Pharmacological effects of leucine-associated phosphatidic acid consumption on the rapamycin synthesis
2 Physical Education Collegiate, Faculdade Guairacá, Guarapuava, Paraná, Brazil, Email: lasilva7@hotmail.com
Received: 06-Mar-2018 Accepted Date: Apr 30, 2018; Published: 10-May-2018
Citation: de Oliveira LEC, da Silva LA. Pharmacological effects of leucine-associated phosphatidic acid consumption on the rapamycin synthesis. J Pharm Toxicol. 2018;1(2):20-22
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
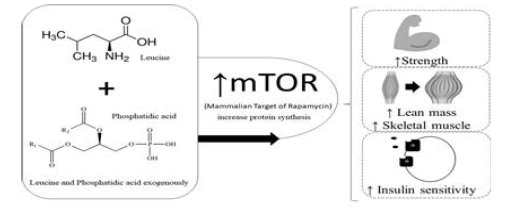
Based on the numerous studies cited and their evidences, the present study comes with the idea of combining both leucine and phosphatidic acid exogenously (supplementary), since the studies cited analyzed the compounds separately and obtained positive results as for its efficiency to increase protein synthesis, and consequently of strength, lean mass, skeletal muscle increase and insulin sensitivity. The objective of the study was to verify the activation of mTOR in animal model due to the supplementation of Leucine and phosphatidic acid. Based on the assumption that both components supplied as food supplements increase their intracellular amounts, the combination of both could potentiate the results of weight training even more, thus providing better health outcomes for practitioners and improvements, enhancing the production of proteins.
Keywords
Protein; Amino acid; Supplementation
Academic visitors generally seek both aesthetic gains and health maintenance, since physical exercise has gained prominence as one of the great allies in the fight against chronic degenerative diseases. In this sense we can highlight a determining factor to achieve these goals: Hypertrophy of the skeletal muscles that will guarantee both health improvements and aesthetic purposes. Consequently to arrive at these results the body triggers several biological processes for the construction of new muscle fibers. These are composed mostly of proteins. For this it is necessary that protein synthesis occurs, that is, the production of more proteins. The increase or maintenance of lean skeletal muscle mass is an important goal. Skeletal muscle tissue is largely dependent on muscle protein synthesis and thus a protein kinase called Mammalian Target of Rapamycin (mTOR) has been widely recognized as a key regulator of muscle growth. The protein synthesis pathway, which makes cell growth through phosphorylation, proliferation of skeletal muscle cells and also of transcription highlighted will be mTOR a protein kinase, so, people who seek an increase of muscles in the body need to increase the activation of mTOR. Resistance training (bodybuilding) alone increases mTOR activation. The activation of mTOR depends on several components, among them, nutrients (glucose), the intracellular concentrations of phosphatidic acid (phospholipid that is part of the cell membrane, which increases the protein synthesis resulting from weight training), and branched chain amino acid leucine. It is proven by numerous studies [1,2] that the addition of leucine and phosphatidic acid exogenously increase their intracellular amounts and thus reestablish protein synthesis in the post-exercise. However, few studies until then have observed the action of the two components together, concomitant with training with weights, which will be approached in this work in a more direct way. Briefly, the exercise highlights the importance of a reestablishment of protein synthesis in the period that happens. After exercise, protein volume and protein balance synthesis is slower than protein breakdown; this negative balance reflects the inhibition of various translation initiation components. Resistance exercise generally does not affect AMPK (which acts as a “switch”, which tells cells when to generate and store molecules such as fat for example and when to use existing energy reserves). When AMPK is activated it triggers the use of energy accumulated from fats, increasing the removal of fats and blood glucose, increasing the production of mitochondria and reducing inflammation), i.e., increasing the total capacity for protein synthesis through P13-kinase and activation of PKB and rpS6. In this case recovery is apparently dependent on supplemental leucine in order to increase the intracellular concentrations of that amino acid that activates mTOR and begin the translation process to resume protein synthesis. Based on the numerous studies cited and their evidences, the present study comes with the idea of combining both leucine and phosphatidic acid exogenously (supplementary), since the studies cited analyzed the compounds separately and obtained positive results as for its efficiency to increase protein synthesis, and consequently of strength, lean mass, skeletal muscle increase and insulin sensitivity. Based on the assumption that both components supplied as food supplements increase their intracellular amounts, the combination of both could potentiate the results of weight training even more, thus providing better health outcomes for practitioners and improvements, enhancing the production of proteins. The objective of the study was to verify the activation of mTOR in animal model due to the supplementation of Leucine and phosphatidic acid.
