Nitric oxide–mediated regulation of retinoic acid–induced cartilage breakdown
2 Division of Biomedical Sciences, University of Bradford, Bradford, UK, Email: joseph.bird@aru.ac.uk
Received: 16-Jan-2018 Accepted Date: Feb 26, 2018; Published: 15-Mar-2018
Citation: Yaya M, Bird J, Idris B, et al. Nitric oxide–mediated regulation of retinoic acid–induced cartilage breakdown. J Histol Histopathol Res 2018;2[1]: 1-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: The aim of this research is to characterize the most appropriate concentration of Foetalbovine serum [FBS] for culture maintenance, and determine the apoptosis rate in the culture system, and also to investigate the induction of proteoglycan catabolism and the role of nitric oxide [NO] on proteoglycans [PG] loss. Furthermore it also aims to study the involvement of specific nitric oxide signaling pathways and to determine the involvement of matrix metalloproteinase [MMPs] in the degradation of proteoglycans.
METHODS: Proteoglycan degradation was investigated with 1 μmol/L retinoic acid [RA] and NO donor Diethylenetriamine DETA-NONOate in bovine cartilage explant cultures. Signaling pathways were investigated using specific inhibitors. Nitrite, an end product of NO metabolism, was measured in media by the Griess reaction. Matrix metalloproteinase [MMP] activity was investigated by gelatinase zymography and chondrocyte apoptosis in cultures was assessed using active caspase-3 immunohistochemistry.
RESULTS: Low rates of apoptosis were identified relative to positive control samples. Retinoic acid at 1μmol/L caused a significant increase in proteoglycan loss and this effect was completely reversed in the presence of NO. The zymography results did not show a difference in MMP activity. It was also found that p38 and p42/44 MAPKs, ROCK and soluble guanylate cyclase activities were involved in RA induced PG loss but not in NO mediated anti-catabolic.
CONCLUSION: This study demonstrates that 1% FBS is the most appropriate concentration to study PG loss in bovine culture model. In our culture system there are likely to be low levels of apoptosis due to the tissue culturing procedure and PG loss in RA-induced cultures is mediated by proteinases other than MMPs.
Abbreviations
ADAMTS: A disintegrin and metalloproteinease with thrombospondin motifs; APS: Ammonium persulphate; c-GMP: Cyclic – Guanin monophosphate; DAB: 3,3’-diaminobenzidine tetrahydrochloride; DETA: Diethylenetriamine; DMEM: Dulbeccos Modified Eagles medium; DMMB: Dimethylmethylene blue; DSMO: Dimethyl sulfoxide; ECM: Extracellular matrix; FBS: Foetal bovine serum; GAG: Glycosaminoglycan; H2O2: Hydrogen peroxide; IGD: Interglobular domain; IL-1: Interlukin-1; iNOS: Inducible nitric oxide synthase enzyme; MAPK: Mitogen-activated protein kinase; MMP: Matrix metalloproteinase; MT-MMPs: Membrane type MMPs; NaOH: Sodium hydroxide; NO: Nitric oxide; OA: Osteoarthritis; OCT: Optimal cutting temperature compound; ODQ: Zero– direct–quadrature; PBS-T: Triton X-100, PBS; PG: Proteoglycans; RA: Retinoic acid; ROCK: Rho-associated coiled-coil kinase; SDS: Sodium dodecylsulphate; TEMED: Tetramethylethylenediamine; TIMP: Tissue inhibitors of MMPs; TNF: Tumor necrotic factor
Introduction
Pro-inflammatory cytokines plays an important role in the regulation of articular cartilage metabolism. They induce cartilage degradation through the action of nitric oxide [NO]. However, NO has also been demonstrated to antagonize the degenerative effect of interlukin-1 [IL-1] and retinoic acid [RA] in explant cultures. This research investigates the biphasic role of NO in cartilage breakdown and identifies the pathways involved in mediating this effect.
Joint diseases are the main cause of movement disability and pain in old people. Osteoarthritis [OA] is a common chronic pathology affecting one in five people per year in the United Kingdom and is the main reason for 98% of joint replacement [1]. Presently, there is no curative treatment for OA and currently available drugs only alleviate pain. A balance between extracellular matrix ECM production and degradation is the main factor in maintaining cartilage integrity. Once this equilibrium changes, cartilage breakdown starts [2]. Chondrocytes express surface receptors for inflammatory mediators such as cytokines [3] and inducible nitric oxide synthase enzyme [iNOS]. In inflammatory conditions, chondrocytes become activated by different mediators which can induce chondrocyte phenotypic change with disturbance of the anabolic-catabolic balance and subsequent cartilage degradation [4]. These molecules induce the up-regulation of matrix metalloproteinase MMP and non-MMP enzyme gene expression coupled with the inhibition of chondrocyte synthetic pathways [5]. Cytokines represent the key players in cartilage degradation. They are categorized into three groups; catabolic cytokines including interlukin-1 IL-1 α and IL-1β, regulatory cytokines such as such as IL-6, IL-8 and tumor necrotic factor TNF, anabolic factors such as growth factors and complement [6]. The IL-1 and TNF are the major cytokines involved in cartilage catabolism and in OA they are responsible for the initiation of degradation process, followed by their stimulation of IL-6 release with subsequent release of other cytokines, alteration in ECM structure and quality and increased MMP expression [7,8]. In addition, IL1 and TNF induce NO production which is believed to be involved in cartilage catabolism [9]. It has also been implicated in chondrocyte apoptosis signaling as well as the induction of MMPs [10]. Another important signaling pathway involved in cartilage breakdown is the inducible nitric oxide synthase [iNOS] pathway [11]. It mediates catabolic effects either by the activation of the cyclic –Guanine monophosphate c-GMP pathway, with subsequent activation of different biological processes or by the production of large quantities of NO [12].
The mitogen-activated protein kinase [MAPK] pathways have been demonstrated to have a regulatory role on pro-inflammatory mediators as well as the production of MMPs [13]. Levels of activated MAPK have been reported to increase in osteoarthritis cartilage. P38 MAPK pathway inhibition has been identified to reverse cartilage destruction in mouse models [14]. Mitogen-activated protein kinase pathways become activated in response to a variety of stimuli such as cytokines and inflammatory mediators [15]. Rho kinases are among the downstream signaling of RhoA that play important roles in chondrocyte differentiation and actin cytoskeletal regulation. The RhoA/ROCK signaling pathway has been shown to be involved in cartilage degradation [16].
