Getting started, the research question and onwards “research spiral”
Received: 31-Oct-2017 Accepted Date: Dec 11, 2017; Published: 10-Jan-2018
Citation: Gupta VL. Getting started, the research question and onwards “research spiral”. J Nurs Res Pract. 2018;2(1):16-17.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
The research question is the objective of the study, the uncertainty the investigator wants to resolve. Research question often begin with vague and general concern that needs to be narrowed down to a concrete researchable issue. These are the same questions confronting the doctor/ nurse and patient and are at issue in most doctor-nurse-patient encounters.
The following table (Table 1) lists these questions and clinical issue that is related with it [1-3].
| Questions | Issue |
|---|---|
| Is a person sick or well? | Normality |
| What abnormalities are associated with having disease? | Abnormality |
| How accurate are diagnostic tests or strategies used to find a disease? | Diagnosis |
| How often does a disease occur? | Frequency |
| What factors are associated with an increased likelihood of disease? | Risk |
| What are consequences of having a disease? | Prognosis |
| How does the treatment change the future course of a disease? | Treatment |
| Does the intervention on people without disease keep disease from arising? | Prevention |
| Does early detection and treatment improve the course of disease? | |
| What conditions result in disease? | Cause |
| What are the pathogenetic mechanisms of disease? |
Table 1: Questions and clinical issue related
At this stage it is important to understand to which category your question belong to thereby knowing which clinical issue it is going to address. To proceed further in searching the evidence, it is important to recognize the clinical issue that is involved as this will decide the research design which will be used to answer clinical question [4,5].
Most of the evidence that is there in literature is in the form of published studies. Once you have identified the clinical issue and matched study design (Table 2). Clinician will know for sure which studies he or she is going to search for, e.g. case-control study, cohort, or clinical trial, or any other design to address the clinical issue already identified.
| Clinical Issue | Research Design |
|---|---|
| Diagnosis | Prevalence |
| Prevalence | Prevalence |
| Incidence | Cohort |
| Risk | Cohort Case Control |
| Prognosis | Cohort |
| Treatment | Clinical Trial |
| Cause | Cohort Case Control |
Table 2: Clinical issues and appropriate research design
Regarding the strength of various research designs, it could be as simple as, “Case report” and as exhaustive and demanding as “Randomized Clinical Trial” (RCT). Case report, though simple is weakest and RCT though exhaustive is strongest research design. As you go from case report to clinical trial, the strength of research design increases. (Table 3) RCT is the research design par excellence, evidence derived from its strongest, more valid and reproducible [6].
| Design | Strength |
|---|---|
| Case report | Weak |
| Case series | 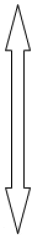 |
| Aggregate risk Study | |
| Cross. Sectional Study | |
| Case Control Study | |
| Cohort Study | |
| Clinical Trial | Strong |
Table 3: Types of research design and their strength
Research designs can be further grouped as, 1. Descriptive and 2. Interventional.
1. Descriptive designs, are hypothesis generating, and includes, a. case reports b. case series, c. aggregate risk studies, d. cross sectional studies, e. case control studies and f. cohort studies.
2. Interventional designs are hypothesis testing and are mainly clinical trials.
Qualitative or social science research
Qualitative research has its roots in social science and is more concerned with understanding why people behave as they do, their knowledge, attitudes, beliefs, fears etc. (e.g. why do patients prefer to be involved in decision making about their treatment?) It is a system of inquiry which seeks to build a holistic, largely narrative, description to inform the researcher’s understanding of a social or cultural phenomenon. Qualitative research takes place in natural settings employing a combination of observations, interviews, and document reviews.
Using an inductive mode of inquiry, qualitative researchers, refrain from posting firm hypotheses or any hypotheses at all. General research questions are typically posed and as data collection and analysis proceed, more specific questions usually emerge. These more specific questions and/or hypotheses may be extended, deleted, or reframed as data collection and analysis continues. The objective of this process is the emergence of a comprehensive, accurate description of the phenomena being investigated from the perspective of those who experience it.
Qualitative Research Design Strategies, include; 1. Case study, 2. Ethinography, 3. Phenomenology, 4. Grounded Theory, 5. Focus Groups, 6. Historical Research.
Quantitative Vs Qualitative Research Methods, Not competing, but complimentary. They Provide different perspectives, answering different sets of research questions. Qualitative research is forerunner to quantitative research, and is hypothesis generating. Quantitative research is done in medical science has more objectivity, while qualitative research done mostly in social science has more of subjectivity. There are modified quantitative or qualitative methods, hybrid designs where there is trade off between objectivity and subjectivity [7].
Your research question should have sound biological rationale, plausibility. It should be relevant, feasible, novel, and ethical. Next step is to identify clinical issue and accordingly research design. Do literature search, critical appraisal of published studies. Conduct your study, data compilation, analysis on to publication of results. This is the research spiral, getting started to the logical end [8].
REFERENCES
- Feinstein AR. The Architecture of Clinical Research. Boston Little Brown and Co. Clinical Epidemiology. 1985.
- Fletcher RH, Fletcher SW. Clinical epidemiology: a new discipline for an old art. Ann Int Med. 1983;99:401-3.
- Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1-2):51-63.
- Sackett DL, Haynes RB, Tugwell P. Clinical Epidemiology: a Basic Science for Clinical Medicine. Boston Little Brown and Co. 1985.
- Robert H, Fletcher, Suzanne W, et al. Clinical Epidemiology: the essentials, Second Edition Williams and Wilkins. 1986.
- Gupta VL. Evidence Based Medicine, Proceed of Indo-Global Health summit, Hyderabad. 2015.
- Gupta VL. Social science research, Qualitative Research Designs, Proceed. Of Annual Indian Society of Nephrology west zone conf. Bhopal. 2011.
- Gupta VL. Manual of Clinical Epidemiology, medical research methodology, Indian Society of Nephrology, west zone publication, Workshop on “Research Methodology” Mumbai. 2013.