Leucine Protein Synthesis And Insulin Release
According to Norton and Layman [2], after exercise, muscle recovery requires dietary proteins to increase leucine levels in the muscle cell in order to inhibit the four-factor complex through the activation of the target protein kinase rapamycin (mTOR). The effect of mTOR is synergistic with insulin via the phosphoinositol-3-kinase signaling pathway. Together, leucine and insulin allow the skeletal muscle to coordinate protein synthesis according to the physiological state and diet balance [2]. Resistance exercise produces numerous changes in amino acid metabolism and protein turnover in skeletal muscle, according to Rennie and Tipton [3] and Gautsch et al. [4]. The large and continuous alterations are based on the need for energy and the availability of amino acids; long-term changes require the adaptation of proteins for structural and performance purposes. The process of catabolism is determined by the type of exercise, exercise clearly does not cause loss of skeletal muscle tissue, but rather, it is an optimizer of muscle growth and anabolic processes such as hypertrophy [2]. In this way the training requires the body to trigger metabolic adjustments from the period of catabolism (during practice) to the period of anabolism (recovery).
Resistance exercise is a great stimulator of protein and amino acid metabolism in the skeletal muscle, and this process is limited to six amino acids (aspartate, asparagine, glutamate, leucine, isoleucine and valine) [3]. Among these the most notable effects were with the branched-chain amino acid leucine [5,6]. Leucine has a role in several metabolic processes, as Layman [5] corroborated in his study that leucine also acts as a regulator for the initiation of protein synthesis, such as the insulin modulator phosphoinositol 3-kinase (PI3-kinase), a key compound for the production of alanine and glutamine, thus having great impact on insulin signaling, translation initiation and production of alanine and glutamine. Intracellular concentrations of leucine represent a balance between leucine in plasma, absorption of intracellular proteins and rates of leucine removal through the oxidative process of intracellular amino acids and protein synthesis [7]. Leucine is the only amino acid to play its regulatory role in metabolism, including protein translation control and glycemic regulation [8]. These assertions are made taking into account the role of leucine as regulator of skeletal muscle protein synthesis by initiating 4E (elF4E), 4G (eLF4G) and ribosomal protein S6 (rpS6) [8]. There are also other metabolic aspects that are sensitive to intracellular concentrations of leucine, including alphaketo- chain branched-chain dehydrogenase (BCKDH), which is a limiting factor in the rate of branched-chain amino acid degradation [9]. Pyruvate dehydrogenase, a key enzyme in glucose that controls the entry of pyruvate into the TCA cycle [10]; the insulin receptor substrate-1 (IRS-1), the first result of insulin receptor phosphorylation; and the pancreatic beta cell, relative to insulin release [8]. In their entirety all these diverse metabolic functions allow leucine to have great influence on the rate of muscle protein synthesis, insulin action and glucose homeostasis.
Activation of mTOR is influenced by several regulatory proteins, including the tuberous sclerosis complex (TSC1 and TSC2), Rheb (which is an activator of mTOR and is of extreme importance for the progression of the transcription process. The more isosorylated S6K is, the more active the mTOR pathway will be) and AMP kinase (AMPK) [11]. TSC1/TSC2 and Rheb are essential regulators located between protein kinase B (PKB) and mTOR. Rheb, a GTPase-Ras like, is a positive regulator of mTOR. The Rheb action is opposed by the TSC1/TSC2 complex, which acts as GTPase Rheb, to promote the conversion of GTP to Rheb-Rheb-PIB, inhibition of the positive effect. Tsc2 is very sensitive to growth and energy factors, such as AMPK, but not to amino acids [12]. In TSC2 knockout cells, amino acid restriction impairs mTOR signaling, giving the idea that the principal site for the effects of leucine is derived from TSC2 most likely through Rheb [13].
