Nodal, Haematological, Bicameral: Lymphoblastic lymphoma
Received: 26-Oct-2018 Accepted Date: Nov 02, 2018; Published: 12-Nov-2018
Citation: Bajaj A. Nodal, Haematological, Bicameral: Lymphoblastic Lymphoma. J Blood Disord Treat. 2018;1(2):21-27.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
An exceptional and aggressive neoplasm with a 2% estimated prevalence of adult Non Hodgkin’s lymphoma is the precursor T cell lymphoblastic lymphoma (T cell LBL). It frequently presents as a cervical, supraclavicular or an axillary lymph node enlargement with a mediastinal tumour in young adults. The Ï«á´½ T cell Acute Lymphoblastic Leukaemia (ALL) roughly exhibits 9%-12% of acute T cell leukaemia in children and adults. The monomorphic dissemination and proliferation of malignant lymphoblast depict a cytology of a medium sized cell with minimal cytoplasma delicate, fine, well dispersed chromatin, a convoluted nuclear membrane and miniature, distinct nucleoli. Lymphoblastic lymphoma is characteristically restricted to the thymic dependent para-cortical zones of the lymph nodes The lymphoma demonstrates pan T cell antigens CD1a, CD2+,CD5+,CD7+, cytoplasmic CD3+ (cyCD3) , CD43+(1,3) and CD71+(transferring receptor antigen) on immune- histochemistry. Chromosomal translocations recognized in T cell lymphoblastic lymphoma are within the alpha and delta T Cell Receptor (TCR) loci situated at chromosome 14q11.2, the beta locus located at chromosome 7q35 and the gamma locus visualized at chromosome 7p14-15 along with partner genes MYC, TAL1, RBTN1,RBTN2, HOX, TAL1, LMO2, FBXW7, NOTCH1, CDKN2A, IL7R, PHF6 TLX1, WT1, MYB & PTEN genes. A whole body Computerized Tomography (CT) scan of head & neck, thorax, abdomen and pelvis or a positron emission tomography (PET-CT) scan is mandated for disease staging and assessment of therapeutic response. An unfavourable outcome in adults is seen with a) age >30 years. b) locally advanced, progressive disease stage III or IV. c) elevated values of serum Lactate Dehydrogensae (LDH) beyond 1.5 times the upper normal limit. d) a central nervous system incrimination. e) malignant cells infiltrating the bone marrow or mediastinum. Distinct therapeutic phases elucidated are the Induction, Consolidation and Maintenance phase.
Keywords
Lymphoblastic lymphoma; Lymphoblastic Leukaemia; T cell neoplasm.
Precursor T cell Lymphoblastic Lymphoma (T LBL) may be designated as an exceptional and aggressive neoplasm with a prevalence of an estimated 2% of adult Non Hodgkin’s lymphoma [1]. Engendered from the T cell lymphoblast, it frequently implicates young adults who depict a cervical, supraclavicular or an axillary lymph node enlargement along with enormous mediastinal tumour aggregates [1,2]. Superior vena cava syndrome or tracheal obstruction with pleural and/or pericardial effusion may accompany the neoplasm [2,3]. Extra nodal disease of the skin, testis, liver, spleen or the bone may infrequently coexist. The central nervous system may be implicated concomitantly with the bone marrow lesions. The contemporarily classified Gamma Delta T cell neoplasm may be characterized by the elucidation of γδ T Cell Receptor (TCR) within the tumour cells. The γδ T cell Acute Lymphoblastic Leukaemia (ALL) roughly exhibits 9%-12% of acute T cell leukaemia in children and adults. Generally a γδ T cell ALL may be similar to the γδ T cell ALL in a majority of clinical and haematological aspects [4,5].