High levels of proteinases are the main cause of cartilage breakdown; they have been demonstrated both in osteoarthritis and rheumatoid arthritis cartilage [17].
Matrix metalloproteinases in cartilage are secreted by chondrocytes, and are secreted as pro-enzymes, becoming activated after the dissociation of cysteine residues in the pro-peptide domain exposing the zinc atom-conserved active site in the latent form. They can be activated by proteolytic and oxidative nitrosylation [18] or by phosphorylation [19]. Under normal conditions, MMP activity in joints is controlled by the tissue inhibitors of MMPs [TIMP]. They can be inhibited by binding to TIMP in a 1:1 ratio [20]. Imbalance between TIMPs and MMPs was demonstrated in osteoarthritis [5].
Intracellular activation was first demonstrated in pro-MMP-11 activation by furin. Extracellular activation is the major mechanism of activation of pro-MMPs and is mediated either by proteases, plasmin cleavage [21] or the involvement of membrane type MMPs [MT-MMPs] such as the activation of MMP-2. The aggrecan molecule has two main cleavage sites in the interglobular domain [IGD], the first at Asn341–Phe342 which is targeted by MMPs generating a G1 fragment with the VDIPEN[431] sequence on the COOH terminus [22]. The second site is at Glu373-Ala374, which has been identified to be cleaved by another group of proteinase known as the aggrecanases, producing a NITEGEN[373] sequence-terminating G1 fragment. Aggrecanases are zinc-dependent enzymes that contain an amino terminal peptide domain, MMP domain, disintegrin like domain and thrombospondin motif at C-terminus, known as a disintegrin and metalloproteinease with thrombospondin motifs [ADAMTS], which are membrane bound enzymes that are secreted in an active form [23].
Nitric oxide has been demonstrated as a key player in the pathogenesis of osteoarthritis [7]. In cartilage tissue NO is produced by chondrocytes in response to cytokines [24]. Nitric oxide is believed to mediate a catabolic effect on cartilage as it inhibits collagen production and promotes the release of proteoglycans. In addition [and more importantly], it up-regulates the activity of MMPs [25]. High levels of NO were found in the synovial fluid of arthritis joints. Nitric oxide has also been found to mediate IL-1 and TNF-α mediated aggrecan degradation [26].
Apoptosis has a key role in tissue remodelling and development. The molecular mechanism of apoptosis involves the activation of a group of proteinases known as caspases [Cysteine Aspartyl-specific Proteases]. Chondrocyte apoptosis is mediated by complex pathways including the imbalance between anti-apoptotic and pro-apoptotic proto-oncogene [Bcl2/ Bax] intracellular levels, the Fas/Fas ligand and iNOS pathways [27]. Nitric oxide has been shown to induce apoptosis in different cell types such as macrophages and endothelial cells. It was reported that exogenous NO can induce apoptosis of chondrocytes, while the presence of oxygen free radical scavenger is necessary for endogenous NO to induce apoptosis. However, there is a variation in the degree of apoptosis between different cartilage layers as it appears to be higher in superficial layers than the deep zones [28].
Materials and Methods
Preparation of articular cartilage explants and culture treatment
Bovine articular cartilage was obtained from the metacarpophalengeal joints of steers less than 36 months old. Tissue was collected under aseptic conditions, diced into 1-2 mm2 and maintained in Dulbeccos Modified Eagles medium [DMEM] [Gibco® Life technologies UK] containing [4.5 g/L glucose, 110 mg/L sodium pyruvate and 2 mM L-glutamine] supplemented with 100 U/ml penicillin, 100 µg/ml streptomycin and 1 mg/ml fungizone. Tissue was incubated at 37°C with 1% [v/v] foetal bovine serum [Biosera UK] for 24 hrs, and then tissue was aliquoted in to 24 well culture trays at 100-300 mg/well. Four replicates for each treatment were prepared. Cartilage degradation was induced with 1 μmol/L all trans-retinoic acid [Sigma–Aldrich UK] and culture conditions included 466 μmol/L [Z]-1-[N-[2-aminoethyl]-N- [2-ammoniioethyl]amino] diazen-1-ium-1,2-diolate [DETA-NONOate], 466 μmol/L Disodium [E]-1-sulphonatodiazen-1-ium-1,2-diolate [S-NONOate], 10 μmol/L SB 203580, 10 μmol/L PD 98059, 5 μmol/L ODQ [all from Cambridge Bioscience], 10 μmol/L Y-27632 [Abcam Biomedical]. Dimethyl sulfoxide [DSMO] was used as inhibitor vehicle, sodium hydroxide [NaOH] was used for nitrite donor dilution. Both were shown to have no effect on the metabolism of proteoglycans [data not shown]. Culture media was replaced after 24hrs and then incubated for 72 hrs. Media then was collected on ice for analysis; tissue samples were washed 3 times in PBS at room temperature and kept at -20°C for further analysis [29].
Measurement of nitrite production
Nitric oxide synthase activity was investigated indirectly by measuring nitrite, the end product of NO, in triplicate cultures using a spectrophotometerbased nitrite assay [30]. Concentrations were calculated from a sodium nitrite standard curve produced from a 0.1M stock solution. 100 μl of samples were added in 1:1 ratio to freshly prepared Greiss reagent [1% [w/v] sulphanilamide and 0.1% [w/v] naphthylethelenamide, 5% [v/v] phosphoric acid] in 96 well plates. Only samples containing [DETA –NONOate] were diluted at 1:2 in water. The remaining samples were measured undiluted. Absorbance was measured using a 96 well microplate reader [Jenway 6300] at 570 nm.
Measurement of glycosaminoglycan [GAG] release by spectrophotometer based assay
Proteoglycan release to culture medium was measured in triplicate using 96 well plates, by measuring the absorbance of dimethylmethylene blue [DMMB] dye [16 mg DMMB in 5 ml ethanol, 993 ml H2O, 2 ml formic acid, 2 g sodium formate, pH 3.5]. Dilutions in water of 1:20 were prepared for all samples except for RA treated samples which were diluted 1:50. 250 μL of DMMB dye were added to 40 μL of conditioned media in triplicates and absorbance was measured at 620 nm. Shark chondroitin sulphate [Sigma- Aldrich UK] was used to prepare a standard curve [31].
