Parathyroid adenoma in pregnancy: A case report and systematic review of the literature
2 Department of Surgery, AHEPA University Hospital, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece, Email: chorange2404@gmail.com
3 Department of Obstetrics and Gynecology, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
Received: 03-Jun-2022, Manuscript No. PULJEDS-22-5022; Editor assigned: 06-Jun-2022, Pre QC No. PULJEDS-22-5022 (PQ); Accepted Date: Aug 07, 2022; Reviewed: 20-Jun-2022 QC No. PULJEDS-22-5022 (Q) ; Revised: 03-Aug-2022, Manuscript No. PULJEDS-22-5022 (R); Published: 10-Aug-2022, DOI: 10.37532/puljeds2022.6 (4).34-46
Citation: Ioannis P, Angeliki C, Moisis M, et al.. Parathyroid adenoma in pregnancy: A case report and systematic review of the literature. J Endocrine Disorders & Surgery 2022;6(4):34-46.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Primary hyperparathyroidism is a common disorder of the parathyroid glands. Parathyroid Adenoma (PA) in pregnancy is a relatively rare disease, which diagnosis and treatment is a challenging task. The aim of the present study is to present a new case of parathyroid adenoma during pregnancy and to give a detailed account of all reported cases of PA during pregnancy in the literature.
Methods: A bibliographic research was performed and characteristics of parathyroid adenomas in pregnancy such as age, gestational week at diagnosis, ionized calcium levels, genetic testing result, symptomatology, radiological method of localization, treatment method, gestational week at operation and maternal/fetal complications were recorded.
Results: A 34-years-old woman at her 25 weeks' gestation was diagnosed with parathyroid adenoma and was referred to our surgical department due to contraindication for conservative treatment. A parathyroidectomy was performed and the maternal and fetal postoperative period was uneventful. 211 cases of parathyroid adenoma in pregnancy were recorded in the literature and statistical analysis was performed. Median gestational week at diagnosis was 21 ± 9.61 weeks. Mean level of ionized calcium was 2.69 mmol/L (SD=0.75, (2.55-2.84 95%CI)). Most cases were familiar (72.4%), while surgery was the preferred treatment option (67.3%). The majority of cases were asymptomatic (21.3%) and the main radiological method applied for localization was ultrasound (63.4%) is of great importance as surgical treatment at the second trimester of pregnancy outweighs the maternal and fetal risks.
Conclusion: Parathyroid adenoma in pregnancy is a rare condition. The early diagnosis
Keywords
Parathyroid adenoma; Pregnancy; Endocrine surgery; Parathyroid surgery; Primary hyperparathyroidism
Abbreviations
PTH: Parathormone; PA: Parathyroid Adenoma; PHPT: Primary Hyperparathyroidism; MEN: Multiple Endocrine Neoplasia; FHH: Familiar Hypocalciuric Hypercalcemia
Introduction
Primary Hyperparathyroidism (PHPT) is relatively common disorder of parathyroid glands, causing skeletal, renal and cardiac complications.
As etiological features of PHPT, single adenoma, hyperplasia, carcinoma and familiar causes (MEN, FHH, and Hyperparathyroidism jaw tumor syndrome) are presented [1]. Primary hyperparathyroidism in pregnancy has an incidence of 1%, which is underestimated due to the plethora of undiagnosed cases [2,3]. The main symptomatology may mimic this of pregnancy, although severe maternal and fetal complications occur in untreated hypercalcemia. Mild hyperparathyroidism could be treated conservatively, but in cases of severe or symptomatic hypercalcemia, parathyroidectomy is the treatment of choice.
The aim of the present study is to present a new case of parathyroid adenoma during pregnancy and its diagnostic and therapeutic management. Furthermore, we aimed to give a detailed account of all reported cases of PA during pregnancy in the literature and to analyze the available data.
Case Presentation
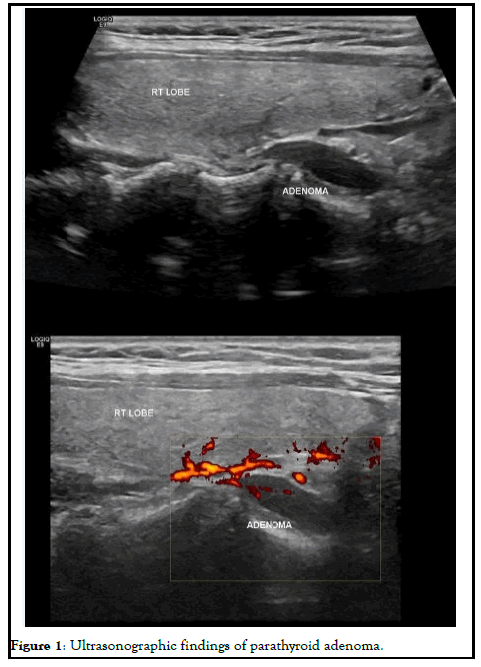
A 34-years-old woman at her 25 weeks' gestation was referred to our department of endocrine surgery due to a parathyroid adenoma diagnosed by her endocrinologist. The main symptom was hyperemesis during her second trimester of pregnancy. It is essential that she was free of symptomatology during her first trimester of pregnancy and hyperemesis of second trimester troubled her gynecologist, who referred her to the endocrinologist. Her past medical history was unremarkable and her family history revealed no evidence of familiar endocrine disease. Her initial laboratory examination revealed high ionized serum calcium 1.47 mmol/L (normal range 1.15-1.33 mmol/L), increased PTH 75.8 pg/ml (normal range 15-65 pg/ml), decreased serum phosphorus 2.4 mg/dl (normal range 2.7-4.5 mg/dl), increased 24 h urine calcium 592 mg/24 h (normal range 100-300 mg/24 h) and decreased vitamin D levels 6.15 ng/dl (normal range >20 ng/ dl). She was not advised for oral supplementation with vitamin D due to hypercalciuria and the risk of renal complications. Cervical ultrasonography revealed a flattened, hypoechoic, solid, homogenous nodule, located under the inferior pole of the right lobe of thyroid gland, on its posterior part and proximal to trachea, measuring 25 × 6.3 × 10 mm, indicative of parathyroid adenoma. The patient was advised about surgical treatment as conservative treatment with vitamin D supplements was not an option and the parathyroid adenoma increased the risk for both preeclampsia and abortion. Her preoperative laboratory results revealed total serum calcium 10.7 mg/dl (normal range 8.5-10.2 mg/dl), serum phosphorus 2.6 mg/dl (normal range 2.6-4.5 mg/dl) and PTH 78.1 (normal range 15-65 mg/dl). She underwent para-thyroidectomy, during which the right superior parathyroid gland was found enlarged and was resected. The intraoperative PTH level at the beginning of the operation and 15 minutes after the specimen removal were 176.9 pg/dl and 30.29 pg/dl, respectively. The post-operative maternal and natal course was uneventful. Chvostek and Trousseau signs were negative. Postoperative laboratory examination 8 hours after the operation was serum calcium 9.4 mg/dl, PTH 5.59 pg/dl and phosphorus 3.3 mg/dl. The patient was treated with intravenous calcium supplementation with 6 amp calcium gluconate (1 amp=1 gr calcium gluconate) in 500 cc Normal saline 0.9% and oral calcium and vitamin D3 supplements (continued upon discharge). The following day her laboratory exams were improved (Ca: 9.0 mg/dl, P: 3.1 mg/dl, PTH: 2.06 pg/dl) and, after the advisory of her endocrinologist and gynecologist, N/S 1000 ml with 10 amp calcium gluconate was administered to the patient in order to avoid hypocalcemia and the possible maternal and fetal complications. The second postoperative day, her laboratory findings were Ca: 9.4 mg/dl, P: 4.7 mg/dl, PTH: 3.71 pg/dl and she was discharged. The histological report concluded to a parathyroid adenoma measuring 2.4 cm and weighting 2.5 gr without evidence of malignancy. During postoperative follow-up period, she had an uneventful vaginal delivery and both mother and the newborn were normocalcemic (Figure 1).