Disease Characterization
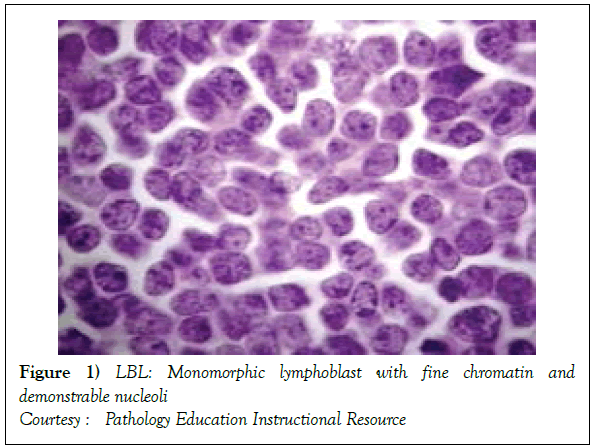
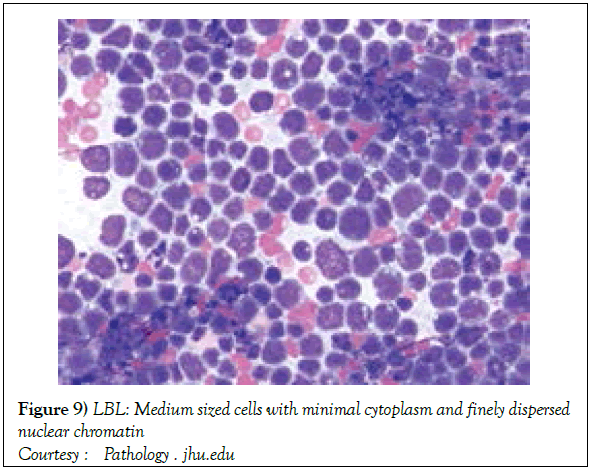
The disorder may preponderantly be confined to the lymph nodes with a usual codification as a Lymphoblastic Lymphoma (LBL). A malignant transformation of T lymphocytes primarily confined to the bone marrow or peripheral blood may be categorized as Acute Lymphoblastic Leukaemia (ALL) [1,3]. Lymphoblastic lymphoma (Figure 1) [6] may chiefly be elucidated in children and adolescents though the disease may manifest in adults. The neoplasm depicts characteristic clinical attributes with an estimated half (50%) the instances demonstrating a mediastinal mass. The tumour may generally be confined to the thymic region (thus a label of a Sternberg sarcoma). In the absence of therapy, the disorder may be particularly aggressive and with accompanying expeditious multisystem dissemination, a peripheral blood elucidation of Acute Lymphoblastic Leukaemia (ALL) like picture and a termination or demise within a brief duration [2,3]
Tumour Morphology
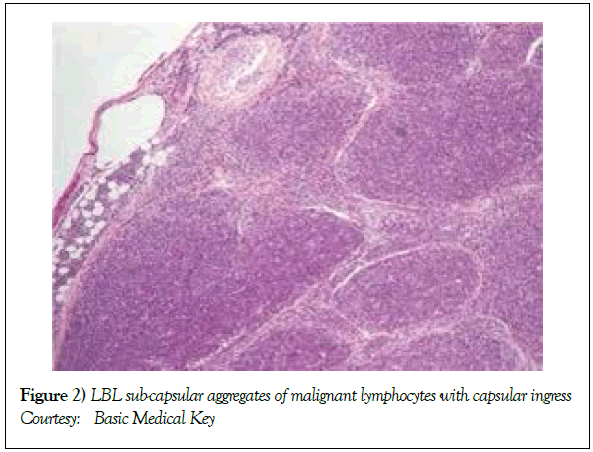
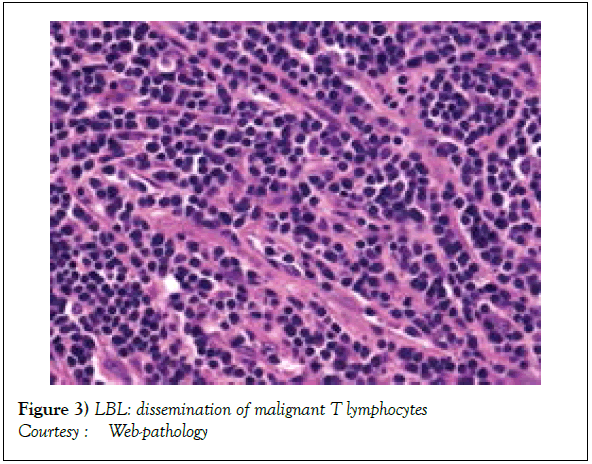
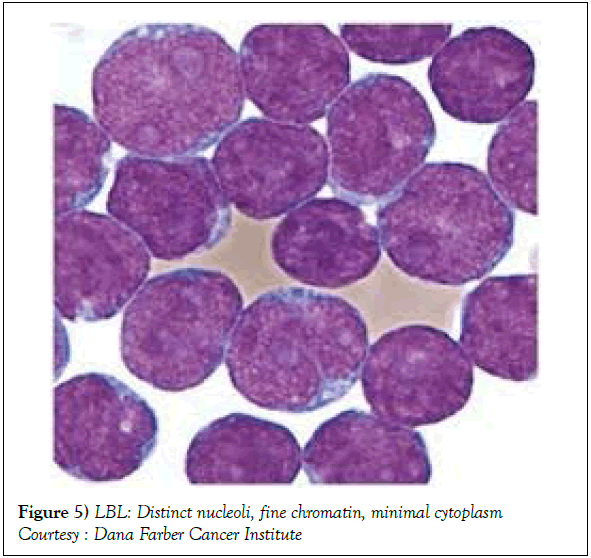
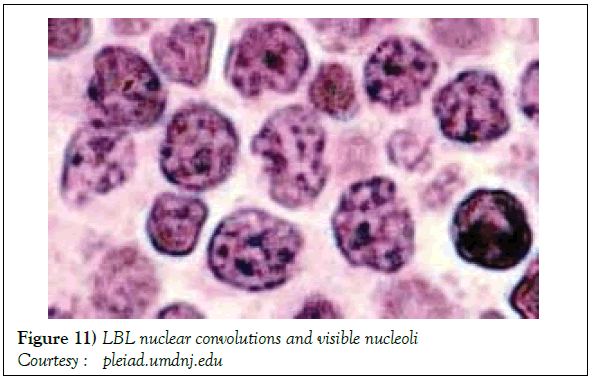
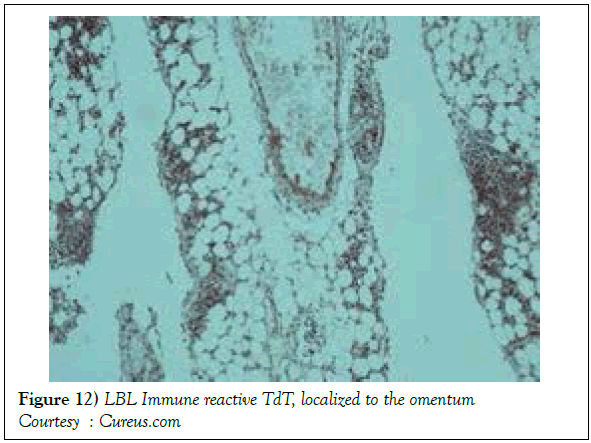
On gross examination, the tumour may display a whitish external surface, be soft in consistency and may demonstrate foci of haemorrhage and necrosis [7]. On microscopic elucidation, the neoplasm may delineate a monomorphic proliferation and dissemination of malignant lymphocytes. Certain instances of malignant lymphoid proliferation may be interspersed with numerous mitotic figures, thus exemplifying a focal starry sky configuration, a feature delineated with adjunctive malignant lymphomas such as Burkitt’s Lymphoma. The tumor may expand beyond the confines of a lymph node or thymus in order to diffusely infiltrate the circumscribing adipose tissue. The malignant lymphocytes may infiltrate the incorporated blood vessel wall in a targetoid manner. A cytological evaluation of the malignant lymphoblast may exhibit a medium sized cell with a delicate, fine, well dispersed chromatin, a convoluted nuclear membrane and miniature, distinct nucleoli [7]. The tumour cells may cogitate a minimal cytoplasm, a spherically outlined nucleus (in contrast to an angulated nucleus of a follicular lymphoma) with delicate convolutions originating from numerous miniature invaginations of the nuclear membrane (Figure 2) [8]. Thin sections of formalin fixed paraffin embedded tissue examined under an oil immersion lens may display the aspect in a fraction of the tumor cells. Finely dispersed chromatin may incorporate miniature, visible nucleoli. Innumerable mitotic figures may engender a markedly elevated mitotic count [7]. The convoluted lymphoid cells may be identical to the cerebroid cells of the Mycosis Fungoides- Sezary syndrome, conditions with divergent clinical manifestations. However, the malignant lymphocytes of lymphoblastic lymphoma enunciate a thinner nuclear membrane, an appropriate dispersion of nuclear chromatin with thin, delicate nuclear invaginations. An atypical or a large cell variant of lymphoblastic lymphoma may be exemplified in an estimated 10% of the neoplasm. The mediastinal tumour mass may