Phosphatidic Acid, Protein Synthesis From Resistance Training
Phosphatidic acid (AP) appears to be a crucial figure in the stimulation of mTOR signaling, but the mechanisms by which this compound currently stimulates mTOR is not fully understood. Recent studies suggest that phosphatidic acid significantly activates mTOR, bringing better results for skeletal muscle hypertrophy, maximal strength and lean mass [14].
In addition, studies have also shown that mTOR signaling is necessary to induce increases in protein synthesis and final hypertrophic response resulting from training [15,16]. PA is a diacylglycerophospholipio in which two fatty acids and one phosphate group are covalently linked to a molecule of glycerol through ester bonds and can act as a signaling lipid, being a percussor for the biosynthesis of other lipids and is one of major components of cell membranes [17]. Studies have shown that mechanical stimuli may induce increases in intracellular PA levels and that the increase contributes to signaling dependent mTOR events, such as phosphorylation 9 (P-P70-389) th9 (p-P70) thrombin ribosome S6 kinase 1 (p70) [14]. It has also been shown that phosphatidic acid can bind directly to mTOR domain FKBP12-rapamycin (FRB) binding and thereby activates mTOR signaling [18]. Concomitantly it has been researched and proven that exogenous sources of phosphatidic acid can promote the activation of mTOR signaling, however the effects of the supplemental substance to be driven through various mechanisms [19]. Still further, Winter et al. [19] demonstrated that the exogenous addition of phosphatidic acid to fibroblasts results in the activation of mTOR through an indirect mechanism that is dependent on phosphatidic acid being metabolized to lysophosphatidic acid (LPA) and the family receptors of LPA, this study also shows that the activation of LPA receptors induces mTOR signaling. It has been demonstrated that the passively elongated skeletal muscles lead to an increase in the intracellular signaling of PA and mTOR, suggesting that the exogenous supply of PA and resistance training can activate mTOR through distinct pathways and that is activation of these same pathways may have additive effects on mTOR signaling.
While PA plays a crucial role in the stimulation of mTOR signaling and that an increase in BP is sufficient for the activation of mTOR signaling, the mechanisms are not known with exactness. PA can be synthesized by a variety of reactions through various reagents, but it is unclear whether other precursors (glycerol-3-phosphate (G3P), LPA or dicy-glycerol (DAG), have a similar ability in activation mTOR In addition, different sources of PA (soy and egg) may have several degrees of unsaturated or saturated fatty acid chains and this may influence the behavior of PA, it has been suggested that two saturated fatty acids will promote storage, but a saturated fatty acid and an unsaturated fatty acid will promote signaling [20].
Absorption of 1.5 g of PA was observed in studies and an increase was seen in plasma concentration after 30 min, BP concentrations appear to remain in plateau between 1 and 3 h after ingestion. After 7 h PA contractions remained elevated.
Thus elevations by exogenous pathways of PA can be provided through oral supplementation, while endogenous production can be promoted through a resistance training stimulus [14]. However, a few studies have investigated supplementation of oral PA combined with resistance training [1]. More specifically, Hoffman et al. [1] concluded that it is very likely that PA supplementation in humans provides greater increases in lean mass and strength in squatting exercise over placebo, but this study is not able to satisfy all doubts about it.
In the study by Joy et al. [14] in their study the effects of various PA precursors on their ability to stimulate mTOR signaling and whether any other species of phospholipids are also capable of stimulating mTOR signaling was compared a double-blind, placebo-controlled study, which was designed to assess the effects of orally administered PA on skeletal muscle hypertrophy, strength, and energy when consumed during a periodized resistance training program, and suggest that a combined combination of 750 mg of combined BP with a periodized resistance training program appears to have a likely benefit in strength improvement, and an advantage in lean mass gain in young trained individuals, also observed an increase in lean mass and strength of the groups supplemented with supplemental fostatidic acid versus the placebo group, highlighting the soybean component, which, when compared to the egg obtained (+636%) against (+221%). In stage two, the supplemented group significantly increased their lean body mass (2.4 kg) and strength (+51.9 kg) relative to the placebo group. Both studies used a dose of 750mg daily.