Methodology
A bibliographic research was performed using Medline (Pubmed), Scopus, Embase and Google Scholar. The search terms employed were "parathyroid adenoma" or "primary hyperparathyroidism and "pregnancy" or "postpartum". Since 1953, when the first description of PA in pregnancy was made, 281 articles were published. In the latest 20 years (2000-2021), 141 articles were found. The primary objective of this study was to assess the treatment options in PA during pregnancy regarding maternal and fetal complications. As secondary outcome, symptomatology and diagnostic options were considered. Inclusion criteria were all papers reports cases of PA in pregnancy and their treatment. Exclusion criteria were articles published before 2000 (due to missing data and lack of modern imaging), cases with hyperplasia or secondary hyperparathyroidism or parathyroid carcinoma and reviews of the literature. In total, 93 articles were included in our review.
In order to express results descriptive statistics were used appropriately. Means, medians and standard deviations were used for continuous variables [4]. The normal distribution of quantitative data has been checked using Kolmogorov Smirnov test. Non-parametric tests (Mann-Whitney U test) have been applied for the non-normal distribution data. Ttest was used for normal distributed data. Chi-square test has been applied for the data correlated. ROC curve has been calculated for the detection of a cutoff point level. Excel 2007 and SPSS 22.0 were employed to statistically analyze the data.
Results
Characteristics of PAs were determined concerning age, gestational week at diagnosis, ionized calcium levels, genetic testing result, symptomatology, radiological method of localization, treatment method, gestational week at operation and maternal/fetal complications. In the literature searched, 211 cases of parathyroid adenoma in pregnancy were recorded.
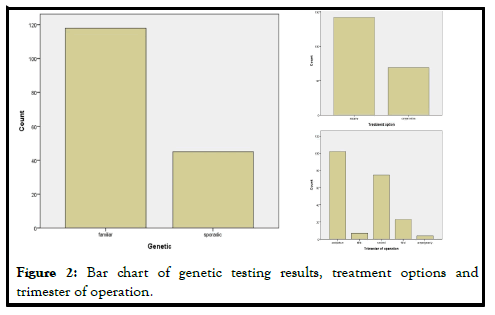
Mean age of pregnant women was 30.85 ± 5.57 years old, ranging from 18 to 43 years old. Median gestational week at diagnosis was 21 ± 9.61 weeks. Mean level of ionized calcium was 2.69 mmol/L (SD=0.75, (2.55-2.84 95%CI)). The genetic testing results showed that 118/163 (72.4%) of cases were familiar, while 45/163 (27.6%) were sporadic (Figure 2).
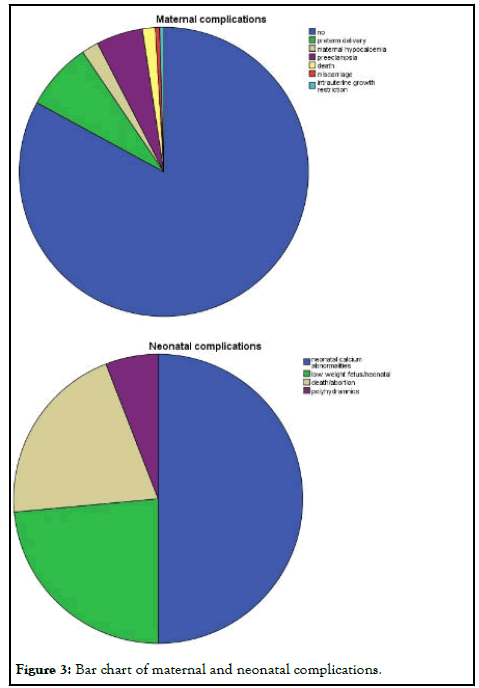
Surgery was the favorite treatment option 142/211 (67.3%) in comparison with conservative treatment 69/211 (32.7%), either with hydration, vitamin D supplementation and steroids and furosemide administration or with medical treatment with cinacalcet, calcitonin and bisphosphonates. In 6 cases, cinacalcet was administered, but surgery was afterwards applied. The main gestational trimester of operation was the second one (35.5%), while in the first one 3.3% of pregnant were operated and in the third trimester 10.9%. A high percentage of operation was observed in the postpartum (48.3%). Maternal and neonatal complications were observed either in conservative or in surgical treatment group. No maternal complications were described in 82.6% of cases, while preterm delivery (7.6%) and preeclampsia (5.2%) were the most common complications. In neonatal group, calcium abnormalities (in fact hypocalcemia) were most usually observed as a complication (50%), followed by low weight fetus or newborn (23.6%) (Figure 3).
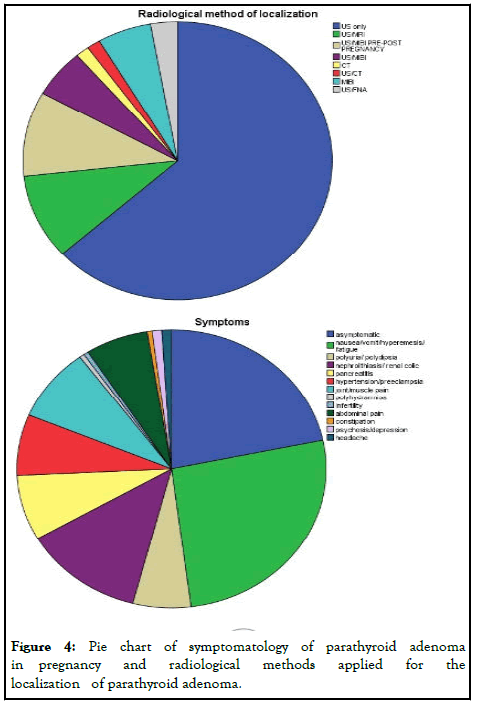
The majority of cases were asymptomatic (21.7%), whereas nausea/vomit/ hyperemesis/fatigue (26.3%) and symptomatology from urinary track (nephrolithiasis) (12.6%) were the main symptoms described. Joint/muscle pain (8.6%), pancreatitis (7.6%), hypertension/preeclampsia (7.1%), abdominal pain (6.6%) and polyuria/polydipsia (6.1%) follow in frequency.
The main radiological method applied for localization of the parathyroid adenoma was ultrasound (63.4%), while combination of ultrasound and MRI was used in 9.9%. Ultrasound and scintigraphy pre or post-pregnancy applied also in 9.9%. Scintigraphy alone during pregnancy, following the safety protocols, was used in 5.6% of cases (Figure 4).