display remnants of uninvolved thymic tissue, which may engender a mistaken diagnosis of a thymoma (thymoma rarely manifests in children and depicts a congregation of miniature or activated lymphocytes instead of convoluted malignant lymphoid cells) [7,9]. Lymphoblastic lymphoma may characteristically be restricted to the thymic dependent para-cortical zones of the lymph nodes. The enzyme histochemistry of lymphoblastic lymphoma may elucidate a β glucuronidase, ᾳ napthyl acetate esterase enzyme along with an indicator of thymocyte presence Terminal deoxynucleotidyl Transferase (TdT) (Figure 3) [10] and a chiefly paranuclear demonstration of acid phosphatase [7]. Immune histochemical elucidation of TdT may be possible on formalin fixed, paraffin embedded sections. The majority (80%-85%) of lymphoblastic lymphoma may enunciate T cell immune markers with reiteration of various phases of intra-thymic T cell differentiation such as the appearance of precursor T lymphoblast. Thus the World Health Organization (WHO) may designate the neoplasm as a precursor T cell lymphoblastic lymphoma ( T- LBL) with a concordant precursor T cell Acute Lymphoblastic Leukaemia (TALL) arising within the bone marrow and peripheral blood [4,11]. The appearance of Terminal deoxynucleotidyl Transferase (TdT), an enzyme characteristic of precursor T lymphoid cells may be considered as a categorical immune histochemical indicator of lymphoblastic lymphoma. A majority (90%) of the instances depict pan T cell antigens such as CD1a, CD2+,CD5+,CD7+, cytoplasmic CD3+ (cyCD3) and CD43+ [1,7]. A CD71+ (transferring receptor antigen) may be comprehensively delineated by the lymphoma (100%), an estimated 20% may exemplify the HLA DR cell surface receptor and approximately 20% instances demonstrate markers of Natural Killer (NK) lymphocytes such as CD16+ & CD57+ [7]. The immune marker CD99+ may be consistently reactive. An estimated one third (33%) of chromosomal translocations may be recognized within the alpha and delta T Cell Receptor (TCR) loci situated at chromosome 14q11.2, the beta locus located at chromosome 7q35 and the gamma locus visualized at chromosome 7p14-15, in concordance with several partner genes (MYC, TAL1, RBTN1, RBTN2 and HOX11). The specified chromosomal translocations may engender the dys-regulated transcription of various partner genes [7,12]. Approximately one fifth (15%-20%) of the lymphoblastic lymphoma cells may exhibit a B lymphocyte instead of a T lymphocyte immune marker profile such as the exemplification of CD19+, CD20+, CD21+ and CD24+ [7]. WHO may designate the particular instances as a precursor B cell lymphoblastic lymphoma or precursor B cell acute lymphoblastic leukaemia with bone marrow and peripheral blood manifestations [4,13]. Besides, the B cell malignant variants may specifically emerge as extra-nodal tumours with a minimal predilection for conversion into leukaemia. The tumour cells may exemplify a cytoplasmic immunoglobulin with a lack of surface immunoglobulin. Lymphoblastic lymphomas may demonstrate attributes of a T cell LBL concordant with a B cell LBL. Lymphoblastic lymphoma mandates a demarcation from a Mediastinal thymoma or a neoplasm with a round cell histology such as an Ewing’s sarcoma/Peripheral neuroectodermal tumour/Burkitt’s Lymphoma and the blastoid variant of Mantle cell lymphoma [7].
