Phosphorylation of AktSer473/Ask1Ser83 in Trolox-induced suppression of doxorubicin-induced changes in rat cardiomyocytes
- *Corresponding Author:
- Dr Pawan K Singal
Institute of Cardiovascular Sciences, St Boniface General Hospital Research Centre, 351 Tache Avenue, Room R3022, Winnipeg, Manitoba R2H 2A6.
Telephone: 204-235-3887
Fax 204-233-6723
E-mail psingal@sbrc.ca
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
Apoptosis signal-regulating kinase 1; Cell survival signalling; Oxidative stress; Reactive oxygen species
Doxorubicin (Dox), an anthracycline antibiotic, is one of the most effective chemotherapeutic agents and is used to treat a wide range of malignancies [1-4]. However, cardiotoxic side effects of its use remain a major concern [2,4-6]. Among different pathological mechanisms, oxidative stress (OS) is widely considered to be the subcellular basis of this cardiotoxicity [4,7,8]. At the molecular level, OS-induced apoptosis has been reported to be influenced by the regulation of protein kinases such as phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt) and mitogen-activated protein (MAP) kinases including apoptosis signal-regulating kinase 1 (Ask1), p38 and c-jun N-terminal kinase (JNK) [9-11].
Akt is a serine/threonine protein kinase and is activated in a PI3Kdependent manner by a variety of stimuli [12,13]. PI3K/Akt is now regarded as a major cell survival signalling pathway in modulating cardiomyocyte apoptosis [14-16]. It has been demonstrated that Dox suppresses PI3K/Akt signalling in isolated neonatal cardiomyocytes [17]. It has also been reported that adenovirus-mediated intracoronary delivery of a constitutively active Akt1 gene protects the myocardium against Dox-induced cardiomyopathy [18].
Ask1, a serine/threonine MAP3 kinase, is sensitive to OS and is known to be phosphorylated at several sites, which either upregulates or downregulates its activity [19]. In this regard, in response to cellular damage due to OS, Ask1 is phosphorylated at Thr845 and dephosphorylated at Ser83, resulting in an increase in its activity [20,21]. Phosphorylated Ask1 activates downstream MAP2 kinase 4/7 and 3/6, which further induce phosphorylation of JNK and p38, respectively [22], resulting in cell apoptosis. In contrast, Akt-induced phosphorylation of Ask1 at Ser83 has been reported to inactivate this kinase and promotes cell survival [23]. Similarly, selenite-induced phosphorylation of PI3K/Akt has also been shown to negatively regulate Ask1 by phosphorylation at Ser83 [24]. Furthermore, Ask1 is an intermediate step between PI3K/Akt and proapoptotic kinases such as p38 and JNK [23].
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is a water-soluble derivative of alpha-tocopherol with a carboxylic group that enables it to permeate cell membrane [25]. This antioxidant has been reported to protect against OS-induced cell damage in various cell types, including cardiomyocytes, both in vivo and in isolated cells [7,26,27]. In the present study, we examined the crosstalk among AktSer473, Ask1Ser83 and Ask1Th845 in adult rat cardiomyocytes exposed to Dox as well as Trolox.
Methods
All experimental protocols were approved by the University of Manitoba (Winnipeg, Manitoba) Animal Care Committee, following the guidelines established by the Canadian Council on Animal Care.
Isolation and treatment of adult rat cardiomyocytes
Primary ventricular cardiomyocytes were isolated from male Sprague Dawley rats weighing 250±10 g as previously described [10], with slight modifications. Briefly, hearts were perfused with a calcium-free Krebs buffer containing 110 mM NaCl, 2.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3 and 11 mM glucose (pH 7.4) for 5 min. The perfusion was then switched to a recirculating mode with buffer containing 20 μM calcium, 0.1% w/v collagenase and 0.1% w/v bovine serum albumin for 30 min. The collagenase-digested ventricles (both left and right) were gently processed to obtain the final isolated cardiomyocyte preparation in M199 medium supplemented with antibiotics (streptomycin-penicillin, 100 mg/mL) containing 1.8 mM CaCl2. After initial culture of cardiomyocytes on laminin-coated dishes (20 μg/mL) for 2 h to remove any dead cells, cardiomyocytes (1×106 per dish) were further incubated overnight in serum-free M199 medium at 37°C in a 5% CO2 and 95% O2 atmosphere. The viability of cardiomyocytes was assessed using 0.4% Trypan blue (1:1).