The ionized calcium levels seemed to have a statistical significant correlation with treatment method (Z=-2.593, p=0.01). The cutoff point of ionized calcium level suggestive of surgery was found 2.86 mmol/L by ROC curve. The complications were not correlated to mother's age (p=0.91) the complication risk was associated with treatment option (p<0.001) and yet by conservative treatment is 19% more possible that complications will arise (Table 1).
| Author | No of cases | Age | Gestation week | Calcium | F/S | Symptoms | Past sym | Localization | C/S* | Oper week | Histology | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McCarthy et al. | 1 | 20 | 5 | 2,74 | F | nausea | no | US | S | 18 | Hyperplasia | No |
| 2 | 40 | 7 | 3,08 | S | asympt | no | US/MIBI/CT PRE | S | 16 | Adenoma | preterm delivery | |
| 3 | 34 | 16 | 2,79 | S | asympt | no | US | S | 27 | Adenoma | no | |
| Ali et al. | 1 | 34 | 7 | F | infertility | no | US/MIBI | S | 24 | Hyperplasia | mat hypocalc/fetal no | |
| Malekar-Raikar et al. | 1 | 29 | 18 | 3,47 | S | nausea | no | US | S | 18 | Adenoma | preeclampsia |
| Herrera-Martinez. | 1 | 31 | 22 | 2,79 | S | asympt | no | US | S | 24 | Adenoma | no |
| 2 | 31 | 11 | 3,17 | S | asympt | nephrolit | US | S | 21 | Adenoma | No | |
| Rchachi et al. | 1 | 26 | 33 | 3,12 | polyur/dips | no | us | S | 33 | Hyperplasia | hypocalc/preterm | |
| 2 | 42 | 32 | 2,99 | nephrolit | no | us | S | 32 | Adenoma | hypocalc | ||
| 3 | 32 | 23 | 2,74 | nephrolit | no | us/MRI | C | |||||
| Ning et al. | 1 | 37 | 32 | 1,49 | hypertension | HIV | C | preeclampsia/neonatal hypocal | ||||
| Perin et al. | 1 | 32 | 8 | 2,03 | S | nausea/weak | no | us/MRI | S | 15 | Adenoma | hypocalc |
| Guo et al. | 1 | 29 | 6 | 4,39 | F | nausea/weak | abortion | us/MIBI | S | Adenoma | no | |
| Kokrdova et al. | 1 | 27 | 27 | 1,43 | S | nausea/weak | no | US | S | 31 | Adenoma | no |
| Sharma et al. | 1 | 25 | 30 | 2,77 | S | joint pain | no | US/MRI | C | preterm del/preecla/neonatal low weight | ||
| Mokrysheva et al. | 1 | 28 | postpartum | 1,57 | S | joint pain | miscarriage/bone pain | CT | C | postpartum | Adenoma | hypocalc/neonatal hypocalc |
| Zanardini et al. | 1 | 28 | 11 | 3,04 | S | nausea/weak | no | US/4 D-CT | S | 14 | Adenoma | no |
| Muscat-Baron et al. | 1 | 26 | 34 | 3,2 | nephrolit/polyhydramnios | no | US | S | 34 | Adenoma | no | |
| Truong et al. | 1 | 38 | 36 | 2,02 | flank pain | no | US | S | 36 | Adenoma | no | |
| 2 | 35 | second trimester | 1,43 | nephrol | no | US/MIBI PRE | S | second trimester | double adenoma | no | ||
| 3 | 28 | 20 | 2,89 | nausea | PHPT first pregnancy/nephro/nausea | MIBI PRE | S | 20 | double adenoma | no | ||
| Nilsson et al. | 1 | 34 | 28 | 1,7 | asympt | nephrolit | US | S | 30 | Adenoma | no | |
| 2 | 29 | 35 | 2,02 | premature delivery/nausea/hypertension | miscarriage/extrauterine pregnancy | US | C | postpartum | Adenoma | neonatal hypocalc | ||
| Jesudason et al. | 1 | 25 | 20 | 3,09 | renal colic | renal colic | S | 27 | Adenoma | no | ||
| Naru et al. | 1 | 23 | 22 | 3,17 | asympt | child seizures | US | S | 27 | Adenoma | no | |
| Zhang et al. | 1 | 28 | 22 | 3,49 | nausea | no | US | S | 24 | triple adenoma | preterm del/low weight | |
| Som et al. | 1 | 42 | 29 | 2,79 | S | hypertension | miscarriages | MIBI post | C | postpartum | Adenoma | neonatal death |
| Hession et al. | 1 | 33 | 28 | 3,3 | preeclampsia | miscarriages/ectopic pregnancy/renal calculi | US | C | postpartum | Adenoma | preterm delivery | |
| 2 | 29 | 9 | 3 | abdominal pain/vomit | renal calculi/bone pain/lethargy | US | S | 19 | Adenoma | no | ||
| Mirza et al. | 1 | 31 | 6 | 3,52 | nausea/fatigue/polyur/myalgia/constipation | no | US | S | 7 | adenoma with atypia | no | |
| Davis et al. | 1 | 35 | 13 | 3,6 | S | hyperemesis | constipation/nephrolit | US | S | adenoma with atypia | preeclampsia/preterm del/neonatal low weight | |
| Diaz-Soto et al. | 1 | 28 | 29 | 3,48 | S | nausea | pyelonephritis | US | S | 30 | adenoma | no |
| 2 | 40 | 3 | S | asympt | pre PHPT diagnosis | MIBI pre | C | no | ||||
| Ince et al. | 1 | 34 | 2,74 | S | asympt | nephrolit | US/MIBI | S | 23 | adenoma | no | |
| Godinez-Vidal et al. | 1 | 27 | 27 | pancreatitis | nephrolit | S | adenoma | no | ||||
| Thomas et al. | 1 | 32 | 8 | 3,12 | hyperemesis/psychosis | no | US | S | 18 | no | ||
| Morrison et al. | 1 | 29 | 36 | 2,98 | asympt | US | C | no | ||||
| 2 | 27 | 10 | 2,74 | asympt | C | adenoma | ||||||
| 3 | 20 | 24 | 3,1 | asympt | US | C | preterm delivery | |||||
| Cuhaci et al. | 1 | 20 | 8 | 3,04 | abdominal pain | US | S | 9 | adenoma | |||
| 2 | 38 | 8 | 2,87 | asympt | US | S | second trimester | adenoma | ||||
| Dogru et al. | 1 | 27 | 4 | 3,42 | nausea | US | S | second trimester | adenoma | no | ||
| Manjunatha et al. | 1 | 22 | 12 | 3,16 | hyperemesis | US | S | second trimester | adenoma | no | ||
| Gastelum et al. | 1 | 40 | 30 | 2,04 | abdominal pain | US | S | 30 | adenoma | |||
| 2 | 30 | 40 | 1,61 | preterm delivery | US | C | postpartum | adenoma | ||||
| Sanderson et al. | 1 | 31 | 26 | 2,94 | flank pain | US | S | 26 | triple adenoma | |||
| Dokmetas et al. | 1 | 40 | 14 | 2,75 | asympt | US | S | 17 | adenoma | |||
| Bitar et al. | 1 | 33 | 15 | 3,27 | bone pain/nausea | US | S | 19 | adenoma | no | ||
| Baumann et al. | 1 | 23 | 31 | 3,07 | nausea/weak | no | US | S | 23 | adenoma | preeclampsia/preterm delivery | |
| Li et al. | 1 | 27 | 16 | 3,48 | S | nausea | miscarriages | US/MRI | S | 18 | adenoma | no |
| Arnez et al. | 1 | 40 | 25 | 3,02 | polyur/dips | miscarriages | US | S | 28 | adenoma | no | |
| Razavi et al. | 1 | 42 | postpartum | 2,84 | asympt | US | C | postpartum | adenoma | neonatal hypocal | ||
| Malheiro et al. | 1 | 31 | 16 | 2,95 | asympt | pre PHPT diagnosis | MIBI | S | 16 | adenoma | no | |
| Pothiwala et al. | 1 | 30 | 8,5 | 3,37 | myalgias/bone pain/nausea | nephrolith | MRI/US/FNA | S | 11 | adenoma | no | |
| 2 | 22 | 21 | 3,07 | asympt | intrauterine fetal death | MRI/US | S | 22 | adenoma | no | ||
| McMullen et al. | 1 | 8 | 2,7 | renal calculi | C | postpartum | adenoma | fetal death | ||||