Immune Phenotypes In Lymphoblastic Lymphoma
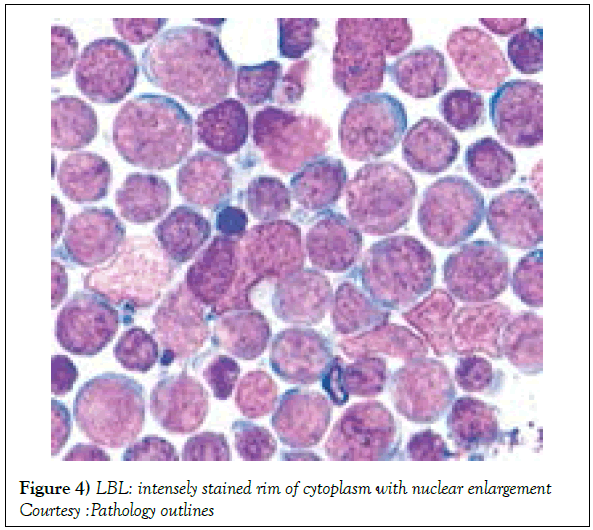
The range of preliminary antigenic evaluation may extend to CD45 (LCA), CD3, CD2, CD5, CD7, Terminal deoxynucleotidyl Transferase (TdT), CD1a, CD10, CD19, CD20, CD79a, kappa/lambda light chains of surface immunoglobulin, CD13 , CD33, myeloperoxidase. CD34, CD45 and TdT, though the B cell and myeloid markers may not be elucidated [1,3]. The immune phenotype of lymphoblastic T cell lymphoma/ leukaemia may elucidate the T cell antigens as categorized within the pro T lymphoblast, pre T lymphoblast, cortical (thymic) and mature T lymphocytes stages. Acute Lymphoblastic Leukaemia (ALL) may delineate a cyCD3 located within the cytoplasm and an sCD3 situated on the cellular surface [12,13] Immune reactive combinations of lymphoblastic lymphoma may be delineated as CD4-/CD8-, CD4+/CD8+, CD4+/CD8- or CD4-/CD8+ [1,6]. Cytoplasmic CD3 (cyCD3) may be considered as a consistent T cell marker for the maturation of T lymphocytes (Figures 4 and 5) [14,15]. The immune molecules exemplified as per the progressive maturation stage of T lymphocytes may incorporate specific markers such as the pro T (CD7+), pre T (CD2+ and/or CD5+ and /or CD8+) , cortical T (CD1a+) and medullary T( surface CD3+ and CD1a-) subtypes, which may be demonstrable with variants of T cell acute lymphoblastic leukaemia [1,7]. In contrast to ᾳ β T cell ALL, the ϫᴽ T cell ALL may elucidate haemoglobin values which decline in children, a frequently enlarged spleen and a marked leucocytosis in adults along with an elevated CD45RA-/CD45RO+ immune phenotype, detected in the children and adults [5,11]. Though the ϫᴽ T cell ALL may commonly demonstrate the T Cell Receptor (TCR) gamma and TCR delta genetic rearrangements, the TCR beta chain and the dual , clone specific recombination of the vᴽ1 and Vᴽ2 fractions may also be enunciated.
Genetic Modifications
The T cell acute lymphoblastic lymphoma/leukaemia may depict chromosomal mutations in the TAL1, LMO2, FBXW7, NOTCH1, CDKN2A, IL7R, PHF6 TLX1, WT1, MYB & PTEN genes [1]. The JAK/STAT signalling cascade may be de-regulated in T cell Acute Lymphoblastic Lymphoma (T cell ALL). The IL7R may alter the IL7Rᾳ along with the cᶌ molecules located on the cellular membrane. The complex may activate the Janus Kinases (JAK) pathway comprising of JAK1/JAK3 along with PTPN2 genes and the signal transducer of activation (STATS) network. The combination pathway may assist the modulation of RAS, HSP90, MEK and ERK which further induces the T cell translation and propagation [11]. The activations of STATS pathway may concurrently, initiate the IL7Rᾳ via the BCL2, ICN, MAML and ZEB2 genes along with the cooperation of nuclear STAT5. Adjunctive mechanisms of mobilizing NOTCH1 via the cell membrane situated ICN molecules may concomitantly energize the nuclear MAML2 to simultaneously induce the IL7Rᾳ protein [1]. The membrane localized PI3K may additionally synergize the PIP2, PIP3, PTEN markers in order to activate the PDK1 and AKT genes. Concordance of mTOR with the aforementioned pathway may engender the malignant translation, multiplication and extended survival of T lymphocytes (Figure 4). The supplementary appearance of DNM2 gene along with the Clathrin protein may promote endocytosis within the cellular membrane [1]. Apart from the genetic elucidation as discussed, the BCL2 and BCLXL genes may augment the cellular survival or induce single cell apoptosis. Contributions of nuclear proteins such as NUP214 in conjunction with ABL1 may manifest [1]. The oncogenic eventualities may incorporate genetic rearrangements of the TAL1, TLX1, TLX3, HOXA, NKX and LMO2/ LYLl1 pathways which may activate the NOTCH1/FBXW7 network with the additional mobilization of CDKN2A network [1]. Supplementary elucidation of the JAK3/IL7R/STAT5B and PRC2 fusion proteins in conjunction with NUP214, ABL1, PTPN2 and BCL11B/PHF6 may augment the genetic instability. Genetic alterations situated within the WT1 chromosome with concomitant deletions of the PTEN/PI3K 6q chromosomal fractions may be delineated (Figure 5).
