Parotid gland arteriovenous malformation embolisation A novel approach to the use of precipitating hydrophobic injectable liquid (PHIL)
2 Department of Radiology, Pauls Stradins Clinical University Hospital, Latvia, Email: karlis.kupcs@rsu.lv
Received: 19-Nov-2020 Accepted Date: Dec 04, 2020; Published: 09-Dec-2020, DOI: 10.37532/1983-8905.2021.14(1).1-3
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Arteriovenous malformations (AVMs) develop due to a failure of differentiation in the early stages of embryogenesis. AVMs are characterised by an abnormal connection between feeding arteries and draining veins by an intervening of pathologic blood vessels network (nidus). The bypassing of the capillary system results in high-flow arteriovenous blood shunting. AVM of the parotid gland (PG) is an extremely rare condition with very few reported cases in the literature. Treatment options include embolisation, laser, cryotherapy, corticosteroids and the surgical resection of the PG. Embolisation using a wide variety of liquid embolic agents (LEAs) plays a key role in the treatment of PG AVM. The novel use of an iodinated copolymer- based precipitating hydrophobic injectable liquid (PHIL) for trans-arterial embolisation of PG AVM is safe, feasible and effective.Keywords
Parotid gland; Arteriovenous malformation; Embolisation; Precipitating Hydrophobic injectable liquid; PHIL
Introduction
A 46-year-old male was hospitalized in Pauls Stradins Clinical University Hospital (Riga, Latvia) with complaints about a pulsatile right cheek mass, tinnitus in the right ear and the pains that irradiate to the oral cavity, but that did not relate to meals. A swelling in front and below the right ear occurred spontaneously three years ago and gradually progressed in size.
On physical examination, the patient was found to have a soft, tender, non-fluctuant and well-defined mass in the right parotid region measuring around 3 × 4 cm. A palpable thrill over the right parotid gland and well- audible bruit, even from the short distance over the swelling, was noticed.
Case Report
There was no associated lymphadenopathy. The compression of the swelling of the right submandibular region during intra-oral examination resulted in a large amount of clear saliva excretion. Intermittent, pulsatile and dull pain irradiated to the right mandibular molars (4th quadrant; 46, 47, 48) and pre-auricular area.
A positive “turkey wattle” sign was noticed – the swelling increased in size on tilting the face towards the right side.
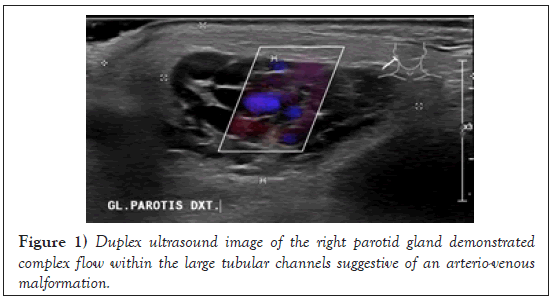
Duplex ultrasound (DUS), of the right parotid region revealed enlarged PG with blunt contours, non-homogeneous echogenicity and hypo-vascular zones. The blood flow in the PG could barely be detected, but a slight compression of it caused an apparent increase in flow rate. A compressible swelling, comprising of tubular channels showed mixed flow on color Doppler, suggestive of an AVM (Figure 1).
No malignant cells were detected in the histopathological investigation of the PG puncture specimen.
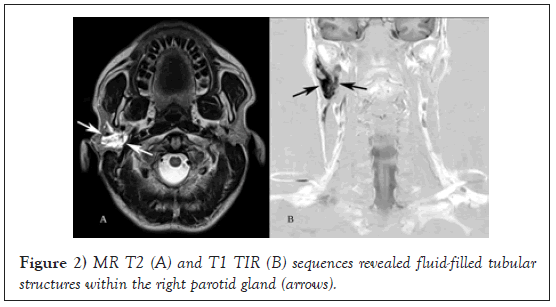
Magnetic Resonance Imaging (MRI) of the right parotid region showed enhancing tubular structures involving the right parotid gland suggestive of a vascular malformation (Figure 2).