| 2 | 20 | 3,2 | C | postpartum | adenoma | preterm delivery | ||||||
| 3 | 34 | 2,7 | C | postpartum | preterm delivery | |||||||
| 4 | 38 | 3,1 | renal calculi | MIBI | C | postpartum | preterm delivery | |||||
| 5 | 10 | 3,5 | renal calculi | MIBI | S | second trimester | adenoma | |||||
| 6 | 21 | 2,8 | S | second trimester | adenoma | |||||||
| 7 | 23 | 3,2 | renal calculi | MIBI | S | second trimester | adenoma | |||||
| Stringer et al. | 1 | 32 | 19 | 2,92 | S | US/MIBI | S | adenoma | no | |||
| 2 | 22 | 20 | 3,45 | F | US | S | adenoma | no | ||||
| 3 | 30 | 22 | 2,78 | F | US | S | adenoma | no | ||||
| 4 | 36 | 17 | 2,67 | S | US | S | adenoma | no | ||||
| 5 | 31 | 23 | 3,09 | S | US | S | adenoma | no | ||||
| 6 | 39 | 23 | 2,72 | S | US | S | adenoma | no | ||||
| 7 | 37 | 32 | 3,06 | S | US | S | adenoma | no | ||||
| 8 | 43 | 25 | 2,7 | S | US | S | adenoma | no | ||||
| Dahan et al. | 1 | 22 | 32 | 2,89 | nausea/pancreatitis | no | US | C | postpartum | adenoma | preterm delivery | |
| Bansal et al. | 1 | 31 | 24 | 3,09 | abdominal pain/vomit pancreatitis | US/MRI/FNA | C | no | ||||
| Dias Leite et al. | 1 | 40 | 27 | 3,49 | S | nausea/hypertension/pancreatitis | US | C | preterm delivery | |||
| Boorugu et al. | 1 | 27 | 26 | 3,37 | S | pancreatitis | no | US/MIBI post | C | postpartum | adenoma | neonatal hypocalc |
| Alharbi et al. | 1 | 28 | 27 | 2,97 | hypertension/preeclampsia | no | US | C | preterm delivery | |||
| Dale et al. | 1 | 31 | 32 | 1,58 | preeclampsia/pancreatitis | no | US | C | preterm delivery | |||
| Alajmi et al. | 1 | 33 | 28 | 2,65 | hypertension | no | MIBI post | C | postpartum | adenoma | preterm delivery | |
| Norman et al. | 1 | 19 | 2,87 | no | S | 17 | adenoma | |||||
| 2 | 21 | 2,92 | miscarriage | S | 15 | adenoma | ||||||
| 3 | 24 | 2,82 | miscarriage | C | ||||||||
| 4 | 25 | 2,75 | miscarriage | S | 14 | adenoma | ||||||
| 5 | 26 | 2,9 | miscarriage | C | ||||||||
| 6 | 27 | 2,7 | C | |||||||||
| 7 | 27 | 2,87 | S | 13 | adenoma | |||||||
| 8 | 27 | 2,8 | miscarriage | C | ||||||||
| 9 | 28 | 2,97 | miscarriage | S | 15 | adenoma | ||||||
| 10 | 28 | 2,87 | S | 17 | adenoma | |||||||
| 11 | 28 | 2,62 | C | |||||||||
| 12 | 29 | 2,82 | miscarriage | C | ||||||||
| 13 | 29 | 2,8 | miscarriage | S | 23 | adenoma | ||||||
| 14 | 30 | 3,1 | miscarriage | C | ||||||||
| 15 | 30 | 2,9 | S | 16 | adenoma | |||||||
| 16 | 31 | 2,85 | miscarriage | S | 14 | adenoma | ||||||
| 17 | 32 | 2,77 | miscarriage | C | ||||||||
| 18 | 32 | 2,82 | S | 16 | adenoma | |||||||
| 19 | 33 | 2,8 | S | 14 | adenoma | |||||||
| 20 | 33 | 2,85 | miscarriage | C | ||||||||
| 21 | 33 | 2,92 | miscarriage | C | ||||||||
| 22 | 33 | 2,67 | miscarriage | C | ||||||||
| 23 | 34 | 2,85 | miscarriage | C | ||||||||
| 24 | 34 | 2,75 | miscarriage | C | ||||||||
| 25 | 36 | 2,82 | S | 18 | adenoma | |||||||
| 26 | 36 | 2,97 | miscarriage | S | 16 | adenoma | ||||||
| 27 | 36 | 2,72 | miscarriage | C | ||||||||
| 28 | 37 | 3,05 | miscarriage | S | 13 | adenoma | ||||||
| 29 | 37 | 3,25 | miscarriage | C | ||||||||
| 30 | 38 | 3,07 | miscarriage | C | ||||||||
| 31 | 38 | 2,97 | miscarriage | S | 17 | adenoma | ||||||
| 32 | 40 | 2,85 | miscarriage | C | ||||||||
| Nash et al. | 1 | 30 | 32 | 3,2 | abdominal pain/vomit | miscarriage/nephrolith/ectopic pregnancy | US/MIBI post | C | postpartum | adenoma | preterm delivery | |
| Krysiak et al. | 1 | 35 | 8 | 2,6 | pancreatitis | miscarriage | CT post | C | postpartum | adenoma | neonatal hypercalc | |
| Tsai et al. | 1 | 31 | 15 | 2,99 | nausea/polyur/malaise/pancreatitis | nephrolith | US | S | 16 | adenoma | no | |
| Yang et al. | 1 | 24 | 37 | 3,11 | S | pancreatitis | no | CT/MIBI post | C | postpartum | adenoma | preterm delivery/maternal death |
| Hong et al. | 1 | 32 | 37 | 1,59 | preeclampsia/intracranial hemorrhage | no | US/CT post | C | postpartum | adenoma | preterm delivery | |
| Petousis et al. | 1 | 28 | 26 | 2,99 | S | abdominal pain | no | US/MRI | S | 29 | adenoma | no |
| Dincer et al. | 1 | 29 | postpartum | 3,24 | muscle pain/pancreatitis | US/MRI/CT/MIBI post | C | postpartum | adenoma | no | ||
| Bendinelli et al. | 1 | 38 | 9 | 2,61 | S | polydip-ur/hyperemesis | no | US | S | 23 | adenoma | no |
| Tachamo et al. | 1 | 32 | 7 | 1,67 | S | lumbar pain | pituitary adenoma | US/FNA | S | first trimester | adenoma | |
| Hui et al. | 1 | 35 | 28 | 3,08 | polur-dip | hypertension first pregnancy | US | S | 33 | adenoma | no | |
| 2 | 29 | 10 | 3,18 | vomit | US | S | 11 | adenoma | no | |||
| 3 | 39 | 15 | 3,2 | asympt | PHPT pre diagnosis | US | S | 22 | adenoma | no | ||
| Sharma et al. | 1 | 31 | 6 | 3,52 | polyu-nausea-weak | US | S | 7 | adenoma with atypia | preeclampsia | ||
| Abusabeib et al. | 1 | 38 | 15 | 3,03 | joint pain | lumbar pain/lymphadenopathy | US/CT/MIBI pre | S | 18 | adenoma | ||
| Horton et al. | 1 | 21 | 10 | 1,69 | vomit/polydip/headache | 4 DCT | S | 14 | adenoma | preeclampsia | ||
| Osuna et al. | 1 | 22 | 8 | 3,87 | nausea/vomit/abdominal pain | hypertension/nephroloth | US | S | 8 | adenoma | no | |
| Gonzalo-Garcia et al. | 1 | 30 | 30 | 3,67 | preeclampsia/intrauterine growth restriction | miscarriage | US/MRI-CT/MIBI post | C | preeclampsia/preterm delivery/neonatal hypocal | |||
| Saad et al. | 1 | 18 | 23 | 3,15 | weak/pain | nephrolit/polyur/vomit/weak | US/MIBI | S | 24 | adenoma | no | |
| Haciyanli et al. | 1 | 25 | 19 | 2,94 | nausea/muscle pain | no | US | S | adenoma | no | ||
| 2 | 36 | 24 | 3,09 | asympt | no | US | S | adenoma | no | |||
| Vera et al | 1 | 34 | 10 | 3,02 | S | nephrolith | US/MIBI/CT pre-US/FNA | C | postpartum | adenoma | neonatal hypocalc | |
| Pal et al. | 1 | 26 | 28 | 2,87 | bone pain/renal colic | US | C | postpartum | adenoma | neonatal hypocalc | ||
| 2 | 38 | 20 | 3,02 | renal colic/weak | miscarriage/infertility | US | C | postpartum | adenoma | no | ||
| 3 | 36 | 16 | 2,59 | pancreatitis | miscarriage | US | C | postpartum | adenoma | no | ||
| 4 | 37 | 25 | 3,09 | bone pain/renal colic | miscarriage/PA excision | US | C | postpartum | double adenoma | no | ||