Radiological Investigations
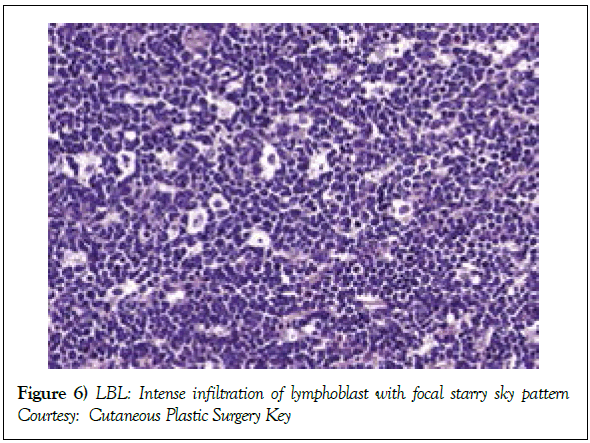
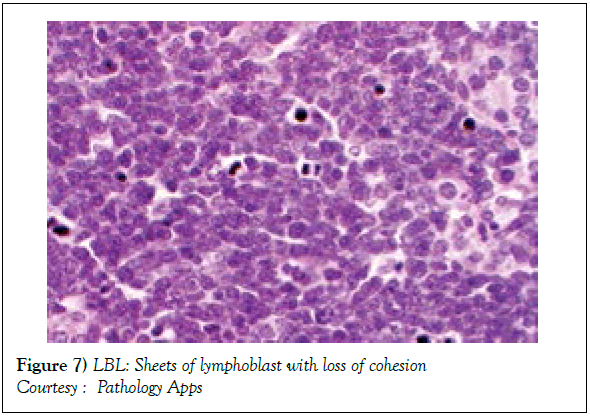
Modalities of imaging such as a whole body Computerized Tomography (CT) scan of the head & neck, thorax, abdomen and pelvis or a Positron Emission Tomography (PET-CT) scan may be mandated for suiTable disease staging and assessment of therapeutic response [2]. Response evaluation following induction of chemotherapy may require a Computerized Tomography (CT) scan, particularly for assessing the occurrence and volume of intra thoracic and abdominal disease (Table 1). Assessment of mediastinal disease may be challenging with the emergence of a T cell large granular lymphoblastic lymphoma (T LBL) variant. A residual or relapsing mass may emerge and persist in spite of adequate therapy. Majority of the T cell large granular lymphoblastic (LBL) lymphoma (Figures 6 and 7) may demonstrate an avidity for 18 Fluoro-deoxy Glucose during a positron Emission Tomography (FDG PETCT) scan [2]. PET CT may be a pre-requisite prior to the initiation of therapy [7,8,12]. Although a PET CT may not indicate long term therapeutic outcome of the disease [10], an absence of characteristic features of disease resolution on a PET CT may eliminate the requirement of a chemo-therapeutic augmentation or a mediastinal irradiation [2].
Figure 6: LBL: Intense infiltration of lymphoblast with focal starry sky pattern
Courtesy: Cutaneous Plastic Surgery Key
Figure 7: LBL: Sheets of lymphoblast with loss of cohesion
Courtesy : Pathology Apps
| Name | cyCD3 | CD7 | CD5 | CD2 | CD1a | sCD3 | CD34 |
|---|---|---|---|---|---|---|---|
| Pro –T | + | + | -- | -- | -- | -- | +/- |
| Pre -T | + | + | + | + | -- | -- | +/- |
| Cortical (thymic) T lymphocytes | + | + | +/- | +/- | + | +/- | -- |
| Mature T lymphocytes | + | + | +/- | + | -- | + | -- |
Table 1: Antigenic elucidation with stages of T cell ALL (Acute Lymphoblastic Leukemia)
Prognostic Determinants
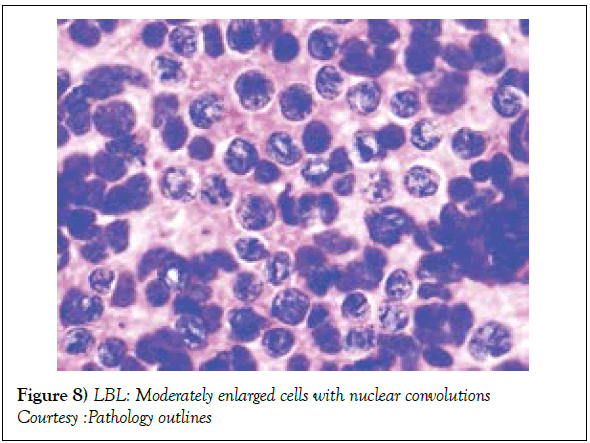
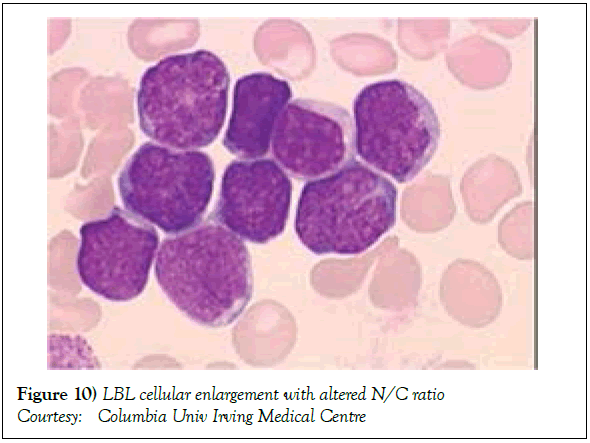
Risk factors implying an unfavourable outcome in adult patients may incorporate: a) age>30 years. b) advanced, progressive disease stage III or IV. c) elevated values of serum Lactate Dehydrogensae (LDH ) beyond 1.5 times the upper normal limit. d) a central nervous system incrimination. e) malignant cell infiltration of the bone marrow or mediastinum [1,2]. Individuals depicting one or more of the aforementioned features with an inferior prognosis may mandate an intense chemotherapeutic induction/ regimen with a Haematopoietic Stem Cell Transplant (HCT) [2]. The Event Free Survival (EFS) and Overall Survival (OS) of individuals with T Cell Lymphoblastic Lymphoma (T LBL) (Figures 8 and 9) with a preceding HCT may be superior, in contrast to patients on singular chemotherapeutic regimens. Following a haematopoietic stem cell transplant, a reoccurring disease may be the primary cause of mortality along with features such as superimposed infections, Graft Versus Host (GVH) disease, respiratory, cardiovascular and associated complications, secondary to chemotherapy [2]. Early T Precursor (ETP) cell acute lymphoblastic lymphoma/leukaemia elucidating stem cell or myeloid immune markers may depict inferior prognostic responses, in contrast to associated forms of T cell acute lymphoblastic lymphoma/leukaemia [1,2].
