Peripheral Soft Tissue Masses: Present Day Diagnosis and Treatment
Received: 24-Jan-2018 Accepted Date: Jan 30, 2018; Published: 06-Feb-2018
Citation: Hoadley A, Dow T, Akhnoukh I, et al. Peripheral Soft Tissue Masses: Present Day Diagnosis and Treatment. Surg Case Rep 2018;2(1):1-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
INTRODUCTION: Peripheral soft tissue masses are problematic for the clinician in terms of diagnostic steps and definitive treatments. Patients usually present with large soft tissue masses and imaging with CT scans usually shows a fat density mass with a “pushing” type of growth and no invasion of peripheral structures. The mass may be superficial to the muscular layers, beneath the muscle or located in both places. Muscle invasion is usually a sign of malignancy.
METHODS: 3 patients presented to the Surgical Oncology Service with large, neglected soft tissue masses of the extremity and peripheral tissues of the back. The three patients were referred by general surgeons from the community due to suspecting the lesions were malignant. All 3 patients underwent resection of the soft tissue tumors.
RESULTS: Histologic examination of the specimens from the 3 patients yielded a benign lipoma in the first, an atypical lipomatous tumor/well differentiated liposarcoma in a 2nd and a definitive liposarcoma in a 3rd case.
CONCLUSION: Large, neglected soft tissue tumors of the periphery should be handled with percutaneous or open biopsy followed by surgical resection. If attempts at diagnosis are inadequate, surgical resection to obtain clear margins should be performed. Close correlation with pathology is essential for subsequent treatment decisions.
Keywords
Soft tissue mass; Sarcoma; Lipoma; Diagnosis; Treatment
Introduction
Soft tissue masses originate from mesenchymal tissues and are classified based on tissue. Lipomatous masses, composed of adipose tissue, are the most common type of benign soft tissue mass with an incidence rate in the general population of 2.1 per 100 persons [1]. Classic lipomas are welloutlined tumors entirely comprised of fat without evidence of nodules or septations. Lipomas may also occur as intramuscular masses presenting with inflammation, poor circumscription, and infiltration. These tumors may present similarly to well-differentiated liposarcomas or atypical lipomatous tumor which arise from the torso wall or extremity (peripheral), making diagnosis difficult [2]. Further complicating the diagnosis is the classification system for liposarcoma including dedifferentiated, myxoid, and the pleomorphic subtypes. By definition, well-differentiated liposarcomas usually present with less than 75% adipose tissue, larger than 10 cm and containing thick septae and nodular areas of no adipose tissue [1]. Imaging used to evaluate soft tissue tumors often do not provide enough insight for distinguishing between benign and malignant states. Instead, imaging is limited to determining the size, location, composition of the tumor, and any visible invasion of nearby tissues. Used in conjunction with imaging, biopsy of the tumor will allow for more specific diagnosis and treatment plan. The intermediate designation of atypical lipomatous tumors is best treated by complete surgical resection to achieve clear margins and to relieve symptoms and improve quality of life. Failure to remove the tumor with well-established margins leads to risk of recurrence and compromises the quality of life for the patient [2].
Many times general clinicians and general surgeons are confronted with a patient with a large peripheral soft tissue mass where the differential is between a benign lipoma, an atypical lipomatous tumor or a liposarcoma. Clinical signs to help with the differential have been suggested but these are not hard fast rules. Patients with peripheral masses over a certain size may all be referred to a surgical oncology specialist even when they may be treated with a simple surgical resection. Lipomatous tumors compose 50% of all soft-tissue tumors and the benign lipoma is the most common. Lipomas are a benign tumor of adipocytes that are well-circumscribed and lobulated usually having a thin fibrous capsule separating the growth from surrounding tissue [3]. Lipomas can occur anywhere and may infiltrate muscle and be poorly circumscribed, which may blur the diagnosis. Lipomas may have inflammation and fibrosis which are also features of the well-differentiated liposarcoma (WDLS). If available genetic testing [4] may be helpful and has resulted in many previously diagnosed ALT being reclassified as lipomas.
Atypical lipomatous tumors (ALT) are an intermediate lesion between the benign lipoma and the malignant liposarcoma and may represent WDLS’s that occur on the extremity or body wall [5]. ALT is composed of mature fat and a variable number of atypical, enlarged, pleomorphic nuclei, low cellularity and uncommon mitotic figures. The number of mitotic figures is an important distinction between ALT and DDLS. An important prognostic variable is tumor location. Evans [6] showed that patients with peripherally located tumors show no disease-specific mortality contrasting with patients who have retroperitoneal or central tumors. These patients should all be referred to a specialty center with a multidisciplinary team in place. In dealing with ALT the best opportunity for cure is with the initial resection. With incomplete or margin-positive surgeries, recurrence is inevitable. Mortality may not be affected but with each recurrence, morbidity increases and quality of life suffers.