Estimation of MMP activity in the culture media by gelatinase zymography
For the detection of MMP activity in culture, 10 μL volume of conditioned culture medium was incubated with 9 μL sample buffer [6% sodium dodecylsulphate [SDS], 0.04% bromophenol blue, 20% glycerol, 0.256 M phosphoric acid, 0.471 M Tris, pH 6.8] and 1 μL NaOH at 37°C for 4 hrs in four replicates, then centrifuged at 5000 g. 10 μL of molecular weight markers [RPN800E Lifesciences UK], appropriate volumes of Samples [1.5 mg equivalent] were applied on stacking gel [1250 μL 1.5 M Tris buffer, pH 8.8, 500 μL 40% acrylamide [Sigma-Aldrich UK], 3144.5 μL H2O, 50 μL 10% SDS, 5 μL tetramethylethylenediamine [TEMED], 50 μL 10% [w/v] ammonium persulphate [APS]. After separation on 10.5% SDSpolyacrylamide minigels [2500 μL 1.5 M Tris buffer, pH 8.8, 2640 μL 40% acrylamide, 3660 μL H2O, 1000 μL 1% bovine gelatin [Sigma-Aldrich UK], 100 μL 10% SDS, 10 μL TEMED, 100 μL 10% [w/v] APS electrophoresis was run at 30 mA for 6hrs with continuous cooling using an ice container in the electrode buffer [0.1 M Tris-HCL, pH 8.7, 0.768 M glycine, 2 mM ethylenediaminetetraacetic acid [EDTA] using internal and external electrode buffer at 1:4 and 1:8 dilution respectively. After electrophoresis, the prestained molecular weight marker lane was separated and the gel was washed 3 times for 10 minutes each in sequential washing buffer 1 [0.05 M Tris-HCL, pH 7.5, 0.02% sodium azide, 2.5% Tween 80], 3 times for 10 minutes each in sequential washing 2 [0.05 M Tris-HCL, pH 7.5, 0.02% sodium azide, 2.5% Tween 80, 5 mM CaCl2 and 1 μM ZnCl2] at room temperature. Then the gels were incubated overnight at 37oC in [0.05 M Tris-HCL, pH 7.5, 0.02% sodium azide, 5 mM CaCl2 and 1 μM ZnCl2].
The gel then was stained in solution containing 1.2 mM Coomassie blue dye R250 [Thermo Scientific], 30% methanol, 10% acetic acid for 30 minutes followed by destaining in 10% methanol, 5% acetic acid solution for 2 hours until clear bands were seen on the gels, Gels were dried and images taken using digital photography [32].
Immunohistochemical identification of apoptosis
Normal tissue samples were fixed directly in 4% paraformaldehyde in PBS. To induce apoptosis in positive controls, the following treatments were performed: a- tissue was incubated in 1% [v/v] FBS in DMEM containing 500 μM hydrogen peroxide [H2O2] incubated at 37°C for 6hrs. b- 1% FBS/DMEM with 300 μM H2O2 incubated for 6 hrs. c- 1% FBS/DMEM containing 300 μM H2O2 incubated for 24hrs. d- 10% FBS/DMEM with 500 μM H2O2 incubated for 24 hrs. After incubation, the medium was discarded and tissue samples from the experiment and the positive controls were fixed in 4% paraformaldehyde in PBS embedded in optimal cutting temperature compound OCT and sectioned by Leica cryostat at 10 μm thickness. After mounting on pre-coated slides, antigen retrieval was achieved by incubating the slides in citrate buffer [10 mM citric acid, 0.05% [v/v] Tween 20, pH 6.0] for 30 minutes at 95°C, and then cooled for 20 minutes at room temperature. Sections were washed twice in 1% [v/v] goat serum, 0.04% Triton X-100, PBS [PBS-T] and blocked for non-specific binding with 5% goat serum PBS-T for 30 minutes. Endogenous peroxidase was blocked with 0.3% [v/v] H2O2 in PBS-T for 15 minutes. Slides then were washed with 1% [v/v] goat serum in PBS-T for a few seconds before incubation with active caspase 3 antibody [NB 600-1235, Novus Biologicals UK], diluted 1:100 in 1% goat serum PBS-T overnight at 4°C.
Sections then were washed twice in 1% [v/v] goat serum in PBS-T for 10 minutes each prior to secondary antibody incubation [goat anti-rabbit IgG FFc, horse radish peroxidase conjugate [Life Technologies USA], 1:1000 dilution in 1% [v/v] goat serum in PBS-T for an hour at room temperature. Sections were washed twice in PBS for 10 minutes each before applying freshly prepared 3,3’-diaminobenzidine tetrahydrochloride [DAB] reagent before washing with deionised water and counterstaining with Harris’s haematoxylin [Sigma-Aldrich UK] for 1 minute, washed in distilled water twice for 2 minutes each, then dehydrated in an ascending ethanol gradient and cleared in Histoclear and applying mounting media. Samples were visualized using a light microscope [Nikon] and digital images were captured [28,33].
Statistical analysis
The results were expressed as mean +/- standard deviation [S.D]. All culture experiments were conducted as four replicates. Significance of differences between samples was compared using the Student’s T-test. Statistical significance was defined as [p<0.05].
Results
Retinoic acid and nitric oxide regulation of cartilage metabolism
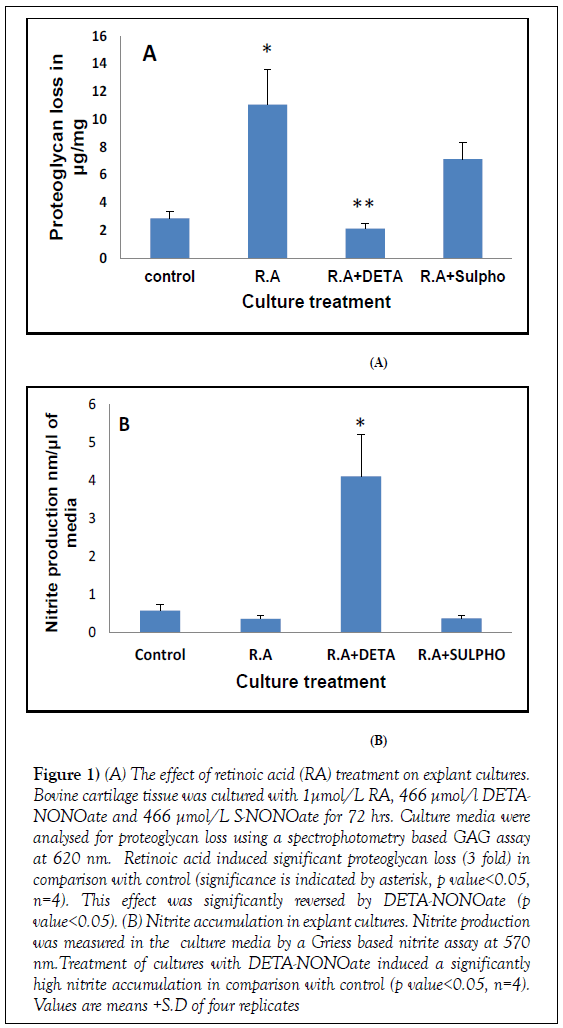
As expected proteoglycan loss in control cultures was consistent throughout the experiments ranging between [1.2 and 3.5 μg/mg]. Treatment of cultures with 1 μmol/L all trans--RA significantly induced glycosaminoglycan [GAG] release from tissue to culture media which was measured by the DMMB assay, producing a 3 fold increase of GAG in media [11 μg/mg, RA treated culture; 2.8 μg/mg control cultures] (Figure 1). This effect was significantly inhibited in the presence of NO donor [DETA-NONOate] to below control levels, concomitant with a significantly higher nitrite accumulation [DETANONOate cultures, 4.1 nmol/μL; control cultures 0.6 nmol/μL] [p<0.05, n=4]. S-NONOate was used as negative control for NO production and produced no significant inhibition of GAG loss indicating that NO produced by DETA-NONOate has an inhibitory effect on RA induced proteoglycan catabolism.