Figure 8: LBL: Moderately enlarged cells with nuclear convolutions
Courtesy :Pathology outlines
Therapeutic Protocols
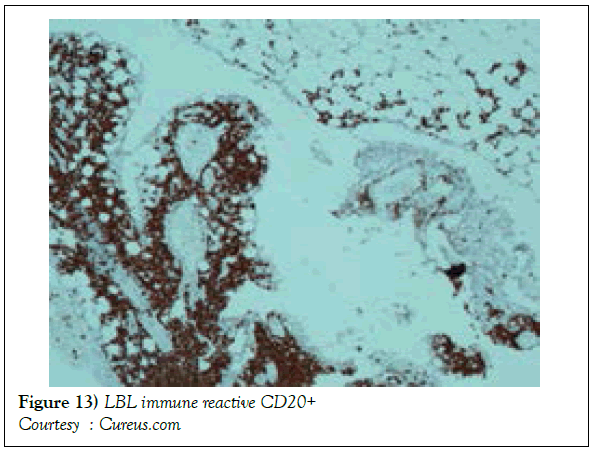
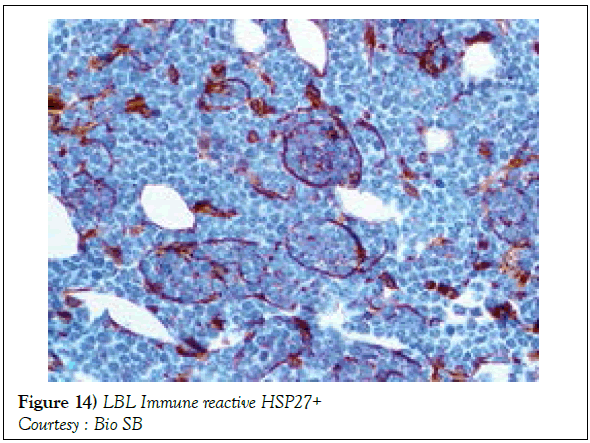
A definitive therapeutic protocol for alleviating the T cell acute lymphoblastic lymphoma/leukaemia may currently be lacking, though several combinations may be employed. The chief objective of therapy may be to achieve and maintain a Complete Remission (CR) (Figure 10). The perceived treatment may enunciate distinct phases such as the Induction, Consolidation and Maintenance phase [1]. Numerous therapeutic options may be available for approaching a T cell large granular lymphoblastic lymphoma (T LBL), particularly the intense chemotherapeutic protocols applicable in the aggressive, high grade Non-Hodgkin’s lymphoma, which may be adopted along with a Haematopoietic stem Cell Transplantation (HCT). A Complete Response Rate (CRR) of greater than 80% and a Disease Free Survival Rate (DFS) of roughly 56% may be achieved with adults managed with intensive chemotherapy (regimen suiTable for acute lymphoblastic leukaemia) [1] (Figure 11). Tumour infiltrating the Central Nervous System (CNS) may be administered a prophylaxis comprising of intrathecal methotrexate (IT-MTX) amidst the induction and consolidation phase. A consolidation mode of therapy extending to several months followed by a maintenance stage lasting roughly two to three years may be considered as an appropriate management of the neoplasm (Figure 12). The paediatric protocols of intensive chemotherapy and CNS prophylaxis may be adopted and may deliver a superior prognosis with adults [1]. Multitudinous chemotherapy or radiotherapy regimens may be identical with the dosage and administration frequency to those employed for acute lymphoblastic leukaemia. The therapeutic simulations may mandate an induction therapy, a CNS prophylaxis (Figures 13 and 14) and a consolidation protocol followed by suiTable maintenance doses for a period of 12-18 months. A Disease Free Survival (DFS) of estimated 40%-70% may be achieved [1,2]. Supportive therapy administered for acute lymphoblastic lymphoma may include: a) allopurinol during the first ten days of induction therapy in order to reduce the incidence of hyper-uricaemia. b) Prophylaxis including antibiotics with anti- viral agents and to eradicate microbes inducing pneumonia such as Pneumocystis jiroveci (Table 2). The administration of the aforementioned prophylaxis may be mandated during the entire chemotherapeutic schedule. c) employment of antifungal drugs in order to prevent the configuration of moulds may be necessitated in the induction phase. d) Asparaginase related toxicities (appearing in B cell acute lymphoblastic leukaemia) or hyper sensitivity reaction may arise in 20% of children and adults and may necessitate appropriate management [1].