Viable cardiomyocytes (>95%) were treated with Dox (5 μM) for 24 h; Trolox (20 μM) for 24 h; or Trolox (20 μM) for 4 h followed by Dox (5 μM) for 24 h; cardiomyocytes without any treatment served as controls. The treatment duration as well as concentrations were based on the authors’ preliminary studies as well as on the reports in the literature [28], as described in the results section. At the end of the various treatment protocols, the cells were harvested and stored at −80°C for biochemical analysis, and were processed fresh for the measurement of reactive oxygen species (ROS) and activity of the apoptotic protein caspase 3/7. Frozen cardiomyocytes were analyzed for phosphorylation of Ask1Ser83, Ask1Thr845 and AktSer473, as well as apoptotic (poly-ADP ribose polymerase [PARP], Bax) and antiapoptotic (Bcl-xL) proteins.
For the time-course studies, phosphorylated proteins (AktSer473, p38, JNK and p53) were detected using a phosphoprotein immunoassay kit according to the manufacturer’s protocol (Bio-Rad Laboratories, USA). Data acquisition and analysis were performed using Bio-Plex Manager software version 4.1.1. Apoptotic (PARP, cleaved caspase 3, Bax) and antiapoptotic (Bcl-xL) proteins were also analyzed.
Akt inhibition studies
Wortmannin treatment: Isolated cardiomyocytes were pretreated overnight with and without the PI3K/Akt inhibitor wortmannin (1 μM), before treating with Dox, Trolox and Trolox + Dox.
Akt Adenovirus culture: Cardiomyocytes were infected with adenovirus constructs according to previously described methods [29], with some modifications. Virus containing enhanced green fluorescent protein (Ad.eGFP) was used as a control as well as to optimize viral infection conditions. The cardiomyocytes were cultured in M199 medium containing 10% fetal calf serum overnight. Ad.eGFP was added to the cardiomyocytes in the medium containing 2% fetal calf serum at different multiplicities of infection (MOI) (20, 50 and 100) for up to 48 h. Transfection efficiency of Ad.eGFP was evaluated at 24 h and 48 h by measuring GFP expression by fluorescence microscopy. Adenovirus vectors containing HA-tagged constitutively active Akt1 (CA-Akt1) or dominant-negative mutant Akt1 (DN-Akt1) (Vector Biolabs, USA) were used at the MOIs and time points indicated in Figure 5. The viral suspension was removed and the cardiomyocytes were subjected to different treatments as described above. At the end of the treatment period, cells were harvested and stored (−80°C) for further analysis of Ask1Ser83, Ask1Thr845 and AktSer473 phosphorylation by Western blotting.
Protein isolation: Cardiomyocytes from different treatment groups were homogenized in RIPA buffer containing protease inhibitor cocktail (Sigma Aldrich, USA) and phosphatase inhibitor (Santa Cruz Biotechnology, USA). The lysates were centrifuged at 14,000 rpm for 10 min at 4°C. The upper layer containing protein fraction was frozen in liquid nitrogen and stored at −80°C. Total protein concentration was determined using bovine serum albumin as the standard according to the modified Bio-Rad (Bio-Rad, USA) microassay procedure [30].
Analysis of cell signalling proteins: The protein samples were subjected to one-dimensional 8% to 12% sodium dodecylsulfate polyacrylamide gel electrophoresis in a discontinuous system. Equal loading of protein was confirmed using Coomassie blue staining and antiactin antibody as an internal control (Santa Cruz Biotechnology, USA). Separated proteins were transferred to 0.45 μm polyvinylidene fluoride membranes and incubated overnight with phospho- or total rabbit antibodies to the cell signalling molecules AktSer473, Ask1Ser83 and Ask1Thr845 (Cell Signaling, USA). Primary antibodies were detected using a goat antirabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibody (Bio-Rad, USA) using the BM Chemiluminiscence (POD) kit (Roche Diagnostics, Canada). The protein bands were visualized using Flour S-Multi- Imager MAX system (Bio-Rad, USA) or x-ray films (Thermo Scientific, USA) and quantified using image-analysis software (Quantity One, Bio-Rad, USA).