Figure 1: (A) The effect of retinoic acid (RA) treatment on explant cultures. Bovine cartilage tissue was cultured with 1μmol/L RA, 466 μmol/l DETANONOate and 466 μmol/L S-NONOate for 72 hrs. Culture media were analysed for proteoglycan loss using a spectrophotometry based GAG assay at 620 nm. Retinoic acid induced significant proteoglycan loss (3 fold) in comparison with control (significance is indicated by asterisk, p value<0.05, n=4). This effect was significantly reversed by DETA-NONOate (p value<0.05). (B) Nitrite accumulation in explant cultures. Nitrite production was measured in the culture media by a Griess based nitrite assay at 570 nm.Treatment of cultures with DETA-NONOate induced a significantly high nitrite accumulation in comparison with control (p value<0.05, n=4). Values are means +S.D of four replicates
The effect of different concentrations of foetal bovine serum on culture conditions
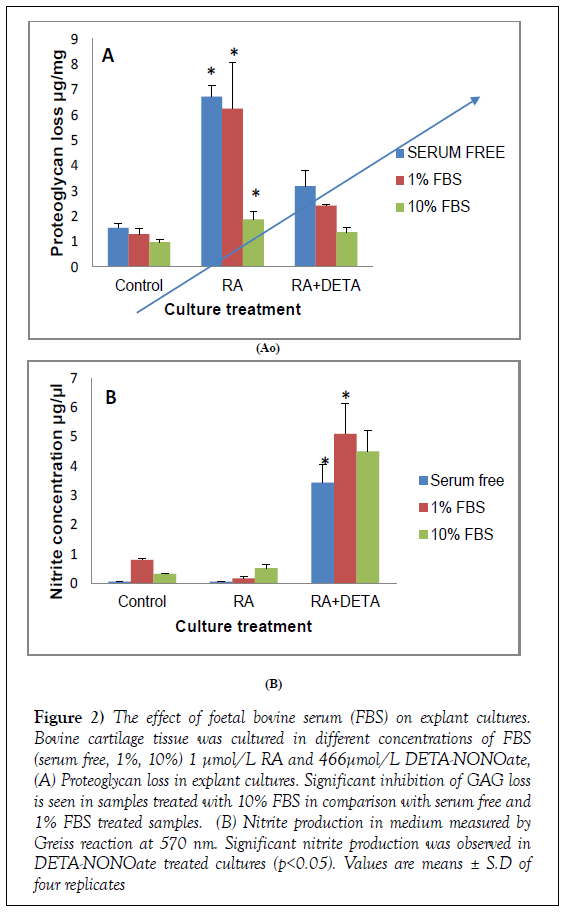
Foetal bovine serum was used as a maintenance factor in these cultures. The impact of FBS on the RA and NO responses was assessed. Tissue was cultured in medium containing 3 different concentrations of FBS [serum free, 1% and 10%]. Aggrecan loss was measured in the presence of 1 μmol/L all trans- RA and DETA-NONOate. As shown in Figure 2, in control samples there was no effect of FBS on control cultures. When stimulated by 1 μmol/L RA, GAG loss was significantly blocked with 10% FBS compared with serum-free cultures [p<0.05] [serum free, 6.6 μg/mg; 1% FBS, 6.2 μg/mg; 10% FBS, 1.8 μg/mg]. Samples incubated in 1% FBS showed no significant reduction in GAG loss compared to control samples. There was a significant increase in nitrite accumulation in DETA-NONOate cultures at all FBS concentrations. These findings suggest that 1% FBS medium can be used to support tissue survival without interfering with culture conditions. As cartilage is nonvascular.
Figure 2: The effect of foetal bovine serum (FBS) on explant cultures. Bovine cartilage tissue was cultured in different concentrations of FBS (serum free, 1%, 10%) 1 μmol/L RA and 466μmol/L DETA-NONOate, (A) Proteoglycan loss in explant cultures. Significant inhibition of GAG loss is seen in samples treated with 10% FBS in comparison with serum free and 1% FBS treated samples. (B) Nitrite production in medium measured by Greiss reaction at 570 nm. Significant nitrite production was observed in DETA-NONOate treated cultures (p<0.05). Values are means ± S.D of four replicates
Identification of signal transduction pathways involved in proteoglycan degradation
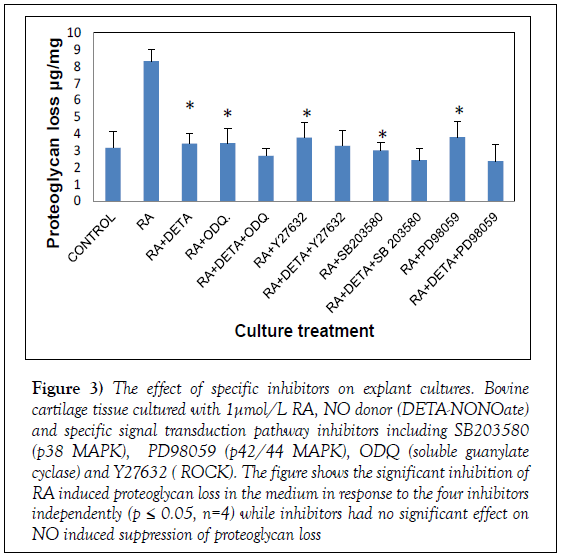
Mitogen activating protein kinases are known to have a role in MMP activation. To elucidate the pathways involved in the regulation of proteoglycan by NO and RA, specific signal pathways were tested using specific inhibitors; we tested p38 MAPK using SB203580, p44/42 MAPK using PD98059, soluble guanylate cyclase activity using ODQ and Y-27632 as a selective ROCK inhibitor. Treatment of RA cultures resulted in significant inhibition of PG loss in response to the four inhibitors [p<0.05, n=4], with the largest effect with SB203580, inhibiting RA-induced catabolism to below control levels [102.9% inhibition]. Potent inhibition was also found with ODQ [94.55%], PD98059 [88.15%] and Y23632 [87.59%] (Figure 3). To test their contribution to NO induced effect, we treated the cultures with signal transduction inhibitors; RA and DETA-NONOate., as shown in Figure 4 none of the inhibitors had any significant effect on NO treatment. Our findings suggest the involvement of these pathways in mediating RA induced GAG loss and that NO is not inhibiting GAG loss through theses pathways.