| Disease Subtype | Immune phenotype |
|---|---|
| T-cell lineage | TdT+, cyCD3+,CD7+ |
| Early T lymphocyte | CD2-. sCD3-, CD1a- |
| Thymic T lymphocyte | sCD3+/-, Cd1a+ |
| Mature T lymphocyte | sCD3+, Cd1a- |
Table 2: Immune phenotypes in T cell ALL
Contemporary Therapeutic Agent
Nelarabine as a contemporary molecule may be employed for treating T cell acute lymphoblastic lymphoma/leukaemia. The mechanism of action of the arabino-nucleoside may be as an anti-metabolite with anti-tumour like function. The metabolized end product ara-G may inhibit the cellular DNA synthesis (Figure 15), thereby inducing apoptosis. A Complete Response (CR) to therapy may be attained with Nelarabine. The drug may be beneficial in children and adults beneath 21 years of age [1]. The administration of Nelarabine may induce “Adverse Events” (AEs-46%) and treatment related adverse events (21%). Infrequent haematological adverse events with milder forms of haematological AEs may be elucidated. Neurological Adverse Events (AEs) may be defined by sensory alterations in the peripheral and central nervous system. Modification of the mental status, peripheral and central nervous system AEs and uncategorized AEs may be exhibited (Table 3). However, adverse events associated with elimination or withdrawal of treatment may be absent. A median Overall Survival (OS) may not be attained with individuals who respond to the contemporary drug [1]. Therapies for Acute Lymphoblastic Leukaemia (Figures 16) may include the phases of induction, consolidation, intensification, maintenance and CNS prophylaxis. The standard induction chemotherapy incorporates agents such as L asparaginase, vincristine and dexamethasone. Consolidation phase includes agents such as Methotrexate, Etoposide, Ifosfamide Imatinib, Filgrastim, Intrathecal, Methotrexate, Intrathecal Hydrocortisone, Intrathecal Cytarabine (Table 4). The intensification stage involves intrathecal Methotrexate, Intrathecal hydrocortisone, Intrathecal cytarabine along with Imatinib, Leucovorin, Cyclophosphamide, Mesna, Filgrastim, Asparaginase and Etoposide. Maintenance stage mandates the employment of Intrathecal Methotrexate, Intrathecal Hydrocortisone, Intrathecal Cytarabine besides agents such as Vincristine, Methotrexate, Dexamethasone, Imatinib Leucovorin, Mercaptopurine, Etoposide, Cyclophosphamide and Filgrastim apart from cranial irradiation, 6 Mercaptopurine (Table 5, 6).
| Characteristic aspects | T ALL | T LBL |
|---|---|---|
| Median age of disease appearance | 30 years | 25 years |
| Male sex | 70 % | 73 % |
| Mediastinal tumour | 66 % | 91 % |
| Pleural effusion | 1 % | 40 % |
| Central nervous system | 7 % | 0-10 % |
| Bone marrow involvement | 100 % | 0-23 % |
GMALL: German Multicentre study group for Adult T ALL
Table 3: Clinical Attributes in Adult T cell ALL/ T cell LBL ( GMALL outcomes)
| Regimen | Response rate | Failure free survival | Overall survival |
|---|---|---|---|
| Two ALL type protocols with intensified CNS regimen | 100% | 3 year FFS 56% | NA |
| Diverse ALL protocols | 80% CR for “Non leukemic” . 77% CR for leukemic individuals | NA | 5 year actual OS 45% |
| Diverse ALL protocols | 77% OR | 3 year RFS 45% | 3 year OS 59% |
| Modified LSA2L2 | 73% CR , 27% PR | 5 year actual FFS 35% | 5 year actual OS 40% |
| APO | 95% CR | 3 year actual FFS 58% | 5 year actual OS 69% |
| Two types of ALL protocols incorporating regimens for CNS disease. | 93% CR | 7 year actual DFS 62% | 7 year actual OS 51% |
| Hyper CVAD | 91% | 3 year PFS 66% | 3 year OS 70% |
| LMT89 (ALL type Induction regimen) | 85% OR | 5 year FFP 44% | 5 year OS 63% |
| “Hybrid” NHL/ALL regimen | 100% OR | 4 year EFS 68% | 4 year OS 72% |
APO: Doxorubicine Prednisone Vincristine; CR: Complete Response; EFS; Event Free Survival; FFP: Freedom From Progression; FFS: Failure Free Survival; Hyper CVAD: Hyper-Fractionated Cyclophosphamide, Vincristine, Doxorubicin, Dexamethasone, Cytarabine, Methotrexate; OR: Overall Response; PR: Partial Response; RFS: Relapse Free Survival
Table 4: Intensive induction regimens in Adult T cell LBL
| Therapeutic Outcomes | Disease free survival |