Apoptosis: Apoptosis was estimated by analyzing the cleavage of PARP and caspase 3, activity of caspase 3/7 and the ratio of Bax to Bcl-xL. PARP, caspase 3, Bax and Bcl-xL were analyzed using Western blotting. Caspase 3/7 activity was measured using Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, USA) following the instructions provided. Briefly, Apo-ONE Caspase-3/7 reagent was added to the wells containing samples, blank and control. Fluorescence of each well was measured at 521 nm after incubation of the samples for 18 h at room temperature. The amount of fluorescence is proportional to the amount of caspase 3/7 activity.
Measurement of ROS: OS in isolated cardiomyocytes was assessed by quantifying the endogenous production of ROS by incubating the cardiomyocytes with 10 μM of 5-(6)-chloromethyl-2’7’-dihydroflourescein diacetate probe (CM-H2 DCFDA) (Molecular Probes, USA) for 30 min at 37°C in a humidified chamber [30]. The study was performed in triplicate in small dishes (35 mm2 × 10 mm) for each treatment group. Fluorescence of 100 cells from multiple fields per dish was recorded using an Olympus BX51 fluorescence microscope (Olympus Corporation, Japan). Fluorescence intensity, which is proportional to the level of ROS, was measured using a digital imaging processing software (Image Pro Plus). An excitation wavelength of 485 nm and emission wavelength of 530 nm were used. Quantitation was performed by normalizing the fluorescence intensity per unit area to that of the control group.
Statistical analysis: Data are expressed as mean ± SEM. Groups were compared using one-way ANOVA, or two-way ANOVA when more than one variable was analyzed for different groups. Bonferroni’s test was performed to identify differences between groups; P<0.05 was considered to be statistically significant. All statistical analyses were performed using Origin version 6 (OriginLab, USA).
Results
Time-course of Dox effects
The concentration of 5 μM Dox was selected for the present study because this concentration has been shown to reproduce plasma peak values achieved in patients receiving standard infusion [28]. To establish an optimal time of exposure to Dox, a time-course study of the effects of Dox was also conducted. Cardiomyocytes showed timedependent changes due to Dox (5 μM) treatment in the phosphorylation of AktSer473, p53, p38 and JNK as well as in the levels of cleaved caspase 3 and the ratio of Bax to Bcl-xL. Because the maximum change in all of the parameters studied was observed at 24 h (data not shown), the remainder of the study was performed at this time point.
Effect of Trolox on Dox-induced OS
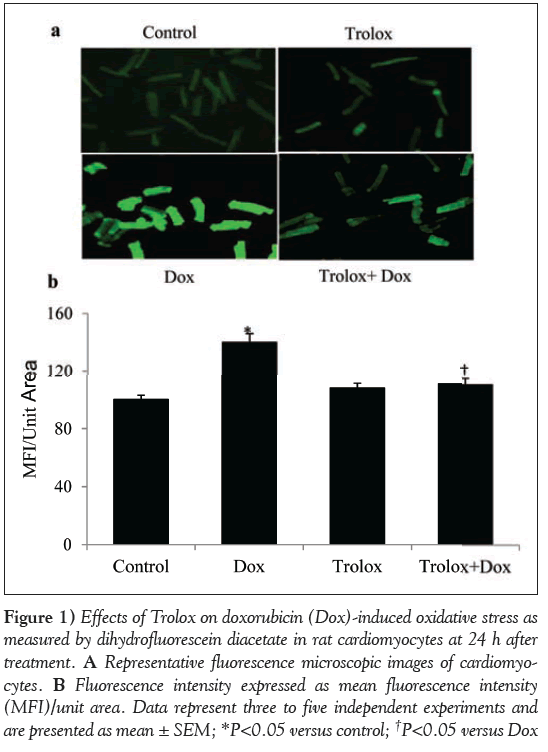
OS in the cardiomyocytes was analyzed by measuring ROS fluorescence of DCF at 24 h (Figure 1A). The fluorescence intensity was quantified and the data are presented as mean fluorescence intensity per unit area (Figure 1B). Dox-treated cardiomyocytes showed a significant (P<0.05) increase in the fluorescence intensity per unit area over the control cardiomyocytes at 24 h of treatment (Figures 1A and 1B). Trolox was used as an antioxidant to study its protective role in Dox-induced OS. ROS production in Trolox treated cardiomyocytes was comparable with those of control cardiomyocytes, and pretreatment with Trolox for 4 h significantly (P<0.05) reduced Dox-induced ROS production in the Trolox + Dox group.