| 5 | 28 | 7 | 3,04 | cerebral venous thrombosis | nephrolith | US | C | postpartum | normal PGs | no | ||
| 6 | 32 | 28 | 2,99 | pancreatitis | nephrolith/miscarriage | US | C | postpartum | no | |||
| 7 | 32 | 24 | 3,64 | pancreatitis | nephrolith/infertility | US | C | postpartum | no | |||
| 8 | 34 | postpartum | 2,82 | asympt | miscarriage | US | C | postpartum | adenoma | neonatal hypocalc | ||
| Song et al. | 1 | 29 | 5 | 1,73 | nausea | S | 18 | adenoma | no | |||
| 2 | 35 | postpartum | 1,69 | joint pain | C | postpartum | adenoma | neonatal hypocalc | ||||
| 3 | 29 | postpartum | 1,36 | joint pain | C | postpartum | carcinoma | neonatal hypocalc | ||||
| 4 | 28 | 14 | 1,7 | nausea | S | 15 | adenoma with atypia | no | ||||
| 5 | 35 | 12 | 2,1 | nausea | S | 18 | adenoma | intrauterine death | ||||
| 6 | 31 | 27 | 1,8 | nausea/unconsiousness | S | 28 | adenoma | pancreatitis/preeclampsia/HELLP | ||||
| 7 | 35 | 8 | 1,34 | polydips | C | |||||||
| 8 | 27 | 12 | 1,46 | asympt | C | neonatal heart disease | ||||||
| DiMarco et al. | 1 | 37 | 3,04 | S | asympt | US/MIBI PRE | S | 19 | ||||
| 2 | 35 | 3 | S | headache | US | S | 18 | |||||
| 3 | 37 | 2,63 | S | asympt | US | S | 19 | preterm delivery | ||||
| 4 | 40 | 2,78 | S | miscarriage | US/MIBI PRE | S | miscarriage | miscarriage | ||||
| 5 | 40 | 2,74 | S | hypertension | US | S | 19 | |||||
| 6 | 41 | 2,8 | S | asympt | US | S | second trimester | |||||
| 7 | 30 | 2,56 | S | asympt | US/MIBI PRE | S | 24 | |||||
| 8 | 32 | 3 | S | asympt | S | 28 | ||||||
| 9 | 36 | 2,66 | S | asympt | US | S | 19 | |||||
| 10 | 33 | 3,3 | S | asympt | US | S | second trimester | |||||
| 11 | 25 | 3,16 | F | MEN1 | US/MIBI PRE | S | 14 | |||||
| 12 | 52 | 3 | S | asympt | US | S | 21 | |||||
| 13 | 37 | 2,75 | S | renal calculi | US | S | intrauterine growth restriction | |||||
| 14 | 29 | 3,35 | S | nausea | US | S | 12 | |||||
| 15 | 32 | 2,85 | S | IVF workup | US/MIBI PRE | S | 24 | |||||
| 16 | 34 | 2,88 | S | asympt | US | S | second trimester | |||||
| 17 | 27 | S | asympt | US/MIBI PRE | S | second trimester | ||||||
| Walker et al. | 1 | 31 | prepregnancy | 2,88 | asympt | Li-fraumeni syndrome | US/MIBI PRE | S | second trimester | |||
| 2 | 24 | second trimester | 3,64 | nephrolit | US | S | second trimester | |||||
| 3 | 29 | second trimester | 3,29 | nephrolit | HIV | US | S | second trimester | ||||
| 4 | 28 | second trimester | 2,78 | asympt | Crohn's disease | US | S | second trimester | ||||
| 5 | 29 | second trimester | 3,18 | abdominal pain | US | S | second trimester | hypercalcemia/neonatal hypercalcemia | ||||
| Cassir et al. | 1 | 17 | 2,8 | polydips/weak/vomit | hypertension/urinary track infections | S | 33 | no | ||||
| 2 | 2 | 1,4 | renal calculi/weak/depression | nephrolith/IBS | S | 26 | no | |||||
| 3 | prepregnancy | 2,3 | renal calculi | prolactinoma/MEN1 | S | prepregnancy | pregnancy termination | |||||
| 4 | 10 | 3,1 | hyperemesis | S | 16 | low weight fetus | ||||||
| 5 | prepregnancy | 2,6 | renal calculi | hypothyroidism | S | prepregnancy | low weight fetus | |||||
| 6 | prepregnancy | 2,7 | renal calculi/muscle weak/fatigue | hypothyroidism/osteopenia | S | prepregnancy | low weight fetus | |||||
| 7 | prepregnancy | 2,5 | asympt | hypothyroidism/radioactive iodine | S | prepregnancy | preeclampsia | |||||
| 8 | 25 | 2,9 | fatigue/polydisp | S | 30 | no | ||||||
| 9 | 27 | 2,8 | asympt | S | 31 | no | ||||||
| 10 | 11 | 2,7 | renal calculi | S | 16 | |||||||
| 11 | 20 | 2,9 | hyperemesis | nephrolith | S | 22 | no | |||||
| 12 | 34 | 2,9 | hyperemesis/abdominal pain | bipolar disease | S | 35 | no | |||||
| 13 | 12 | 3,1 | hypertension | depression | S | 13 | low weight fetus | |||||
| 14 | 33 | 2,7 | asympt | C | polyhydramnios | |||||||
| 15 | 30 | 3,1 | polyur/poydips | hypertension | S | 34 | no | |||||
| 16 | 25 | 2,9 | abdominal pain | C | no | |||||||
| 17 | 19 | 3,1 | abdominal pain/hyperemesis | S | 27 | hypertension | ||||||
| 18 | 31 | 3,6 | asympt | nephrolit | S | 32 | polyhydramnios | |||||
| 19 | 15 | 3,7 | hyperemesis | depression | S | 16 | low weight fetus | |||||
| Hu et al. | 1 | 37 | 7 | 1,34 | asympt | S | adenoma with atypia | |||||
| 2 | 31 | 25 | 1,79 | asympt | S | adenoma with atypia | ||||||
| 3 | 28 | 11 | 1,75 | nausea | S | adenoma | ||||||
| 4 | 29 | 5 | 1,73 | nausea | S | adenoma | ||||||
| 5 | 32 | 27 | 2,29 | pancreatitis/nephrolith/hypertension | S | adenoma | abortion | |||||
| 6 | 22 | 24 | 1,63 | headache/unconsiousness/hypertension | S | adenoma | abortion | |||||
| 7 | 35 | 18 | 3,07 | nausea/nephrolit | S | adenoma | abortion | |||||
| 8 | 34 | 6 | 3,15 | vomit/unconsiousness/nephrolith | S | adenoma | abortion | |||||
| 9 | 26 | postpartum | 1,39 | asympt | C | postpartum | hyperplasia | neonatal hypocalc | ||||
| 10 | 28 | postpartum | 1,47 | asympt | C | postpartum | adenoma | neonatal hypocalc | ||||
| 11 | 26 | postpartum | 1,48 | backache/vomit/nephrolith/hypertension | C | postpartum | adenoma | neonatal hypocalc | ||||
| 12 | 29 | postpartum | 1,36 | asympt | C | postpartum | carcinoma | neonatal hypocalc | ||||
| Jiao et al. | 1 | 37 | postpartum | 2,87 | asympt | |||||||
| 2 | 32 | prepregnancy | 2,95 | joint pain | ||||||||
| 3 | 27 | second trimester | 3,28 | nausea/weak | ||||||||
| 4 | 38 | second trimester | 4,21 | nausea | ||||||||
| 5 | 34 | prepregnancy | 2,77 | nausea/weak | ||||||||
| 6 | 28 | second trimester | 3 | nausea/weak | ||||||||
| 7 | 25 | second trimester | 3,49 | nausea/weak | ||||||||
| 8 | 30 | second trimester | 3,17 | joint pain | ||||||||
| 9 | 32 | first trimester | 3,08 | nausea/weak | ||||||||
| Rey et al | 1 | 40 | 28 | 1,44 | bone pain/polyur | sickle cell disease | US | C | postpartum | adenoma | no | |
| 2 | 30 | 36 | 1,68 | nephrolit | nephrolith | MIBI post | C | postpartum | adenoma | no | ||
| 3 | 26 | 14 | 2,05 | nausea/joint pain | fibroma | C | postpartum | fibroma | pregnancy termination |
Abbreviations: F: familiar; S: Sporadic; US: Ultrasound; MIBI: 99 mTC-scintigraphy; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; C: Conservative; S*: Surgery
Table 1: Characteristics of cases included in the study.
