action of UV light as the pathogenesis of these tumors may be an important factor for the accumulation of these variants.
Variants
Variants are considered to be variations found in a genomic sequence in relation to the reference sequence. It is of fundamental importance, in genetic research, to identify whether the variants found are from a somatic mutation, from alterations caused by the processes involved in the production of FFPE or from an alteration in the NGS pipelines. Therefore, it is necessary to be careful when designing each step of the workflow to minimize the production of sequence artifacts. In addition, validation of mutations, performed by an appropriate experimental design or by traditional methods should be considered. Some hypotheses were considered by different authors to explain the presence of the variants, among them, the cytosine deamination process, the quality levels of the DNA used and the analysis of the parameters used in the pipeline protocols.
DNA deamination
Deamination is contemplated by some authors to be responsible for the emergence of undesirable variants and for contributing to the background noise that occurs during DNA sequencing. This is because if cytosine deamination occurs in FFPE, unrepaired uracil lesions will cause C>T (and therefore G>A) sequence artifacts after PCR amplification. It has been shown that cytosine deamination occurs frequently in FFPE samples of tumoral origin, however, these two factors are not determinant for the process to occur, occurring also, less frequently in normal and non-fixed tissues, such as fresh tissue and peripheral blood.
Some authors have verified that in non-mutated tissue samples, no artifactual mutation occur during FFPE preparation, and have demonstrated this fact by accurately comparing NGS results from FFPE DNA with frozen tissue DNA. This suggests that this process would be related to intrinsic aspects of the samples and not to the processes of FFPE sample preparation, PCR amplification and/or sequencing. The gradual accumulation of genetic mutations in adult human stem cells during life, for example, is associated with several age-related diseases, especially cancer. This is due to unavoidable random mutations that arise during disorderly DNA replication. In normal tissues, these mutations accumulate steadily over time in all tissue types, at a rate of approximately 40 new mutations per year.
However, other authors claim that formalin fixation can trigger this process, only in a small proportion of C bases, and explain that deamination can occur randomly, resulting in low frequency artefactual Single Nucleotide (SNV) variants (approximately 1%) and unpredictable. However, low frequency C>T mutations also occur in cancer and can be clinically important, therefore, these two deamination pathways are important as their knowledge can alert to the possibility of the existence of false variants. Currently, there are protocols that allow the restoration of this process through the use of a repair enzyme. Uracil DNA glycosylase-UDG, is a DNA repair enzyme that removes uracil lesions through hydrolysis of the Nglycosidic bond between the uracil base and the phosphate sugar backbone in DNA. This pretreatment with UDG can reduce the frequency of SNVs (C:G>T:A) in up to 60% of samples in highly fragmented DNA.
However, although the effect of deamination is important, it is necessary to consider DNA integrity as the strongest factor in the successful use of NGS in FFPE samples. Therefore, a prior analysis of the quantity and quality of DNA needs to be performed before pre-treatment with UDG, which is a crucial factor for the efficient execution of any molecular test.
DNA quantity and quality
An important factor and prior to any molecular procedure is that the FFPE sample is prepared according to well-known quality protocols, such as formalin inclusion time and formalin type used, which are sufficient to guarantee the improvement of FFPE for use in techniques molecules, including NGS. However, it is important to consider that even following the manufacturer's instructions of any Kit available on the market; no single nucleic acid extraction protocol allows the rescue of high yields of nucleic acids and high quality nucleic acids in all sample types. Even so, many clinical samples from hospitals are processed or stored incorrectly and may be degraded or too small to extract sufficient amounts of high-quality DNA, for example in cases of melanomas.
It is well known that paraffin samples present fragmented DNA, due to the block production process itself. An alternative to deal with low quantity and low quality DNA is to increase the number of viable templates (targets), because when the number of quality templates is limited, usually by the use of low concentrations of DNA, the damaged templates and reduced size become artificially over-represented occupying a substantial fraction of sequenced reads. It is necessary to consider that the assessment of the amount of DNA in a sample can be performed by different methods, such as spectrophotometric, fluorimetric and by qPCR. The average concentration of DNA extracted from FFPE tissue measured using the spectrophotometric method (Nanodrop) is much higher than the concentration measured using the Qubit® fluorimetric method and qPCR. A fluorimetric analysis could, for example, be more suitable for the quantification of DNA samples extracted from FFPE tissue compared to spectrophotometric analysis, despite the fact that it presents purity parameters, which fluorimetric analysis does not. However, qPCR is considered the best technique, as it details the amount of DNA together with the quality of the amplifiable DNA of the FFPE tissue, having only the cost aspect as a limiting factor to its use.