Figure 1: Effects of Trolox on doxorubicin (Dox)-induced oxidative stress as measured by dihydrofluorescein diacetate in rat cardiomyocytes at 24 h after treatment. A Representative fluorescence microscopic images of cardiomyocytes. B Fluorescence intensity expressed as mean fluorescence intensity (MFI)/unit area. Data represent three to five independent experiments and are presented as mean ± SEM; *P<0.05 versus control; †P<0.05 versus Dox
Effect of Trolox on Dox-induced apoptotic and antiapoptotic proteins
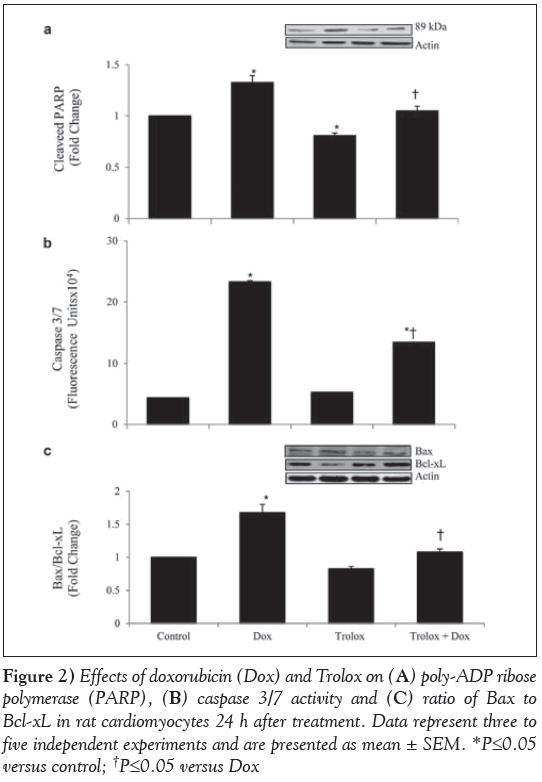
To study the modulation of Dox-induced apoptosis by Trolox, the cellular extracts were analyzed for PARP cleavage, activity of caspase 3/7, and Bax and Bcl-xL protein levels (Figure 2). The ratio of cleaved PARP (Figure 2A) was significantly higher in Dox-treated cardiomyocytes (P<0.05) and significantly lower in Trolox-treated cardiomyocytes (P<0.05) compared with the control. When compared with Dox-only treated cardiomyocytes, Trolox treatment significantly lowered the cleaved PARP in Trolox + Dox cardiomyocytes (P<0.05). Caspase 3/7 activity (Figure 2B) was significantly higher in Dox-treated cardiomyocytes (P<0.05). Trolox pretreatment showed a significant reduction in caspase 3/7 activity in Dox-treated cardiomyocytes (P<0.05). However, the ratio in the Trolox+Dox group was still significantly higher than the control group (P<0.05). The ratio of Bax to Bcl-xL was also significantly higher in Dox-treated cardiomyocytes (P<0.05), and Trolox treatment reduced this ratio significantly compared with Dox treatment (P<0.05) (Figure 2C).