Discussion
Prevalence
The incidence of primary hyperparathyroidism in general population is estimated about 0.15-0.4%. Nowadays, the incidence approaches 1% due to the increase in serum calcium measurements routinely. This prevalence in pregnancy is calculated <1% in West countries, a low incidence rate, as PHPT remains often undiagnosed in pregnant women. The etiology is that serum calcium measurement is not included in the routine gestational examination, while the normal changes in homeostasis of calcium metabolism in pregnancy may cover PA diagnosis. Besides, the vast majority of pregnant women are asymptomatic or the symptoms overlap these of pregnancy (fatigue, nausea, vomiting) [5]. In an Indian large cohort study, the prevalence of PHPT in pregnancy was calculated relatively high, 2.1%, fact that could be explained by the younger age of PHPT appearance in east countries [6].
Physiological maternal and fetal changes in calcium metabolism
During pregnancy, the serum calcium and phosphate homeostasis as long as PTH and 1,25-dihydroxycholecalciferol regulation change. Maternal hemodilution due to plasma volume increase and hypoalbuminemia causes lower total serum calcium [6]. On the contrary, the levels of ionized serum calcium remain unchanged and its measurement is a reliable mark of calcium in gestation. Furthermore, increased renal calcium excretion caused by increased glomerular infiltration results in maternal hypercalciuria. As a consequence, the risk for renal calculi in gestation is higher. The fetal requirement for calcium is 20-30 g for normal development and bone mineralization, passing through placenta. The increased maternal absorption of calcium starts early at 12 week and the pregnant remains in positive calcium balance until third trimester, when there is a peak excretion of calcium. Calcium homeostasis is not regulated by maternal PTH, but by PTH-related protein, produced by placenta, fetal tissues and maternal breast. PTH in first trimester is decreased to low normal values and to mid-normal values in 2nd and 3rd trimester, whereas PTH does not mediate 1 a-hydroxylase secretion, also regulated by PTH-related protein. 1,25-dihydroxycholecalciferol (calcitriol) production, mediated by 1 ahydroxylase, is 2 to 3-fold elevated in pregnancy, regulated by PTHrp, oestrogen, estradiol, prolactin and lactogen. 1,25-dihydroxycholecalciferol mediates the maternal intestinal reabsorption of calcium that is also increased in gestation. PTH, PTHrp, calcitriol and calcitonin do not pass placenta, while fetus is dependent on maternal 25-hydroxyvitamin D, passing through placenta. Fetal ionized calcium and phosphate are higher that maternal, essential for right skeletal development, and neonatal hypocalcemia is rare [5,7].
Clinical manifestations
The above mentioned physiological maternal changes in calcium metabolism can mask PHPT diagnosis [6,8]. Nephrolithiasis and osteopenia are osteopenia are expected in gestation [5,7]. The main manifestations of PHPT can mimic pregnancy symptoms. Mild PHPT is usually asymptomatic, whereas non-specific symptoms such as fatigue, malaise, nausea, vomiting, dizziness, headache, constipation, polydipsia, polyuria, bone and muscle aches are not indicative of PHPT [5]. Severe manifestations have been reported as complications of PHPT. Acute or recurrent pancreatitis caused by hypercalcemia has been reported in high frequency in pregnant women [9-14]. Pancreatitis should be excluded in cases of gestational hyperemesis [7,15]. Hyperemesis gravidarum due to PHPT is reported in 21-37% of cases [5]. The gastrointestinal track symptoms seems to have a leader role in PHPT in pregnancy (87.5% rate of appearance) in contrast to non-pregnant PHPT cases (31.82%) [16]. Urinary track symptoms (50%) and joint pain (50%) follow in frequency [16]. Hypertensive crisis leading to preeclampsia is another severe complication of PHPT during pregnancy. In a vast majority of women, preeclampsia was the main clinical manifestation of PHPT [12,17-20]. In an Indian cohort study, the rate of pancreatitis and preeclampsia appearance was 50 % and 88%, respectively [6]. A case of uraemic encephalopathy related to hypercalcemic crisis and a case of intracerebral hemorrhage are mentioned in the literature [20,21]. In cases of unexplained polyhydramnios, PHPT should be excluded [5,22]. Miscarriage rates are 3 to 5-fold elevated in cases of undiagnosed PHPT and an correlation between higher levels of calcium >11.4 mg/dl and early pregnancy loss has been described [2,22]. Prior miscarriage has been reported in 62.5% according to Pal et al. [6]. The exact relation between levels of serum calcium and the severity of PHPT has not been determined yet. Some studies suggest that higher levels of calcium lead to more severe symptoms, whereas other support the fact that mid hypercalcemia cannot exclude severe complications [22,23]. On the other hand, a large retrospective study concluded that mild elevation of calcium is not associated with obstetrical complications [3]. In our case report, the patient has second trimester hyperemesis without previous symptoms, which made her gynecologist to investigate the case and hypecalcemia was the main clue to the diagnosis of PA.