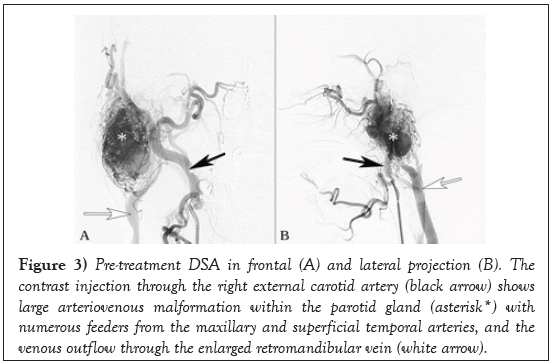
Pre-treatment Digital Subtraction Angiography (DSA) visualised characteristic fast arteriovenous drainage in the nidus and thus confirmed the diagnosis of AVM (Figure 3).
Figure 3: Pre-treatment DSA in frontal (A) and lateral projection (B). The contrast injection through the right external carotid artery (black arrow) shows large arteriovenous malformation within the parotid gland (asterisk*) with numerous feeders from the maxillary and superficial temporal arteries, and the venous outflow through the enlarged retromandibular vein (white arrow).
Endovascular treatment was performed under general anaesthesia with endotracheal intubation. At the beginning of the manipulation 5000 IU of heparin was administered intravenously.
Selective angiography of the right internal carotid artery with 6F guiding catheter Envoy Codman Neuro (Raynham, MA, USA) was performed trough common femoral artery access. Super-selective catheterisation of the AVM feeding arteries under the roadmap guidance with 1,5 F micro catheter Marathon Medtronic (Minneapolis, MN, USA) was followed by a controlled 7 ml 25% PHIL injection. Total duration time of the injection was 15 min.
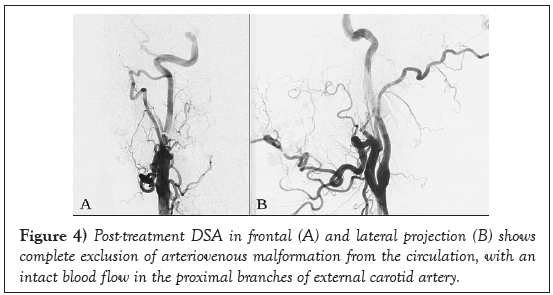
Post treatment Digital Subtraction Angiography (DSA) revealed complete obliteration of the nidus and no further venous distal filling (Figure 4).
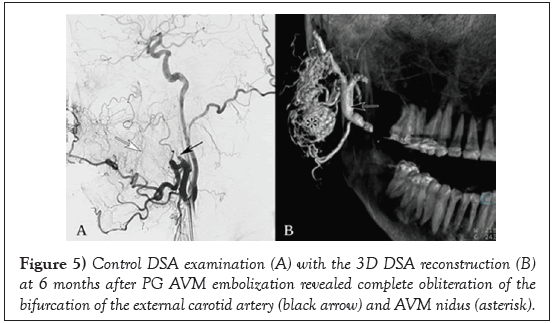
DSA examination at 6 months after PG AVM embolization with PHIL revealed a still complete embolisation of the treated nidus with no obvious recanalization (Figure 5).
Note the prominent collaterals (white arrow) from the facial artery to the vascular territory of maxillary artery.
Six month later, the patient has no complaints about the swelling on the right side of the neck. He should undergo an elective MRI after one year.
Written informed consent was obtained from the patient for his anonymized information to be published in this article.
Ethical approval to report this case was obtained from Pauls Stradins Clinical University Hospital Ethics Committee.
Results and Discussion
According to the International Society for Study of Vascular Anomalies (ISSVA) classification (approved at the 20th ISSVA Workshop, Melbourne, April 2014, last revision May 2018), AVMs are caused by innately perturbed vascular morphogenesis, contrary to the vascular tumours, identified by clear manifestations of cell pathological proliferation.