|---|---|
| Autologous CR 1 | 61 % |
| Autologous CR >1 | 47 % |
| Allogeneic CR 1 | 74 % |
| Allogeneic CR >1 | 16 % |
CR 1: First complete remission
Table 5: Stem cell transplantation in adult B /T cell LBL
| Gene | Frequency (%) | Mechanism | Cell Function | Prognosis |
|---|---|---|---|---|
| CDKN2A/2B | 60-70 | 9p21 deletion | Cell cycle alteration | Good |
| NOTCH1 | 60 | Activating Mutation | NOTCH1 pathway | Good |
| PHF6 | 20-40 | Inactivation Mutation | chromatin remodeling | No Impact |
| PTEN | 20 | Inactivation Mutation or 10q23 deletion | Tumor Suppressor | No Impact |
| FBXW7 | 10-20 | Inactivation Mutation | NOTCH1 pathway | Unknown |
| RUNX1 | 10-20 | Inactivation Mutation | Transcription Factor | No Impact |
| EZH2 | 10-15 | Inactivation Mutation | chromatin remodeling | Poor |
| LEF1 | 10-15 | Inactivation Mutation | Transcription Factor | Unknown |
| ETV6 | 13 | Inactivation Mutation | Transcription Factor | Poor: Associated with immature T-ALL cases |
| BCL11b | 10 | Inactivation Mutation | Transcription Factor | No Impact |
| WT1 | 10 | Inactivation Mutation | Transcription Factor | No Impact |
| IL7R | 10 | Activating Mutation | Transcription Factor | No Impact |
| SUZ12 | 10 | Inactivation Mutation | chromatin remodeling | No Impact |
| EED | 10 | Inactivation Mutation | chromatin remodeling | No Impact |
| NRAS | 5-10 | Activating Mutation | Signal Transduction | No Impact |
| JAK1 | 5-10 | Activating Mutation | Signal Transduction | No Impact |
T-ALL: T-acute lymphoblastic leukaemia
T-LBL: T-lymphoblastic lymphoma
Table 6: Recurrent (>5%) Molecular Abnormalities in T-ALL/T-LBL
REFERENCES
- Acute T cell lymphoblastic leukaemia/lymphoma. Lymphoma tumour board. 2017.
- Park HS, McIntosh L, Braschi-Amirfarzan M, et al. T cell Non-Hodgkin’s Lymphomas: Spectrum of disease and role of imaging in the management of common subtypes. Korean J Radiol. 2017;18:71-83.
- Cortelazzo S, Ponzoni M, Ferreri AJM, et al. Lymphoblastic lymphoma. Crit Rev Oncol Haematol. 2011; 79:340-3.
- SH, Campo E, Pileri SA, et al. The 2016 revision of world health organization classification of lymphoid neoplasm. Blood. 2016;127:2375-90.
- Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T cell lymphomas. Nature Reviews Clinical Oncology. 2009; 6:707-12.
- Rosai, Ackerman. Surgical Pathology. Tenth Edition, 1841.
- Yabe M, Medeiros LJ, Tang G, et al. Prognostic factors of hepato-splenic T cell lymphoma: clinicopathologic studies of 28 cases. Am J Surg Pathol. 2016;40:676-88.
- Matlawska-Wasowska K, Kang H, Devidas M, et al. MLL rearrangements impact outcomes in HOXA deregulated T lineage acute lymphoblastic leukaemia: A Children’s Oncology Group Study. Leukaemia. 2016;30:1909-12.
- Otero HJ, Jagannathan JP, Prevedello LM, et al. CT and PET/CT findings of T cell lymphoma. AJR, Am J Roentgenol. 2009;193:349-58.
- Tanaka T, Yamamoto H, Elsayed AA, et al. Clinicopathologic spectrum of gastrointestinal T cell lymphoma reappraisal based on T cell receptor immune-phenotypes. Am J Surg Pathol. 2016;40:777-85.
- Yabe M, Medeiros LJ, Wang SA, et al. Distinguishing between hepato-splenic T cell lymphoma and ṿᴽT cell large granular lymphocytic leukaemia. Am J Surg Pathol. 2017;41:82-93.
- Zheng H, Wang X, Ma Y, et al. The TCR ᶌᴽ repertoire and relative gene expression characteristic of T –ALL cases with biclonal malignant vᴽ1 and Vᴽ2 T cells. DNA Cell Biol. 2014;33:49-56.
- Merrill ED, Agbay R, Miranda RN, et al. Primary cutaneous T cell lymphoma showing gamma-delta (ṿᴽ) phenotype and predominantly epidermotropic pattern are clincopathologically distinct from classic primary cutaneous ṿᴽ T cell lymphomas. Am J Surg Pathol. 2017;41:204-15.
- Wei EX, Leventaki V, Choi JK, et al. ᶌᴽ T cell –acute lymphoblastic leukaemia/ lymphoma: discussion of two paediatric cases and it’s distinction from other mature ᶌᴽ T cell malignancies. Case reports in Haematology. 2017;5873015.
- Lepretre S, Touzart A, Vermeulin T, et al. Paediatric type acute lymphoblastic leukaemia therapy in adults with lymphoblastic leukaemia : the GRALL LYSA LL03 study. J Clin Oncol. 2016;34:572-80.