Diagnosis
Normal changes of calcium metabolism also affect the diagnosis of PHPT in pregnancy. The diagnosis of primary hyperparathyroidism in general population is based on PTH and serum calcium elevated values and vitamin D and phosphorus decrease. In pregnancy, serum total calcium is decreased, fact that could mask PHPT [7]. The percentage of undiagnosed cases remains high 80%. The diagnosis during gestation is established by elevated serum ionized or albumin corrected calcium levels with debatable PTH levels. The suspicion for PHPT should always be made in cases of increased ionized calcium levels combined with symptomatology of vomitimg, nausea, polyhydramnios, nephrolithiasis and uncontrolled hypertension [22]. It is crucial that familiar hypocalciuric hypercalcemia syndrome (FHH), that mimics PHPT, is excluded. Familial hypocalciuric hypercalcemia is a rare autosomal dominant condition that causes mild hypercalcemia, hypocalciuria, hypermagnesemia, and hypophosphatemia It occurs as a result of mutations in the Calcium Sensing Receptor gene (CASR) that lead to decreased receptor activity [24]. Genetic testing is essential in cases of PHPT in women <40 years old, in order that Multiple Endocrine Neoplasia Syndromes (MEN) are excluded [5]. Diagnostic algorithms have been proposed in the literature. Early diagnosis is vital, so when elevated calcium levels are detected, PTH levels should be measured. In case of PTH low, vitamin D over supplementation, cancer, lymphoma/myeloma, prolonged immobilization, adrenal insufficiency, sarcoidosis and hyperthyroidism should be evaluated. In case of PTH normal or high and urine calcium low, FHH should be excluded. In case of PTH normal or high and urine calcium high, PHPT and MEN are prominent diagnoses [25].
In the international literature, cases of coexistence of parathyroid adenoma and thyroid adenoma or carcinoma have been reported, suggesting that a preoperative detailed patient workup should be made, aiming the best preoperative preparation [26-28].
The localization method that should be applied in PHPT during pregnancy is a debatable subject in literature. Ultrasonography remains the gold standard for the diagnosis and localization of parathyroid adenoma [25]. Its sensitivity is 69% and specificity 74% in experienced hands as it is a fully objective examination, while these percentages approach 100% when ultrasound is combined with FNA guided PTH measurement of suspected nodule [5]. FNA biopsy has been mentioned as a valuable technology tested in some cases of PHPT during pregnancy [29,30]. Magnetic Resonance Imaging (MRI) is an acceptable alternative when ultrasound cannot localize the adenoma. MRI lacks of sensitivity in small parathyroid adenoma and in high volume endocrine surgeons' teams it is not required [5,30]. Computed tomography and Sestamibi should be avoided in gestation due to radiation exposure of fetus [25]. These examinations are proposed in cases of negative bilateral neck exploration. 99 mTc-MIBI scintigraphy has been suggested as a solution in these cases, but it is crucial that a reduction of 50% of radiation to 10 mCi is made so that the fetus is exposed to less than 5 mGy and the appropriate radiopharmaceutical is selected in order that the best balance between the identification of parathyroid adenoma and the minimal fetal risk is achieved.
A diagnostic and therapeutic algorithm for PHPT during pregnancy cases has been suggested by Walker et al. In cases of suspicion of PHPT, a multidisciplinary team should be approached and advised. Necessary biochemical examination (Calcium, phosphorus, PTH, vitamin D) should be done and in case of uncertainty, genetic testing follows. For the identification of PA, ultrasound examination should be applied and if the result is negative, bilateral neck exploration should be done. In case of ultrasound localization of PA, minimal invasive parathyroidectomy is proposed. If neck exploration is negative, MRI, scintigraphy, intraoperative ultrasound and 4 D-CT are additional radiological examinations that could be helpful. A postoperative follow up for at least 6 months is proposed.
Treatment
The definite treatment of PHPT in general population is surgical excision of the adenoma. In cases of PHPT during pregnancy, there are no guidelines for the management of PHPT and it is controversial regarding the concerns for both mother and fetus arising from general anesthesia and surgery. Some treatment options criteria have been set as guidance for decision. The management of PHPT during pregnancy should be based on gestational age, severity of hypercalcemia and risk-benefit balance. A conservative treatment, including low calcium diet, vitamin D supplementation and hydration, should be chosen for mild to moderate hypercalcemia (ionized serum calcium <2.75 mmol/l), whereas surgery is the treatment of choice in cases of symptomatic hypercalcemia. The most appropriate trimester for surgery is the second one, as in the first trimester fetal organogenesis takes part and in the third trimester the risk for preterm labor increases [5,7]. If the surgical option is declined, medical treatment with cinacalcet, bisphosphonates and calcitonin could be proposed. Cinacalcet is a calciomimetic calcium sensoring receptor activator, decreasing PTH secretion by parathyroid glands. Cinacalcet is a category C drug in pregnancy, so its administration should be avoided. Although, it has been applied in restricted cases without side effects for both mother and fetus, but further studies should be provided for its safety during pregnancy [10]. Bisphosphonates is also a category C drug and are contraindicated in pregnancy due to the induction of fetal bone malformation. Calcitonin is a category B drug in pregnancy and seems to be ineffective as well as develops tachyphylaxis. As an alternative to medical treatment, a method with ethanol ablation of parathyroid adenoma by ultrasound guidance is described with acceptable results [6,10].
The second trimester is considered the safest for surgery in gestation [7,25]. Although, there are studies that concluded in the acceptance of surgery in the 3rd trimester if risk-benefit balance is over benefit [22-25]. Some cases of parathyroidectomy without maternal or fetal complications in first trimester have been also published.
As far as the operational stragedy is concerned, in cases of a good preoperative ultrasound examination with high accuracy at PA identification, minimal invasive parathyroidectomy is the treatment of choice [8,23]. Minimal invasive parathyroidectomy could also be achieved by intraoperative ultrasound guidance. Minimal invasive surgery with cervical plexus block without recurrence and fetal complications has been described as an alternative to general anesthesia [23]. In cases of negative preoperative radiological localization, bilateral neck exploration is mandatory. Regarding embryological features of parathyroid glands, their location may vary. Many cases of ectopic parathyroid adenoma have been reported, making the decision for surgery in pregnant women more difficult. In the majority of cases, the adenoma was not identified preoperatively, the neck exploration was negative and additional imaging methods were applied for their localization.
Parathyroidectomy is considered the treatment of choice regardless pregnant's symptomatology, as surgery lower the risks of maternal and fetal complications in comparison to conservative treatment [16]. The possibility of preeclampsia is higher in conservative treatment. On the other hand, many cases of preeclampsia weeks after parathyroidectomy have been referred [8,26]. A large retrospective study by Hirsch et al concluded that there is no statistical significance of pregnancy related complications between parathyroidectomy and conservative treatment [3].
The maternal complications due to untreated hypercalcemia have been analyzed above as clinical manifestations of PHPT. The fetal complications include spontaneous abortion (15%), intrauterine fetal demise (7%), neonatal death (11-16%), neonatal hypocalcemia-tetany (22-50%), premature birth, and intrauterine growth restriction and low birth weight
A multidisciplinary team should manage PHPT in pregnancy and diagnosis and treatment algorithms should be established. If parathyroid adenoma is diagnosed before fertile and there is high chance of pregnancy, parathyroidectomy is mandatory. During gestation, parathyroidectomy is suggested in second trimester even in mild hypercalcemia. If an elevated serum ionized calcium (>2.43 mmol/l) is detected in early pregnancy or prepregnancy, PTH levels should be measured. In post-operative course, serum calcium and PTH should be measured every 2 weeks in order that hypocalcemia is avoided. In our case, conservative treatment with vitamin D supplementation was not a choice due to hypercalciuria and surgery was mandatory. The second trimester of pregnancy is the appropriate period for operation, as possible maternal and fetal anesthetic and operative risks are lesser.
Conclusion
Primary hyperparathyroidism in pregnancy is a relatively rare condition that remains often undiagnosed. This is a result of the similarity of PHPT symptoms to these of pregnancy as long as of the normal changes taking place in pregnancy that mask PHPT diagnosis. Untreated hypercalcemia can lead to severe maternal and fetal/neonatal complications. Mild to moderate hypercalcemia can be treated conservatively, but severe or symptomatic hypercalcemia should be treated surgically. The benefits of the operation outweigh its risks, especially when it is undertaken in the second trimester of pregnancy. Further studies should be done in order that medical drugs applied for hypercalcemia are evaluated for effectiveness and safety during pregnancy. Parathyroidectomy during pregnancy requires a highly specialized surgical team and pre and postoperative management of these patients is a multidisciplinary task.