Figure 3: The effect of specific inhibitors on explant cultures. Bovine cartilage tissue cultured with 1μmol/L RA, NO donor (DETA-NONOate) and specific signal transduction pathway inhibitors including SB203580 (p38 MAPK), PD98059 (p42/44 MAPK), ODQ (soluble guanylate cyclase) and Y27632 ( ROCK). The figure shows the significant inhibition of RA induced proteoglycan loss in the medium in response to the four inhibitors independently (p ≤ 0.05, n=4) while inhibitors had no significant effect on NO induced suppression of proteoglycan loss
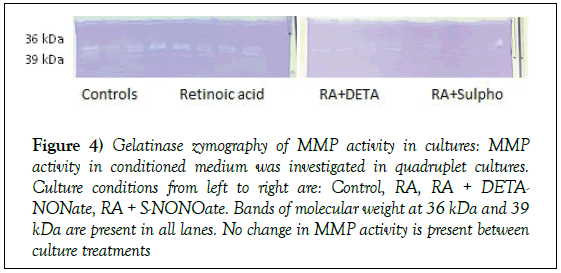
Figure 4: Gelatinase zymography of MMP activity in cultures: MMP activity in conditioned medium was investigated in quadruplet cultures. Culture conditions from left to right are: Control, RA, RA + DETANONate, RA + S-NONOate. Bands of molecular weight at 36 kDa and 39 kDa are present in all lanes. No change in MMP activity is present between culture treatments
Variation in culture sensitivity to specific signalling pathways inhibitors
Different levels of response to inhibitors were seen in different experiments. Table 1 shows variations in the effect of MAPK inhibitors on RA induced PG loss. The range of inhibition for the ROCK inhibitor [Y27632] was 88.15% to 46%. Interestingly, PD 98059 had a wider range, blocking the RA effect to less than control levels [103.39%] in one experiment whilst having a more limited effect of 5.73% in another. Similar variations were observed with SB203580 which has as high as, 102.90% and 103.52% in some experiments and as low as 21.8% in another. The ODQ variation was less marked, ranging between 94.55% and 60.46%.
Table 1: Variation in culture sensitivity to treatment with different signal pathway inhibitors including SB203580 (p38 MAPK), PD98059 (p42/44 MAPK), ODQ (soluable guanylate cyclase) and Y-27632 (ROCK). The values represent the percentage of inhibition of retinoic acid induced proteoglycans loss
| Inhibitor | Experiment 1 | Experiment 2 | Experiment 3 |
|---|---|---|---|
| ODQ | 77.19% | 60.46% | 94.55% |
| Y27632 | 46.99% | 75.26% | 88.15% |
| SB203580 | 21.87% | 103.52% | 102.90% |
| PD98059 | 5.73% | 87.59% | 103.39% |
Matrix metalloproteinase activity in cultures
To investigate whether the GAG degradation in culture is due to MMP activity, we quantified activity in control samples, RA-, DETA-NONOateand S-NONOate-treated cultures by gelatinase zymography. Bands of activity were identified at molecular weight 36 kDa and 39 kDa in all lanes (Figure 4). Currently, there are no MMP-derived gelatinase activities which correspond to these molecular sizes reported in the literature. There was no change in MMP activity between control and other culture treatment conditions suggesting that PG catabolism is mediated by non-MMP enzymes.
Apoptosis in culture model
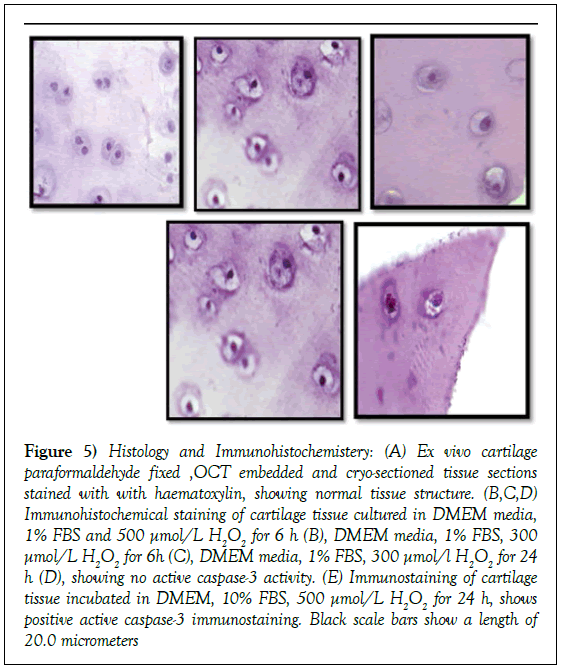
To further investigate if chondrocyte apoptosis in the explant cultures is high enough to contribute to these changes we active caspase 3 expression in ex vivo samples and in positive controls cultured in DMEM media and the following [A]1% FBS and 500 μmol/L H2O2 for 6h, [B]1% FBS, 300 μmol/L H2O2 for 6h, [C]1% FBS, 300 μmol/L H2O2 for 24 h [D]10%FBS, 500 μmol/L H2O2 for 24 hr respectively. As shown in Figure 5, we observed positive active caspase 3 expression in sections treated with 500 μmol/l H2O2 [yellow arrows] while ex vivo tissue and other treatments showed no signs of apoptosis, indicating that our tissue processing procedure does not induce apoptosis in cultures.