AVMs are characterised by an abnormal connection between feeding arteries and draining veins by an intervening network of pathologic blood vessels (nidus). The lack of an intervening capillary arteriovenous connections results in high-flow arteriovenous shunting of blood [1].
The genetic transmission patterns of AVMs are still incomplete, but there are some known genetic mutations (i.e., tumor suppressor PTEN gene in the epithelial line) that can lead to an increased risk of the development of this pathology.
AVM of the PG is a rare pathology with only around 50 cases being reported so far, of which mostly all were about venous malformations [2].
Untreated AVM of the PG can cause increased throbbing of the pulsatile mass, progressive pain, impairing local neurologic disorders, including swallowing difficulties, and in some cases, acute (posttraumatic) bleeding.
It is very important to state the correct clinical diagnosis as early as possible, because irrational avocation of surgical procedures in this location of the AVM of the PG may lead to exsanguination and fatalities [3].
Timely diagnosis plays a key role in the treatment of PG AVMs. The primary goals of the treatment are; to preserve the function of the gland, prevent potential bleeding and restore cosmetics. Treatment options include laser, cryotherapy, embolisation, corticosteroids and finally, the surgical resection of the PG [4].
Embolisation using a wide variety of liquid embolic agents (LEAs) plays a key role in the treatment of AVM. At present, the LEAs such as ethylene- vinyl alcohol copolymer (EVOH) and n-butyl cyanoacrylate are used most frequently in the embolisation of the PG AVMs.
Biocompatible, iodinated copolymer-based precipitating hydrophobic injectable liquid (PHIL) is a novel liquid embolic agent, introduced in 2015 by Micro-Vention (Aliso Viejo, CA, USA). Some studies, although using a limited number of cases, have already reported effective and safe usage of PHIL for the treatment of brain AVM and in cranial and spinal dural arteriovenous fistula embolisation. The usage of PHIL was characterised by authors as easy to use, having faster plug formation, more consistent visibility, fewer artefacts in post-interventional imaging, lower required volumes of LEA, a constant degree of embolisation effect and no intraoperative hazards [5,6].
Conclusion
Although the initial results of PHIL use for embolization of PG AVMs appeared to be pleasing, larger trials are needed to confirm the safety, efficacy and durability of this procedure in particularly this location.
The use of iodinated copolymer-based Precipitating Hydrophobic Injectable Liquid (PHIL) is feasible and effective for trans-arterial embolisation of parotid gland arteriovenous malformation.
REFERENCES
- Vollherbst DF, Sommer CM, Ulfert C, et al. Liquid Embolic Agents for Endovascular Embolization: Evaluation of an Established (Onyx) and a Novel (PHIL) Embolic Agent in an In Vitro AVM Model. AJNR Am J Neuroradiol. 2017; 38(7):1377–82.
- John H, Padmashree S, Pandeshwar P, et al. Arteriovenous malformation of the parotid gland: Diagnostic perspective-A case report. Indian J Dent Res. 2020; 31(1):164–6.
- Achache M, Fakhry N, Varoquaux A, et al. Management of vascular malformations of the parotid area. Eur Ann Otorhinolaryngol Head Neck Dis. 2013; 130(2): 55-60.
- Bhatia C, Dalal S, Sattibabu V, et al. Vascular malformation of the parotid gland: a rare case report. Int Surg J. 2017; 4(6):2081–3.
- Kocer N, Hanımoglu H, Batur S, et al. Preliminary experience with precipitating hydrophobic injectable liquid in brain arteriovenous malformations. Diagn Interv Radiol. 2016; 22(2):184-9.
- Leyon JJ, Chavda S, Thomas A, et al. Preliminary experience with the liquid embolic material agent PHIL (precipitating hydrophobic injectable liquid) in treating cranial and spinal dural arteriovenous fistulas: technical note. J Neurointerv Surg. 2016; 8(6):596–602.