Due to the common nature of the presentation and the difficulty in establishing a diagnosis, this review describes three patients with peripheral or body wall soft tissue tumors and a treatment algorithm for these patients is presented.
Case Reports
Three patients will be presented with peripheral soft tissue tumors to illustrate the spectrum of presentation for these tumors.
Case 1
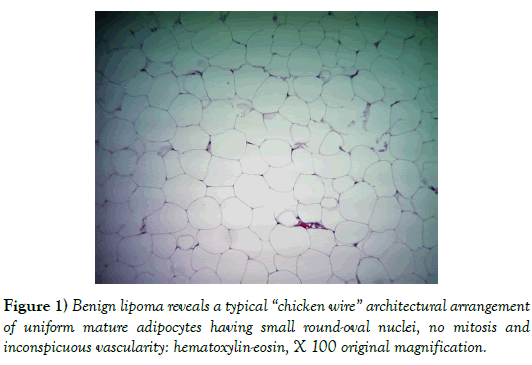
JR is a 59-year-old Caucasian male who presented with the chief complaint of a constant aching in his posterior neck. The patient relates a 25 year history of a soft tissue mass present and increasing in size in this area. The aching had gotten to the point that the patient desired the mass to be removed. The patient’s past medical history is remarkable for prostate cancer, hypertension, splenectomy after a MVI and bilateral inguinal hernia repairs. The patient does not smoke or drink and family history is negative for cancer. On physical exam there was a 10 cm soft tissue mass in the posterior neck. This mass was soft and movable suggesting no attachment to underlying musculature. The patient was taken to the procedure room where under local anesthesia he underwent resection of this mass. Pathology (Figure 1) showed scant vascularity and normal uniform size adipocytes with small nuclei and a lack of mitotic figures. The tumor had a “chicken-wire” appearance typical of a benign lipoma.
Case 2
JL is a 53-year-old female with a past history of hypertension, obesity, tension headache and a previous foot fracture. The patient was initially seen upon referral from her primary nurse practitioner with concerns of progressively enlarging right knee swelling. The onset of the swelling was in 2012 when she fell on her right knee. Over the past year the swelling had worsened with an increase in size as well as an associated numbness. The patient reports that the right knee swelling contributed to her sedentary life-style and her job selection. Over the past year the swelling had doubled in size and is currently interfering with her walking ability due to pain and numbness.
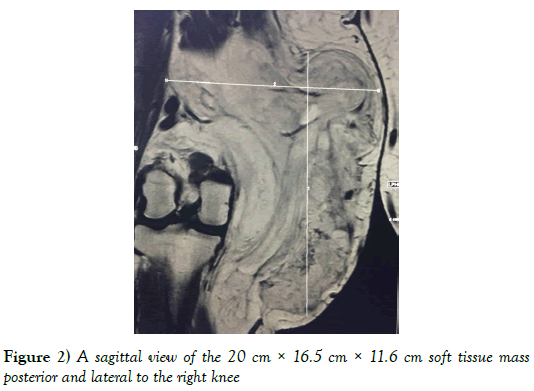
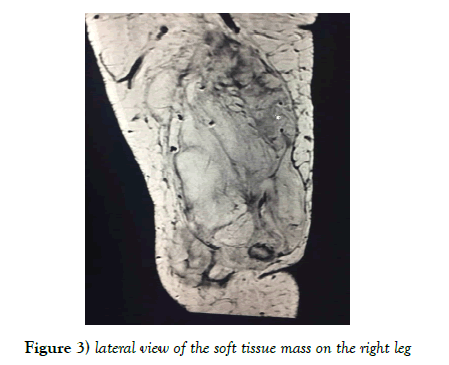
An MRI of the right knee showed a large heterogeneous cyst and predominantly fatty mass involving the medial aspect of the knee measuring 20 cm × 16.5 cm × 11.6 cm. The mass extended posteriorly into the deep compartment between the psoas and gastrocnemius muscles. The lesion was predominantly fatty with internal septation (Figures 2 and 3).
On 06/05/2017 the patient was taken to the OR at Florida Hospital-N Pinellas for a radical resection of this soft tissue mass. At the time of the surgery it was noted that the deep portion of the mass did penetrate the fascia and seem to invade the quadriceps muscle and a resection of some of this muscle was accomplished.