Figure 5: Histology and Immunohistochemistery: (A) Ex vivo cartilage paraformaldehyde fixed ,OCT embedded and cryo-sectioned tissue sections stained with with haematoxylin, showing normal tissue structure. (B,C,D) Immunohistochemical staining of cartilage tissue cultured in DMEM media, 1% FBS and 500 μmol/L H2O2 for 6 h (B), DMEM media, 1% FBS, 300 μmol/L H2O2 for 6h (C), DMEM media, 1% FBS, 300 μmol/l H2O2 for 24 h (D), showing no active caspase-3 activity. (E) Immunostaining of cartilage tissue incubated in DMEM, 10% FBS, 500 μmol/L H2O2 for 24 h, shows positive active caspase-3 immunostaining. Black scale bars show a length of 20.0 micrometers
Discussion
The role of NO in biological and pathological processes has been the subject of much research. In osteoarthritis, NO plays a key role in mediating the catabolic effects of pro-inflammatory cytokines [26], the up regulation of MMPs expression [34], and inhibition of PG synthesis by suppression of chondocyte sensitivity to growth factors [35]. Nitric oxide has long been believed to mediate collagen degradation. However, more recently there is emerging evidence that nitric oxide can antagonise the catabolic processes in cartilage, although its role as an anticatabolic factor has yet to receive significant study. A study has shown that it promotes collagen synthesis in human tendon in vitro, [36]. Other studies showed that NO can modulate MMP activity in a biphasic manner where high levels of NO result in MMP inactivation [37]. In the present study, we found that exogenous NO can significantly block RA-induced proteoglycan degradation in bovine explant cultures [p<0.05]. This finding was consistent with a previous study of the NO effect on RA and IL-1-induced PG loss in equine cartilage [38] as well as a study which reported a significant increase of IL-1β induced PG loss in bovine explants culture in the presence of NO inhibitor [39]. These findings together suggest that there is a basal level of NO that can be protective in cartilage tissue, and this interpretation is supported by a study which suggests that, in the case of low inflammatory conditions, intracellular redox potential is directed toward S-nitrosothiol production which has an inhibitory effect on PG degradation by the inhibition of NF-kB signalling pathway [40]. Although the MAPK pathways are involved as downstream cascade signalling in the various effects of NF-k β, a previous report has shown that they can work independently in bovine cultures [41]. Another possibility is that NO may exert an auto-regulatory feedback inhibition on iNOS activity similar to a mechanism observed in brain endothelium [42]. Mitogen-activated protein kinases are cell signal transduction pathways which communicate extracellular signals and intracellular responses including regulation of protease gene expression via phosphorylation [34]. MAPK activation have been demonstrated in osteoarthritis joints in humans [43]. Thus, inhibition of MAPK signaling pathways is a crucial focus of developing new treatments for osteoarthritis. The p38 MAPK and ERK1/2 pathway [p42/44 MAPK] are involved in high concentration NO-induced chondrocyte apoptosis and PG catabolism in cartilage cultures, as is the ROCK signaling pathway [44]. Also, c-GMP is a second messenger of endogenously produced NO [45]. In this study, we found significant inhibition of PG loss in cultures in response to all the specific inhibitors we used which suggest that retinoic acid-induced PG degradation is mediated by p38, p42/44, ROCK and c-GMP pathways. Our data was consistent with previous studies demonstrating the role of MAPK in PG turnover in response to TNF-α [10], oncostatin M [OSM] and TNF induced cultures. It has also been demonstrated that p38 inhibition reverses cartilage loss in an in vivo model of osteoarthritis [14]. Additionally, p38 pathway has been implicated in transforming growth factor-α [TGFα] induced proteoglycan catabolism [44,46] and chondrocyte apoptosis [47]. Nitric oxide signaling pathways are complex and incompletely understood. It is known to activate p38 phosphorylation in c-GMP/PKG dependent and by independent mechanisms [48]. We tested if the NO protective effect is mediated by the p38 pathway using SB203580 which is reported to inhibit p38 in bovine cartilage [49]. There was no significant effect of p38 inhibition on the NO effect. It was previously reported that NO stimulates c-GMP generation with subsequent activation of protein kinase G. The molecular pathway involving a sGC/PKG-mediated activation of ROCK in response to NO is a possible mechanism though which a basal level of NO has been shown to induce a synaptic inhibitory effect in neonatal motor neurons as well as vascular smooth muscle cells [50].
We used ROCK inhibitor [Y-27632] and a c-GMP inhibitor [ODQ] to investigate if inhibition of these pathways has an effect on NO mechanism of PG maintenance; we did not find significant change with either of them, indicating that both pathways are not involved in the NO protective effect in cartilage. However, investigation of PKG pathway is a necessary step to elucidate the contribution of this mechanism to a potential NO anabolic effect.
Interestingly, we observed some variability in responses from different experiments. The reasons for this are currently unclear. Since all experimental parameters were standardized, the discrepancies are most likely to be the result of factors prior to tissue arrival in the laboratory. Information about breeding conditions, animal health status, farming treatments such as vaccines or hormonal therapy, and animal age are not currently available to us. These variations indicate the need for further confirmation of the role of these pathways.
Nitric oxide is known to play an important role in chondrocyte apoptosis [51]. The contribution of apoptosis to the NO effect on proteoglycan turnover in different culture treatments needs to be fully investigated. Although high concentrations of NO have been implicated in chondrocyte apoptosis, our preliminary results showed only very low levels of apoptosis in our culture model in comparison to cultures treated with 500 µmol/L for 24 h H2O2 to induce apoptosis [active caspase 3 positive]. This is consistent with a previous study of H2O2 induction of apoptosis in cartilage cultures [52].