Figure 2: Effects of doxorubicin (Dox) and Trolox on (A) poly-ADP ribose polymerase (PARP), (B) caspase 3/7 activity and (C) ratio of Bax to Bcl-xL in rat cardiomyocytes 24 h after treatment. Data represent three to five independent experiments and are presented as mean ± SEM. *P≤0.05 versus control; †P≤0.05 versus Dox
Effect of Trolox and interplay of signalling proteins (AktSer473, Ask1Thr845 and Ask1Ser83)
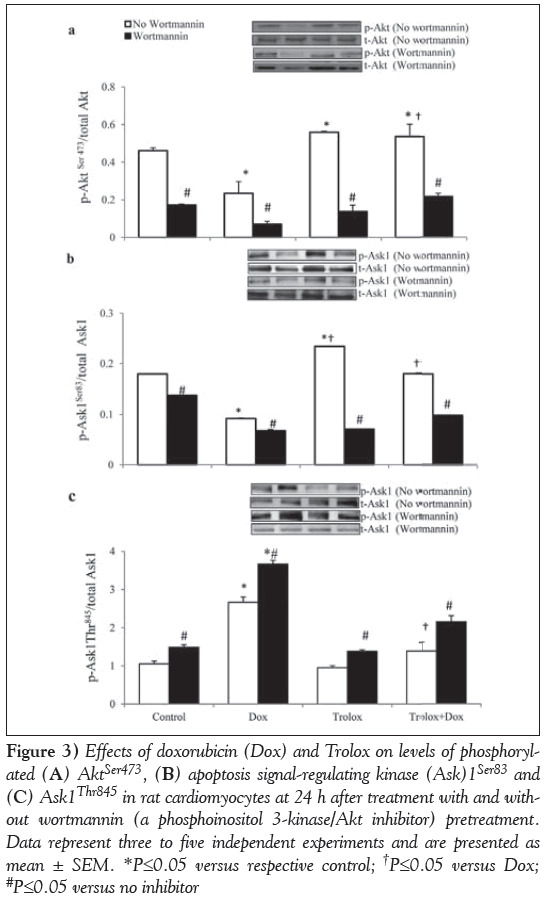
Inhibition of PI3K/Akt: To elucidate the molecular mechanism of protection against Dox-induced changes by Trolox, its effects on the phosphorylation of prosurvival protein Akt at Ser473, and redox sensor Ask1 at Thr845 and Ser83 sites with or without the PI3K/Akt inhibitor wortmannin were studied (Figure 3).
Figure 3: Effects of doxorubicin (Dox) and Trolox on levels of phosphorylated (A) AktSer473, (B) apoptosis signal-regulating kinase (Ask)1Ser83 and (C) Ask1Thr845 in rat cardiomyocytes at 24 h after treatment with and without wortmannin (a phosphoinositol 3-kinase/Akt inhibitor) pretreatment. Data represent three to five independent experiments and are presented as mean ± SEM. *P≤0.05 versus respective control; †P≤0.05 versus Dox; #P≤0.05 versus no inhibitor
Akt phosphorylation was significantly (P<0.05) reduced by Dox (Figure 3A). A small but significant (P<0.05) increase in Akt phosphorylation was recorded in Trolox-treated control cardiomyocytes. Furthermore, pretreatment with Trolox also prevented Dox-induced decline in Akt phosphorylation (Figure 3A). Treatment with wortmannin significantly inhibited the activation of Akt in all the treatment groups (P<0.05), and the inhibition was highest in Dox-treated cardiomyocytes (Figure 3A).