Spectrophotometric analyzes were initially performed (data not shown) and, subsequently, the use of Qubit® 2.0, which uses fluorochromes that specifically bind to dsDNA, in this study, allowed efficient quantification and adequate selection of eligible samples for the study. It is necessary to consider that the amount of DNA does not always represent high quality, which can generate false negatives, and that sometimes low amounts of high quality DNA can also represent a false positive result due to the type of present cells. Thus, considering aspects such as the presence of tissue deamination, quantity and quality of the samples to be studied, we can conclude that FFPE tissues can be used for routine clinical diagnosis, with reliable NGS results, provided that they are in adequate conditions of fixation and validation, after the test, are applied (Figure 2).
NGS and bioinformatics protocols
Compared to Sanger sequencing, NGS is faster and cheaper when considering the number of targets analyzed, moreover, it has the ability to generate large amounts of data. However, this technique requires multiple computational intensive steps for a proper analysis to be performed, and for that, it depends on a highly complex computational data analysis infrastructure.
As the NGS generates quasi-random sample fragments, the use of the VAF value represents the corresponding measure of the proportion of DNA molecules in the original sample that would carry the variant, thus being able to be used for heterogeneity assessments. Therefore, values close to 1 (100%) represent homogeneous samples while values below represent the degree of heterogeneity of that variant. The analysis of VAF in TERT indicated that among all the variants found, only one was not heterogeneous. The clinical relevance of the VAF value for most mutations is still a challenge; however, it is considered that the presence of an elevated VAF is a factor of worse prognosis.
Bioinformatics pipeline are bioinformatics algorithms that run in a predefined sequence to process NGS data. They are fundamental in the process, as the processing of raw sequence data for the detection of genomic alterations has a direct impact on the detection, prognosis and treatment of diseases, consequently on patient care; therefore, they need to be reproducible. Often, the large volume of data obtained by the technique can hide biologically important information amid the large amounts of generated noise, such as false positives and even false negatives 39. Therefore, the call of variants is a fundamental step for the correct analysis of the data generated by the NGS.
There are different programs, also called “callers”, which perform this function, among them we can mention Strelka2, Mutect2, VarScan2 and Shimmer. Choosing these callers and even combining them can be a crucial step in the process, as the variation between the analysis generated by them can be a complicating factor.
Schaetzen et al., explain that combining the variants of different callers can increase the accuracy and sensitivity of the variant call. It states that by using the correct variant calling strategy, most of the clonal SNVs can be recovered in an FFPE sample with high precision and sensitivity and in this way, part of the discrepancy between the results from fresh samples and FFPE can be attributed to tumor heterogeneity within itself.
Variant c.3327delG (p.Gly1109fs)
If we consider the AMP classification, this variant would be considered deleterious in the genomic stability of the gene, and may be associated with the pathogenesis of melanoma, especially when associated with mutations of other genes. However, there is a previous issue to be verified, which is the veracity of this variant. In this study the c.3327delG variant (p.Gly1109fs) and the c.2259delG (Gly753fs), 3MA sample were considered as an artifact. Factors such as low amount of DNA, errors in the alignment of short reads (due to primers with unspecific annealing in the internal region of correct amplicons) and location in the terminal region of the exon must be considered and analyzed, as they may be present in routine variant analysis, but are indicative of false variants.
Several authors report that NGS data are prone to certain types of artifactgenerating variant calls, yet they can be systematically filtered without significantly compromising sensitivity. Thus, as NGS platforms and their language become more common for use in molecular diagnostics, more and more the processes involved with the production of artifacts will be identified and studied, avoiding frequent diagnostic errors in clinical practice. Thus, when we eliminated the variant c.3327delG (p.Gly1109fs) from the analysis, there was a significant reduction of variants in the body of the TERT gene, graph 1. In addition, it made it possible for other variants, also found in terminal regions of several genes studied by our research group (works in the final stage of publication), that were repeated and eliminated by adjustments in the pipelines.
Study limitations
The main limitation of this study was that portions of normal tissue from the margin of the tumors were not used for comparison with the tumor samples. Furthermore, the samples were not previously treated with UNG.