Retinoic acid is known to induce cell trans-differentiation. Whether PG loss is due to alteration in degradation rates only or as part of a phenotypic change, is an important point for further research. This explanation is supported by a study by [53] who reported that ROCK inhibition maintains chondrocyte phenotype; therefore antagonizing the RA induced Transdifferentiation. In addition, inhibition of ROCK signaling has been found to inhibit MMP-3 activity and GAG loss in monolayer human chondrocyte cultures [16]. Our zymography gels did not show changes in MMP activity in response to culture treatments in correlation with the changes we observed with GAG assay. This finding is consistent with previous studies on RA induced PG on bovine explants cultures. Absence of changes in MMP activity may have several interpretations. Firstly, in our culture model, PG degradation might not be due to MMPs activity, and other proteases such as aggrecanases might be implicated in RA-induced proteoglycan catabolism. This finding is consistent with other published data indicating the role of ADAMTSs in aggrecan catabolism in explant cultures [54]. This interpretation is supported by a previous study showing that aggrecanases are more efficient than MMPs in cleaving within aggrecan molecules due to delayed pro-MMP activation and that MMP inhibition in bovine cartilage explant cultures does not attenuate aggrecanase induced proteoglycan degradation. Another suggestion is that our culturing time was not sufficient to achieve pro-MMP activation; this suggestion is supported by previous reports of absent MMP activity in early culture and that the MMP catabolic effect happens in the late phase of culture. Several studies support the interpretation of a role of aggrecanase in RA induced proteoglycan catabolism [53] whilst another study has shown that MMPs are not involved in RA induced PG catabolism [54]. Other possibilities are that PG degradation was mediated by MMPs that are not detected by zymography or that the experimental sensitivity is too low to detect the activities that are present. For the latter, experimental conditions would need to be optimized to increase gel sensitivity by increasing the concentration of conditioned medium. However, the identification of the two of metabolic G1 fragments in culture [G1-NITEGE and G1-FDIPEN] is important to quantify the contribution of each of these enzymes in the degradation process. In contrast to our findings, MMP-13 and MT1-MMP activation in response to RA was demonstrated in untreated metatarsal rudiments [55]. In bovine cartilage, [56] studies have shown MMP1 and MMP13 proteolytic activity in response to IL-1α and oncostatin M cytokines. Additionally, MMP-2 and MMP-9 were identified in bovine culture in response to cytokines stimulation [26]. These data implicate MMPs as having a role in mediating some of the proteoglycan degradation in bovine cartilage cultures.
Foetal bovine serum is used in cultures as a source of nutrients, hormones, and growth factors. In this study, we tested three different concentrations of FBS including serum free, 1% FBS and 10% FBS. We found that 10% FBS blocked RA-induced PG loss in bovine cartilage cultures in comparison with other concentrations. This was consistent with a study demonstrating the reversal of RA effect in response to high concentration of FBS [57]. We found that 1% FBS is the most appropriate concentration for our culture model. However, recent studies suggest serum free culture for more accurate results, as well as better mechanical and biochemical responses [58].
Limitations
There were some limitations of this study including the lack of sufficient information about the condition of steers prior to the experiment which resulted in variable sensitivities to culture treatments. Therefore, further investigation of responses is required. Another limitation of our study was that the antigen retrieval step of the active caspase 3 immunohistochemical analysis resulted in loss of tissue due to the high temperatures required. Further optimization of the antigen retrieval step is required to circumvent this problem. This is a preliminary study therefore extrapolation of the data on the anti-catabolic effects of NO to the human disease is premature. However, the bovine data in this study does agree with data from an equine cartilage model, suggesting that further study of the biphasic effect of NO is warranted.
Clinical Application
Nitric oxide is being used in the treatment of respiratory distress disease [59], and has also shown an enhancing effect on human performance [60]. Thus, the testing of the NO effect in human cartilage collected during joint replacement surgery and identifying the potential signaling pathways involved in this effect would enable a closer exploration to the human disease and it’s management.
Conclusion
We concluded that 1% foetal bovine serum FBS is the most appropriate concentration to study proteoglycans loss in bovine culture model. Furthermore, Retinoic acid stimulates cartilage degradation through the activation of p38, p42/44, ROCK and c-GMP pathways. Whereas, Nitric oxide can antagonize retinoic acid-induced cartilage breakdown and this effect is not mediated by these pathways. In the culture system used, there are likely to be low levels of apoptosis due to the tissue culturing procedure. In addition this study shows that proteoglycans loss in retinoic acid-induced cultures is mediated by proteinases other than matrix metalloproteinase.
REFERENCES
- http://www.arthritisresearchuk.org/arthritis-information/data-and-statistics.aspx
- Lane NE, Brandt K, Hawker G, et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19:478-82.
- Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone 2012;51:249-57.
- Rigoglou S, Papavassiliou AG. The NF-κB signaling pathway in osteoarthritis. The International Journal of Biochemistry & Cell Biology 2013;45:2580-84.
- Murphy G, Nagase H. Reappraising metalloproteinases in rheumatoid arthritis and osteoarthritis: destruction or repair? Nat Clin Pract Rheumatol 2008;4:128-35.
- Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep 2000;2:459-65.
- Ashkavand Z, Malekinejad H, Vishwanath BS. The pathophysiology of osteoarthritis. J Pharm Res 2013;7:132-8.
- Kupai K, Szucs G, Cseh S, et al. Matrix metalloproteinase activity assays: Importance of zymography. J Pharmacol Toxicol Methods 2010;61:205-9.
- Tung JT, Venta PJ, Caron JP. Inducible nitric oxide expression in equine articular chondrocytes: effects of anti-inflammatory compounds. Osteoarthritis and Cartilage 2002;10:5-12.
- Liacini A, Sylvester J, Qing Li W, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-α is mediated by MAP kinases, AP-1, and NF-κB transcription factors in articular chondrocytes. Exp Cell Res 2003;288:208-17.
- Huang CY, Hung LF, Liang CC, et al. COX-2 and iNOS are critical in advanced glycation end product-activated chondrocytes in vitro. Eur J Clin Invest 2009;39:417-28.
- Abramson SB. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage 2008;16[2]:15-20.
- Loeser RF, Erickson EA, Long DL. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr Opin Rheumatol 2008;20:581-6.
- Medicherla S, Ma JY, Mangadu R, et al. A selective p38 alpha mitogen-activated protein kinase inhibitor reverses cartilage and bone destruction in mice with collagen-induced arthritis. J Pharmacol Exp Ther 2006;318:132-41.
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 2012;92:689-737.
- Furumatsu T, Matsumoto-Ogawa E, Tanaka T, et al. Rock inhibition enhances aggrecan deposition and suppresses matrix metalloproteinase-3 production in human articular chondrocytes. Connect Tissue Res 2014;55:89-95.
- Takaishi H, Kimura T, Dalal S, et al. Joint diseases and matrix metalloproteinases: a role for MMP-13. Curr Pharm Biotechnol 2008;9:47-54.
- Svineng G, Ravuri C, Rikardsen O, et al. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res 2008;49:197-202.
- Sariahmetoglu M, Crawford BD, Leon H, et al. Regulation of matrix metalloproteinase-2 [MMP-2] activity by phosphorylation. Faseb J 2007;21:2486-95.
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 2000;1477:267-83.
- Lijnen HR. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb Haemost 2001;86:324-33.
- Arner EC. Aggrecanase-mediated cartilage degradation. Current Opinion in Pharmacology 2002;2:322-9.