Effects Of Exercise On Protein Synthesis
According to, resistance training reduces the rate of muscle protein synthesis proportionally to its duration and intensity, exhaustive exercise or prolonged low frequency stimuli inhibit the mTOR pathways, including inhibition of elF4E and rpS6. After the exercise, there is an increase for the 4E-BP1 inhibitor elF4E and the binding is reduced with the elF4G initiation complex, recent reports suggest that the mechanism occurs because there is increased AMPK activity [12]. Exhaustive exercise decreases ATP, increases AMP concentration and reduces glycogen concentration (4). Increased AMP and reduced glycogen levels stimulate AMPK leading to TSC2 phosphorylation, TSC1/TSC2 formation, and inhibition of Rheb and mTOR. After exercise exogenous (supplementary) leucine increases the concentrations of intracellular leucine that directly stimulates mTOR and elFG4, thus allowing the recovery of muscle protein synthesis. The combination of leucine and carbohydrates appears to produce a synergistic effect on recovery, possibly through the combined effects of leucine on mTOR and PI3-kinase and PKB insulin, resulting in reduced AMPK and TSC2 activity.
Hornberger and Farrar [15] shows that resistance training causes increased protein synthesis that is observed shortly after exercise and persists for up to 48 h. This increase in protein synthesis appears to be mediated through Pl3- kinase and PKB signaling, which are probably stimulated by growth factors such as IGF-1 and myostatin. Studies that used high frequency stimulation in rats showed that there was an increase in PKB, resulting in increased TSC1 phosphorylation and reduced the formation of the TSC1/TSC2 inhibitor complex, allowing the binding of Rheb with mTHOR and the activation of elF4E and rps6, in addition Furthermore, there is evidence for the direct activation of rpS6 by PI3-kinase, which serves to increase the capacity of the cell for protein synthesis after resistance exercise [12]. Although the combination of high-frequency stimulation growth factors increases postexercise protein synthesis, the synthesis is not fully stimulated and the skeletal muscle remains in a catabolic state without additional leucine, either alone or in combination mixture of amino acids. Supplementary leucine allows the muscle to restore protein synthesis and provides a faster recovery and consequently generates anabolism.
REFERENCES
- Hoffman JR, Stout JR, Williams DR. Efficacy of phosphatidic acid ingestion on lean body mass, muscle thickness and strength gains in resistance-trained men. J Int Soc Sports Nutr. 2012;9:47.
- Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr. 2006;136:533-7.
- Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr. 2000;20:457-83.
- Gautsch TA, Anthony JC, Kimball SR, et al. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol. 1998;274:C406-14.
- Layman DK. Role of leucine in protein metabolism during exercise and recovery. Can J Appl Physiol. 2002;27:646-63.
- Wagenmakers AJM. Muscle amino acid metabolism at rest and during exercise: Role in human physiology and metabolism. Exerc Sport Sci Rev. 1998;26:287-314.
- Kimball SR, Jefferson LS, Nguyen HV, et al. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279:1080-7.
- Layman DK, Baum JI. Dietary protein impact on glycemic control during weight loss. J Nutr. 2004;134:S968-73.
- Harris RA, Kobayashi R, Murakami T, et al. Regulation of branched-chain α-keto acid dehydrogenase kinase expression in rat liver. J Nutr. 2001;131:841-5.
- Chang TW, Goldberg AL. Leucine inhibits oxidation of glucose and pyruvate in skeletal muscle during fasting. J Biol Chem. 1978;253:3696-701.
- Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;2:158-66.
- Atherton PJ, Babraj JA, Smith K, et al. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786-8.
- Smith EM, Finn SG, Tee AR, et al. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem.2005;280:18717-27.
- Joy JM, Gundermann DM, Lowery RP, et al. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab. 2014:11:29.
- Hornberger TA, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29:16-31.
- Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014-9.
- Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475-509.
- Fang Y, Vilella-Bach M, Bachmann R, et al. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942-5.
- Winter JN, Fox TE, Kester M, et al. Phosphatidic acid mediates activation of mTORC1 through the ERK signaling pathway. Am J Physiol Cell Physiol. 2010;299:335-44.
- Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1-4.