References
- Pokhrel B, Levine S. Primary Hyperparathyroidism. StatPearls Treasure Island (FL): StatPearls Publishing. 2020.
[Googlescholar] [Indexed]
- Norman J, Politz D, Politz L, et al. Hyperparathyroidism during pregnancy and the effect of rising calcium on pregnancy loss: a call for earlier intervention. Clin Endocrinol. 2009;71(1):104-9.
- Hirsch D, Kopel V, Nadler V, et al. Pregnancy outcomes in women with primary hyperparathyroidism. J Clin Endocrinol Metab. 2015; 100(5):2115-22.
[Crossreff] [Googlescholar] [Indexed]
- Peat J, Barton B. Medical Statistics. A Guide to Data Analysis and Critical Appraisal: Blackwell Publishing; 2005.
- Kochman M. Primary hyperparathyroidism during pregnancy current approach. Wiedza Medyczna. 2020;2(2):77-83.
- Pal R, Bhadada SK, Gupta N, et al. Primary hyperparathyroidism in pregnancy: observations from the Indian PHPT registry. J Endocrinol Investig. 2020.
[Crossreff] [Googlescholar] [Indexed]
- Kamenický P, Lecoq A, Chanson P, et al. Primary hyperparathyroidism in pregnancy. Ann Endocrinol (Paris). 2016; 77(2):169-71.
[Crossreff] [Googlescholar] [Indexed]
- Malekar-Raikar S, Sinnott BP. Primary hyperparathyroidism in pregnancy-a rare cause of life-threatening hypercalcemia: case report and literature review. Case reports in endocrinology. 2011;2011:520516.
[Crossreff] [Googlescholar] [Indexed]
- Dahan M, Chang R. Pancreatitis Secondary to Hyperparathyroidism During Pregnancy. Obstet Gynecol 2001; 98(5 Pt 2):923-925.
[Crossreff] [Googlescholar] [Indexed]
- Bansal S, Kaushik RM, Kaushik R, et al. Primary hyperparathyroidism presenting as severe hypercalcemia with acute pancreatitis in pregnancy. Gynecological endocrinology : Gynecol Endocrinol Gynecol Endocrinol. 2020; 36(5):469-72.
[Crossreff] [Googlescholar] [Indexed]
- Boorugu HK, Surapaneni T, Vadlamani H, et al. Recurrent pancreatitis due to parathyroid adenoma in pregnancy. Indian J Med. 2020;6(5):277-9.
- Dale AG, Holbrook BD, Sobel L, et al. Hyperparathyroidism in Pregnancy Leading to Pancreatitis and Preeclampsia with Severe Features. Case rep obstet gyn. 2017;2017:6061313.
[Crossreff] [Googlescholar] [Indexed]
- Krysiak R, Wilk M, Okopien B, et al. Recurrent pancreatitis induced by hyperparathyroidism in pregnancy. Arch Gynecol Obstet. 2011; 284(3):531-34.
[Crossreff] [Googlescholar] [Indexed]
- Yang J, Dong MJ, Chen F, et al. A rare lethal case of severe acute necrotizing pancreatitis due to a parathyroid adenoma in a third-trimester pregnant woman. BMC endocrine disorders. 2019; 19(1):82.
[Crossreff] [Googlescholar] [Indexed]
- Tsai WH, Lee CC, Cheng SP, et al. Hyperparathyroidism presenting as hyperemesis and acute pancreatitis in pregnancy: A case report. Medicine. 2021;100(14):25451.
[Crossreff] [Googlescholar] [Indexed]
- Song A, Wang W, Chen S, et al. Primary Hyperparathyroidism during Pregnancy: A Case Series of 8 Patients. Endocrine practice. J Endocrinol. 2019;25 (11):1127-36.
- Dias Leite S, Ormonde CC, Ormonde MC, et al. Preeclampsia as an Inaugural Manifestation of Primary Hyperparathyroidism. J Obstet Gynaecol Res. 2020;42(12):841-44.
[Crossreff] [Googlescholar] [Indexed]
- Alharbi BA, Alqahtani MA, Hmoud M, et al. Preeclampsia: A Possible Complication of Primary Hyperparathyroidism. Case rep obstet gyn. 2016; 2016:7501263.
[Crossreff] [Googlescholar] [Indexed]
- Alajmi F, Millo N, Narasimhan S, et al. Primary Hyperparathyroidism during Pregnancy Causing Preeclampsia and HELLP Syndrome. Can J Diabetes. 2017; 41(5):S36.
- Hong MK, Lin YC, Wei YC, et al. Parathyroid adenoma with hypertensive crisis and intracerebral hemorrhage mimicking hemolysis, elevated liver enzymes, low platelets syndrome. Obstet Gynecol. 2011; 117(2 Pt 2):498-500.
[Crossreff] [Googlescholar] [Indexed]
- Nash E, Ranka P, Tarigopula G, et al. Primary hyperparathyroidism in pregnancy leading to hypercalcaemic crisis and uraemic encephalopathy. BMJ Case Rep. 2015. Crossreff]
[Googlescholar] [Indexed]
- Cassir G, Sermer C, Malinowski AK, et al. Impact of Perinatal Primary Hyperparathyroidism on Maternal and Fetal and Neonatal Outcomes: Retrospective Case Series. J obstet gyn Can. 2020;42(6):750-56.
[Crossreff] [Googlescholar] [Indexed]
- Hu Y, Cui M, Sun Z, et al. Clinical Presentation, Management, and Outcomes of Primary Hyperparathyroidism during Pregnancy. Nt J Endocrinol.. 2017;2017:3947423.
[Crossreff] [Googlescholar] [Indexed]
- Afzal M, Kathuria P. Familial Hypocalciuric Hypercalcemia. StatPearls Treasure Island (FL): StatPearls Publishing. 2021.
- Dochez V, Ducarme G. Primary hyperparathyroidism during pregnancy. Arch Gynecol Obstet. 2015;291(2):259-63.
[Crossreff] [Googlescholar] [Indexed]
- Baumann K, Weichert J, Krokowski M, et al. Coexistent parathyroid adenoma and thyroid papillary carcinoma in pregnancy. Arch Gynecol Obstet. 2011;284(1):91-4.
[Crossreff] [Googlescholar] [Indexed]
- Li Q, Xu XZ, Shi JH. Synchronous parathyroid adenoma, papillary thyroid carcinoma and thyroid adenoma in pregnancy: A case report. World J Clin Cases. 2020;8(21):5426-431.
[Crossreff] [Googlescholar] [Indexed]
- Arnez L, Lawrence V. Complex management decisions in a woman with concurrent primary hyperparathyroidism and metastatic papillary thyroid carcinoma, both presenting during pregnancy. Endocrinol Diabetes Metab Case Rep. 2019;2019.
[Crossreff] [Googlescholar] [Indexed]
- Pothiwala P, Levine SN. Parathyroid surgery in pregnancy: review of the literature and localization by aspiration for parathyroid hormone levels. Journal of perinatology. J Neonatal Perinatal Med. 2009;29(12):779-84.
[Crossreff] [Googlescholar] [Indexed]
- Mansoor I, Zalles C, Zahid F, et al. Fine-needle aspiration of follicular adenoma versus parathyroid adenoma: the utility of multispectral imaging in differentiating lesions with subtle cytomorphologic differences. Cancer. 2008;114(1):22-6.
[Crossreff] [Googlescholar] [Indexed]