- Flannery CR, Zeng W, Corcoran C, et al. Autocatalytic cleavage of ADAMTS-4 [Aggrecanase-1] reveals multiple glycosaminoglycan-binding sites. J Biol Chem 2002;277: 42775-80.
- Hiran TS, Moulton PJ, Hancock JT. Detection of superoxide and NADPH oxidase in porcine articular chondrocytes. Free Radic Biol Med 1997;23:736-43.
- Sasaki K, Hattori T, Fujisawa T, et al. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem 1998;123:431-9.
- Stevens AL, Wheeler CA, Tannenbaum SR, et al. Nitric oxide enhances aggrecan degradation by aggrecanase in response to TNF-alpha but not IL-1beta treatment at a post-transcriptional level in bovine cartilage explants. Osteoarthritis Cartilage 2008;16:489-97.
- Van't Hof RJ, Hocking L, Wright PK, et al. Nitric oxide is a mediator of apoptosis in the rheumatoid joint. Rheumatology [Oxford] 2000;39:1004-8.
- Thomas CM, Fuller CJ, Whittles CE, et al. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage 2007;15:27-34.
- Luyten F, Yanagishits Y, Vukicevic S, et al. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem 1991;287:4.
- Gross SS, Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem 1992;267:25722-9.
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173-7.
- Frankowski H, Gu Y, Heo J, et al. Use of gel zymography to examine matrix metalloproteinase [gelatinase] expression in brain tissue or in primary glial cultures. Methods Mol Biol 2012;814:221-33.
- Al Kholaifi A, Amer A, Jeffery B, et al. Species-Specific kinetics and zonation of hepatic DNA synthesis induced by ligands of PPARa. Toxicol Sci 2008;104:74–85.
- Yang L, Guo A, Gu JC. c-Jun N-terminal kinase and nuclear factor kappaB mediate nitric oxide-induced expression of matrix metalloproteinase-13. Int Orthop 2011;35:1261-6.
- Studer RK, Levicoff E, Georgescu H, et al. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRbeta tyrosine phosphorylation. Am J Physiol Cell Physiol 2000;279:C961-9.
- Xia W, Szomor Z, Wang Y, et al. Nitric oxide enhances collagen synthesis in cultured human tendon cells. J Orthop Res 2006;24:159-72.
- Zaragoza C, Balbin M, Lopez-Otin C, et al. Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int 2002;61:804-8.
- Bird JL, May S, Bayliss MT. Nitric oxide inhibits aggrecan degradation in explant cultures of equine articular cartilage. Equine Vet 2000;32:133-9.
- Stefanovic-Racic M, Morales TI, Taskiran D, et al. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. J Immunol 1996;156:1213-20.
- Reynaert NL, Ckless K, Korn SH, et al. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A 2004;101:8945-50.
- Mendes AF, Caramona MM, Carvalho AP, et al. Role of mitogen-activated protein kinases and tyrosine kinases on IL-1-Induced NF-kappaB activation and iNOS expression in bovine articular chondrocytes. Nitric Oxide 2002;6:35-44.
- Blais V, Rivest S. Inhibitory action of nitric oxide on circulating tumor necrosis factor-induced NF-kappaB activity and COX-2 transcription in the endothelium of the brain capillaries. J Neuropathol Exp Neurol 2001;60:893-905.
- Fan Z, Soder S, Oehler S, et al. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am J Pathol 2007;171:938-46.
- Appleton CT, Usmani SE, Mort JS, et al. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab Invest 2010;90: 20-30.
- Abraham RZ, Kobzik L, Moody MR, et al. Cyclic GMP is a second messenger by which nitric oxide inhibits diaphragm contraction. Comp Biochem Physiol A Mol Integr Physiol 1998;119:177-83.
- Jackson M, Moradi B, Smith M, et al. Activation of matrix metalloproteinases 2, 9, and 13 by activated protein C in human osteoarthritic cartilage chondrocytes. Arthritis and rheumatology 2014;66[6]:1525-36.
- Sun HY, Hu KZ, Yin ZS. Inhibition of the p38-MAPK signaling pathway suppresses the apoptosis and expressin of proinflammatory cytokines in human osteoarthritis chondrocytes. Cytokine 2017;90:135-43.
- Rabkin SW, Klassen SS, Tsang MY. Sodium nitroprusside activates p38 mitogen activated protein kinase through a cGMP/PKG independent mechanism. Life Sciences 2007;81:640-6.
- Badger AM, Cook MN, Lark MW, et al. SB 203580 inhibits p38 mitogen-activated protein kinase, nitric oxide production, and inducible nitric oxide synthase in bovine cartilage-derived chondrocytes. J Immunol 1998;161:467-73.
- Sunico CR, Gonzalez-Forero D, Dominguez G, et al. Nitric oxide induces pathological synapse loss by a protein kinase G-, Rho kinase-dependent mechanism preceded by myosin light chain phosphorylation. J Neurosci 2010;30:973-84.
- Prince D, Greisberg J. Nitric oxide-associated chondrocyte apoptosis in trama patients after high-energy lower extremity intra-articular fractures. J Orthopaed Traumatol 2015;16:335-41.
- Asada S, Fukuda K, Nishisaka F, et al. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 2001;50:19-23.
- Matsumoto E, Furumatsu T, Kanazawa T, et al. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun 2012;420: 124-9.
- Sumer EU, Sondergaard BC, Rousseau JC, et al. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis and Cartilage 2007;15:212-21.
- Price JS, Wang-Weigand S, Bohne R, et al. Retinoic acid-induced type II collagen degradation does not correlate with matrix metalloproteinase activity in cartilage explant cultures. Arthritis Rheum 199;42:137-47.
- Jimenez MJ, Balbin M, Alvarez J, et al. A regulatory cascade involving retinoic acid, Cbfa1, and matrix metalloproteinases is coupled to the development of a process of perichondrial invasion and osteogenic differentiation during bone formation. J Cell Biol 2001;155:1333-44.
- Lakey RL, Cawston TE. Sulfasalazine blocks the release of proteoglycan and collagen from cytokine stimulated cartilage and down-regulates metalloproteinases. Rheumatology [Oxford] 2009;48:1208-12.
- Bian L, Lima EG, Angione SL, et al. Mechanical and biochemical characterization of cartilage explants in serum-free culture. J Biomech 2008;41:1153-9.
- Akmal A, Hasan M. Role of nitric oxide in management of acute respiratory distress syndrome. Ann Thorac Med. 2008;3[3]:100–3.
- Besco R, Sureda A, Tur J, et al. The effect of nitric-oxide-related supplements on human performance. 2012;42[2]:99-117.