Conclusion
The causes of artifacts presence in FFPE DNA sequences are still poorly understood and are probably multifactorial, however the presence of artifacts generated by NGS is established. Even if there is not only one strategy to reduce these findings, recognizing the need for a careful analysis of these sequences can avoid false-positive mutations and be the solution to avoid errors. Therefore, considering aspects such as sample type, quantity and quality, analysis of size and frequency of generated fragments allows for the prediction of errors and guarantees correct analyzes in the clinical routine.
This genomic analysis of the TERT gene in melanomas allowed the detection of variants in a group of primary and metastatic melanomas and the critical investigation of the possible reasons for the presence of true variants and of artifacts in the DNA of these samples. All variants considered veracious were classified with uncertain potential as expected in literature. This study shows that it is possible to detect genetic variants by NGS in melanomas from FFPE samples with high precision and sensitivity, determining that appropriate protocols and strategies are used at all stages of the process, from DNA extraction to the use of the alleged correct variant.
References
- Fortunato A, Mallo D, Rupp SM, et al. A new method to accurately identify single nucleotide variants using small FFPE breast samples. Briefing Bioinfo. 2021;22(6):bbab221.
- Vestergaard LK, Oliveira DN, Hogdall CK, et al. Next generation sequencing technology in the clinic and its challenges. Cancers. 2021;13(8):1751.
[Crossref] [Google Scholar] [PubMed]
- Li Z, Wang H, Zhang Z, et al. Germline and somatic mutation profile in cancer patients revealed by a medium-sized pan-Cancer panel. Genomics. 2021;113(4):1930-9.
[Crossref] [Google Scholar] [PubMed]
- Wong SQ, Li J, Tan AY, et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. Bio Med Genom. 2014;7:1-0.
[Crossref] [Google Scholar] [PubMed]
- Rosa-Rosa JM, Caniego-Casas T, Leskela S, et al. Modified sureselect QXT target enrichment protocol for illumina multiplexed sequencing of FFPE samples. Biological Procedures Online. 2018;20:1-1.
[Crossref] [Google Scholar] [PubMed]
- 6 Prentice LM, Miller RR, Knaggs J, et al. Formalin fixation increases deamination mutation signature but should not lead to false positive mutations in clinical practice. PLoS One. 2018;13(4):e0196434.
[Crossref] [Google Scholar] [PubMed]
- Serizawa M, Yokota T, Hosokawa A, et al. The efficacy of uracil DNA glycosylase pretreatment in amplicon-based massively parallel sequencing with DNA extracted from archived formalin-fixed paraffin-embedded esophageal cancer tissues. Cancer Genetics. 2015;208(9):415-27.
[Crossref] [Google Scholar] [PubMed]
- Do H, Dobrovic A. Dramatic reduction of sequence artefacts from DNA isolated from formalin-fixed cancer biopsies by treatment with uracil-DNA glycosylase. Oncotarget. 2012;3(5):546.
[Crossref] [Google Scholar] [PubMed]
- Ofner R, Ritter C, Ugurel S, et al. Non-reproducible sequence artifacts in FFPE tissue: An experience report. J Cancer Res Clin Oncol. 2017;143:1199-207.
[Crossref] [Google Scholar] [PubMed]
- Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: From mutations to medicine. Genes Develop. 2012;26(11):1131-55.
[Crossref] [Google Scholar] [PubMed]
- Ko JM, Velez NF, Tsao H. Pathways to melanoma. Semin Cutan Med Surg, 2010;29(4):210-7.
- Comodo-Navarro AN, Fernandes M, Barcelos D, et al. Intratumor heterogeneity of KIT gene mutations in acral lentiginous melanoma. Am J Dermatopathol. 2020;42(4):265-71.
[Crossref] [Google Scholar] [PubMed]
- Fernandes M, Barcelos D, Comodo AN, et al. Acral lentiginous melanomas harbour intratumor heterogeneity in BRAF exon 15, with mutations distinct from V600E/V600K. Am J Dermatopathol. 2019;41(10):733-40.
[Crossref] [Google Scholar] [PubMed]
- Chang GA, Wiggins JM, Corless BC, et al. TERT, BRAF, and NRAS mutational heterogeneity between paired primary and metastatic melanoma tumors. J Investigative Dermatology. 2020;140(8):1609-18.
[Crossref] [Google Scholar] [PubMed]
- Griewank KG, Murali R, Puig-Butille JA, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. JNCI: J National Cancer Instit. 2014;106(9).
[Crossref] [Google Scholar] [PubMed]