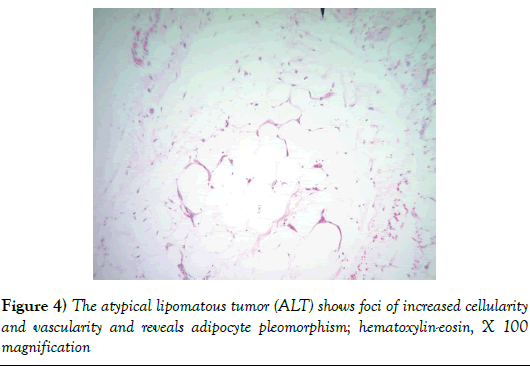
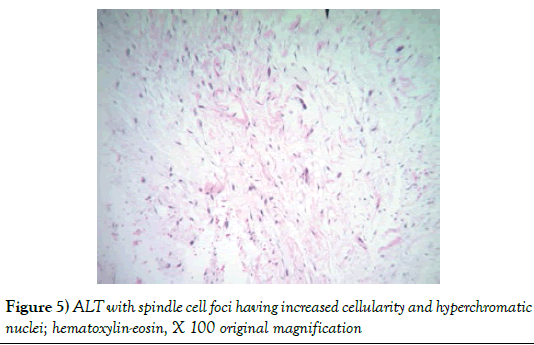
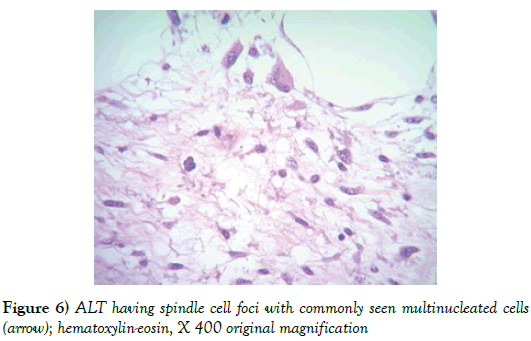
The pathology report showed a 1,940 gram, 20 × 20 × 9 cm soft tissue tumor with the deep margin having skeletal muscle fibers present. Sectioning demonstrated areas of induration, fat necrosis and calcifications. Histology showed the tumor to have increased atypical vascularity and areas of large atypical pleomorphic cells with hyperchromatic nuclei that were multinucleated with possible lipoblasts present. Sections showed variable size adipocytes with nuclear pleomorphism (Figures 4-6). The final report returned atypical lipomatous tumor vs. well-differentiated liposarcoma.
Figure 4: The atypical lipomatous tumor (ALT) shows foci of increased cellularity and vascularity and reveals adipocyte pleomorphism; hematoxylin-eosin, X 100 magnification
Case 3
A 46-year-old female presented to her family physician with a 2.0 cm soft tissue growth on the left forearm. A CT scan of the left forearm showed a soft tissue mass abutting the musculature and the differential diagnosis was a lipoma vs. a sarcoma. A complete excision was performed under local anesthesia and pathology showed a liposarcoma with close if not involved margins. The patient was referred to a comprehensive cancer center and due to the fact that tissue plans were interrupted the patient was treated with radiation therapy to incorporate a larger treatment area. The patient recovered but 1 year later was found to have a local recurrence and Stage IV disease with lung metastases.
Discussion
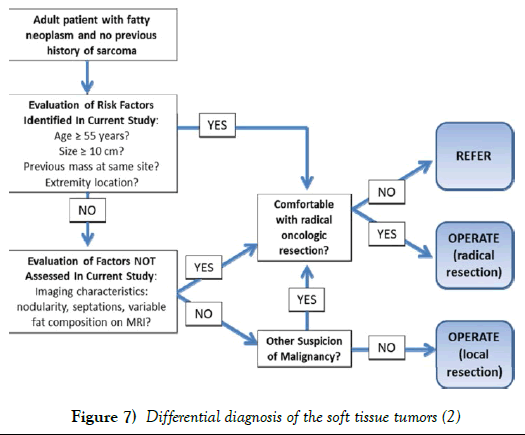
The differential diagnosis of soft tissue tumors of the periphery range from the typical benign lipomas to malignant, highly lethal sarcomas. Benign lipomas are usually observed unless they become symptomatic or are a cosmetic problem. Most benign lipomas are of the size that can be excised in minor surgical treatment rooms under local anesthesia. Recurrences can occur with incomplete resections but these are usually easily handled with a repeat resection (Figure 7).
An intermediate category of peripheral soft tissue tumors is the atypical lipomatous tumor/well differentiated liposarcoma (ALT). This entity is a low grade malignancy of mature to atypical adipocytes that exhibit focal atypia. It is suggested that these tumors be called atypical lipomatous tumor when they occur in the periphery and well differentiated liposarcomas if they are found intra-abdominal or intrathoracic. The common sarcoma subtype is the liposarcoma accounting for 40-45% of the cases. The designation “atypical lipoma” is not recommended.
