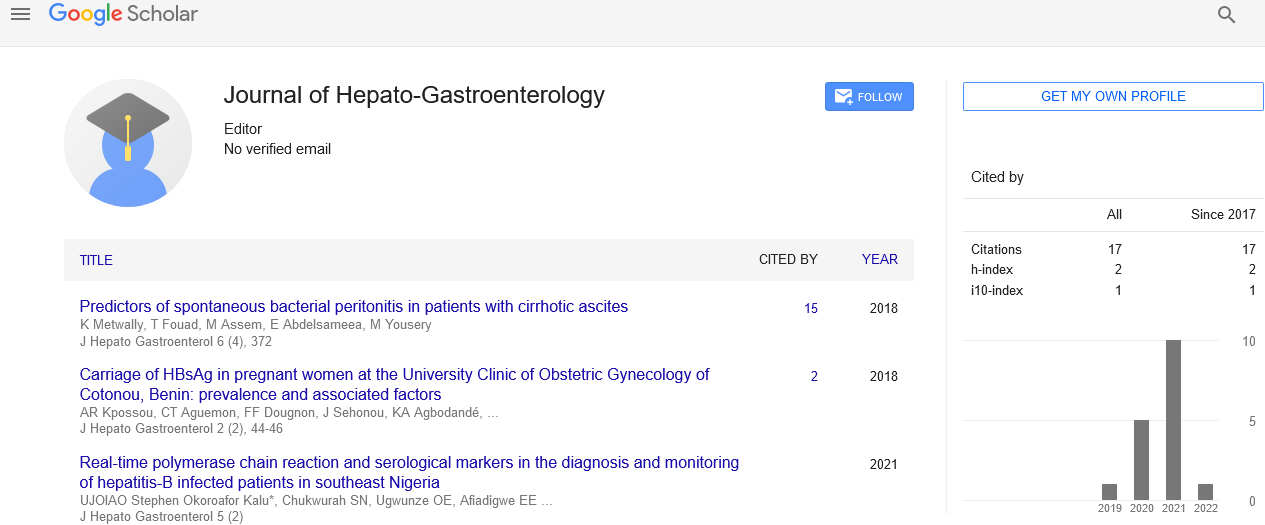
Ombitasvir/paritaprevir/ritonavir plus weight based ribavirin versus ledipasvir/sofosbuvir fixed dose combination in treatment of HCV genotype IV in a patient with idiopathic pulmonary hypertension and renal impairment
Received: 13-Oct-2017 Accepted Date: Oct 13, 2017; Published: 16-Oct-2017
Citation: El-Rahman MIMA. Ombitasvir/paritaprevir/ritonavir plus weight based ribavirin versus ledipasvir/sofosbuvir fixed dose combination in treatment of HCV genotype IV in a patient with idiopathic pulmonary hypertension and renal impairment. J Hepato Gastroenterol 2017;1(1):1.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
In Egypt, genotype 4 is the predominate genotype of HCV. Prevalence of HCV in Egypt ranges from 15% up to 30% in rural areas. Mass treatment of schistosomiasis was implicated in this HCV epidemic that Egypt experiences nowadays. Genotype 4 predominates in a ratio of around 90%.
Treatment of HCV with the new era of DAAD gave a new hope to Egyptian patients. According to the international guidelines Patients infected with HCV genotype 4 can be treated with the fixed-dose combination of ombitasvir, paritaprevir and ritonavir in one single tablet, two tablets given daily with food with weight based ribavirin. The second option available in the Egyptian market is the fixed dose combination of sofosbuvir (400 mg) and ledipasvir (90 mg) in one single tablet administered once daily.
The third option is combining sofosbuvir (400 mg) and Daclatasvir (60 mg) in two separate tablets. The last option available is sofosbuvir (400 mg) together with simeprevir (150 mg). All the above-mentioned treatment options are available only for patients with chronic hepatitis or with compensated cirrhosis for a duration of 12 weeks.
Ombitasvir, paritaprevir and ritonavir or simeprevir are given to child. A patients only, they are not given to patients with decompensated cirrhosis. The other two options addition of weight based ribavirin is done in a case of decompensation.
Ombitasvir, paritaprevir and ritonavir plus ribavirin is the option of choice in case of renal impairment however special precautions are done in this kind of treatment. Any other system uncontrolled decompensate ion is a contraindication. Idiopathic pulmonary arterial hypertension (IPAH) is a condition in which there is an elevated pulmonary artery pressure with no apparent cause.
This is an unusual case that we confronted during the HCV national program of treatment of HCV. A female patient 62-year-old, DM, with mild renal impairment infected with HCV came to the tropical medicine department for the sake of treatment of HCV. In history taken, patient was found to be a relapser to Interferon/Ribavirin 48 weeks.
Initial investigations revealed the following CBC: hemoglobin: 12.6, RBCs: 5.4 million/3 and WBCs 7,900 thousand/cells. Serum albumin 2.8, serum bilirubin (total 0.8 mg/dl, direct 0.2 mg/dl) and a prothrombin activity of 82%. AST 89 IU/l, ALT 45 IU/L, PCR for HCV: 304,030 glycosylated hemoglobin A1c 8 and a serum creatinine of 1.7 mg/dl, Ultrasound revealed early cirrhotic changes. A decision was made to give the patient Ombitasvir/ paritaprevir/ritonavir 2 tablets with food and weight based ribavirin for 12 weeks.
Before initiation of therapy patient had a severe attack of dyspnea upon investigations appeared to be due idiopathic pulmonary hypertension that was treated by sildenafil 50 mg initially then raised to 100 mg/day to alleviant the symptoms. An interaction was found between sildenafil and Ombitasvir/ paritaprevir/ritonavir that we had to shift to sofosbuvir/ledipasvir fixed dose combination with weight based ribavirin for 12 weeks as an alternative treatment protocol, by the end of the first month of treatment, the patient had a negative PCR.
However, recurrence of dyspnea occurred but this time was due to ribavirin associated anemia that aggravated the patient cardiac condition at that time we had to stop ribavirin and maintain ribavirin free for another 2 months. Fortunately, anaemia was corrected and patient achieved end of treatment negative PCR and a sustained virological response 12 weeks and still on follow up.
In conclusion, in an infected patient with HCV genotype IV with idiopathic pulmonary hypertension treated with sildenafil Ombitasvir/paritaprevir/ ritonavir cannot be given as a line of therapy due potential serious drug drug interaction even in the presence of renal impairment. Ledipasivir/sofosbuvir fixed dose combination is an alternative treatment option without ribavirin even in cirrhotic relapses.