The ALT soft tissue tumors are most often found in the legs, retroperitoneum, trunk, head and neck area or mediastinum. The goal for the treatment of these patients is total excision to obtain negative margins, even more important for the sclerosing subtype [7]. The tumor does not metastasize unless it dedifferentiates, which will be associated with a worse survival [6]. It has been noted that subcutaneous or intramuscular tumors may occur but typically do not dedifferentiate or metastasize. Retroperitoneal and thoracic tumors can be difficult to resect with clear margins and thus they frequently recur and may dedifferentiate and cause mortality. Because of these reasons intra-abdominal and retroperitoneal sites are associated with a worse outcome [8].
In pathology the soft tissue masses of ALT are described as circumscribed or infiltrative, coarsely lobulated with pale, firm areas and areas of fat necrosis in the larger size tumors. Grossly they resemble lipomas. Microscopically the tumors are described as having mature fat with variably sized adipocytes and fibromyxoid stroma containing spindle cells with large, deep staining nuclei and marked nuclear enlargement and polymorphism. Cellularity is low but certainly increased compared to the typical lipoma and mitotic figures are uncommonly seen. The fibrous septa commonly contain spindle cells that are pleomorphic with no or very few lipoblasts noted. They rarely have a low-grade osteosarcomatous component [9]. Tumors can be subtyped but the subtype does not seem to have any clinical significance [10].
Fisher and colleagues [2] attempted to provide the practicing clinician with some guidelines based on clinical factors to help with the differential of peripheral soft tissue tumors. In a series of 319 patients, 80% were classified as having benign lipomas and the remainder was diagnosed with ALT. Patients with ALT were older (60.5 vs. 53.5), had larger tumors (16 cm vs. 8.3 cm), had more often extremity tumors (88.9% vs. 60.5%) and were more likely to have a previous attempt at resection. Local recurrence reflected the problem with surgical resection as 22.6% of the patients with ALT suffered a recurrence compared to 5.9% with lipomas. With follow-up of 27.4 months, none of the patients with ALT recurred systemically or died of disease.
The final case illustrates the clinical course of peripheral soft tissues that are unfortunately mishandled. The diagnostic work-up of peripheral soft tissue tumors include eventual tissue diagnosis with either a core biopsy, incisional biopsy for larger tumors or complete excision for smaller tumors. If the latter is chosen care should be taken to not disturb other tissue compartments of the extremity in case a malignant diagnosis is made that would require a more radical resection. With the diagnosis of an ALT or sarcoma referral to a cancer center with multidisciplinary care is warranted.
Conclusion
Peripheral soft tissue tumors may be problematic for the clinician. Once tissue diagnosis is made with as minimal of a technique as possible, definitive therapy may be accomplished with simple excision for benign lipomas or referral to a multidisciplinary cancer center for ALT and sarcomas. Any initial excision for diagnosis or treatment of soft tissue tumors should not contaminate multiple tissue compartments that would compromise further radical resections.
REFERENCES
- Wu JS, Hockman MG. Soft-tissue tumors and tumor like lesions: A systematic imaging approach. Radiology. 2009;253(2):297-316.
- Fisher SB, Baxter KJ, Staley CA, et al. The General Surgeon’s Quandary: Atypical lipomatous tumor vs. lipoma, Who needs a surgical oncologist? Journal of the American College of Surgeons. 2013;217(5):881–888.
- Kooby DA, Antonesca CR, Brennan M, et al. Atypical lipomatous tumor/well differentiated liposarcoma of the extremity and trunk wall. Importance of histologic subtype with treatment recommendations. Ann Surg Oncol. 2004;11:78-84.
- Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008;97:298-313.
- Kashima T, Halai D, Ye H, et. al. Sensitivity of MDM2 amplification and unexpected multiple faint alphoid (alpha 12 satellite sequence) signals in atypical lipomatous tumors. Modern Path. 2012;10:1384-1396.
- Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol. 2007;31(1):1-14.
- Kooby DA, Antonescu CR, Brennan MF, et al. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2004;11(1):78-84.
- Smith CA, Martinez SR, Tseng WH, et al. Predicting survival for well-differentiated liposarcoma: the importance of tumor location. J Surg Res. 2012;175(1):12-7.
- Yoshida A, Ushiku T, Motoi T, et al. Well-differentiated liposarcoma with low-grade osteosarcomatous component: an under recognized variant. Am J Surg Pathol. 2010;34(9):1361-1366.
- Smith CA, Martinez SR, Tseng WH, et al. Predicting survival for well-differentiated liposarcoma: the importance of tumor location. J Surg Res. 2012;175(1):12-7.