Phosphorylation of Ask1Ser83 in Dox-only treated cardiomyocytes declined significantly (P<0.05). Trolox-treated control cardiomyocytes exhibited a significantly higher level of Ser83 phosphorylation (P<0.05). Trolox also prevented Dox-induced decline in Ask1Ser83 phosphorylation, such that these values were no longer different from the control. However, the level was significantly higher compared with Dox-only treated cardiomyocytes (P<0.05) (Figure 3B). Phosphorylation at Ask1Ser83, which is a consensus site for activated Akt, was significantly inhibited by wortmannin in all the treatment groups (P<0.05) (Figure 3B). Phosphorylation of Ask1Thr845 (Figure 3C) was significantly higher in Dox-only treated cardiomyocytes (P<0.05). Phosphorylation in Trolox-treated control and Troloxpretreated Dox cardiomyocytes were comparable (P>0.05) with those of control cardiomyocytes. However, these values were significantly lower compared with Dox-only treated cardiomyocytes (P<0.05) (Figure 3C). Phosphorylation levels of Ask1Thr845 (Figure 3C) were upregulated significantly in the presence of the PI3K inhibitor in all four groups of cardiomyocytes (P<0.05); Dox-treated cardiomyocytes were affected to the greatest extent.
Loss or gain of Akt function
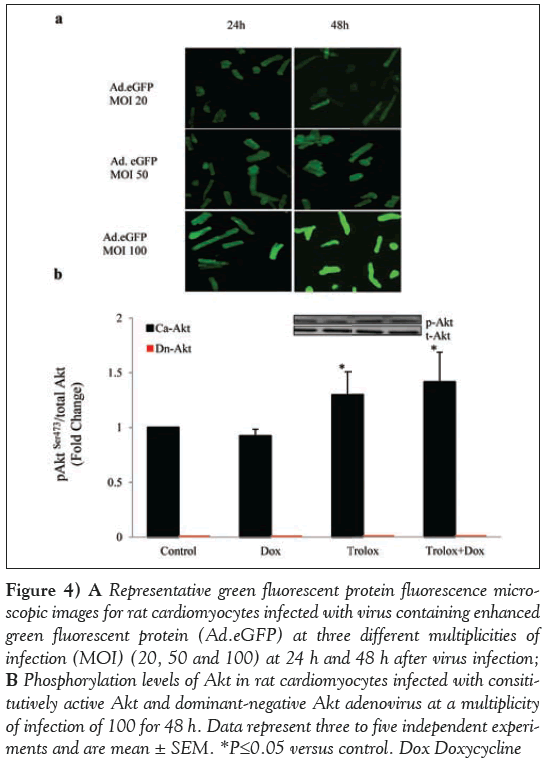
To further substantiate the role of Akt, the gain and loss of Akt gene function with CA-Akt or DN-Akt (T308A; S473A) mutant adenoviruses was also tested in the cardiomyocytes with all treatments. In a dose- (20 MOI, 50 MOI, 100 MOI) as well as time-dependent (24 h or 48 h) study of virus transfection using Ad.eGFP, a maximum expression of GFP was observed at 100 MOI and 48 h (Figure 4A).
Whether the conditions optimized with Ad.eGFP were able to overexpress or block Akt phosphorylation levels was confirmed using immunoblotting with anti-phospho-Akt antibody. Cardiomyocytes infected with CA-Akt showed phosphorylation in all four treatments (Figure 4B). AktSer473 phosphorylation in CA-Akt control cardiomyocytes was normalized to one to compare with all other treatment groups (Dox, Trolox and Dox + Trolox). Akt phosphorylation levels were significantly higher in the Trolox group (P<0.05), but the Trolox + Dox group was not significantly different from the Trolox group (P>0.05). On the other hand, cardiomyocytes infected with DN-Akt gene did not exhibit any phosphorylation at AktSer473 (Figure 4B), although control cardiomyocytes infected with DN-Akt showed some basal-level total Akt protein expression using Akt antibody (data not shown).
Figure 4: A Representative green fluorescent protein fluorescence microscopic images for rat cardiomyocytes infected with virus containing enhanced green fluorescent protein (Ad.eGFP) at three different multiplicities of infection (MOI) (20, 50 and 100) at 24 h and 48 h after virus infection; B Phosphorylation levels of Akt in rat cardiomyocytes infected with consititutively active Akt and dominant-negative Akt adenovirus at a multiplicity of infection of 100 for 48 h. Data represent three to five independent experiments and are mean ± SEM. *P≤0.05 versus control. Dox Doxycycline
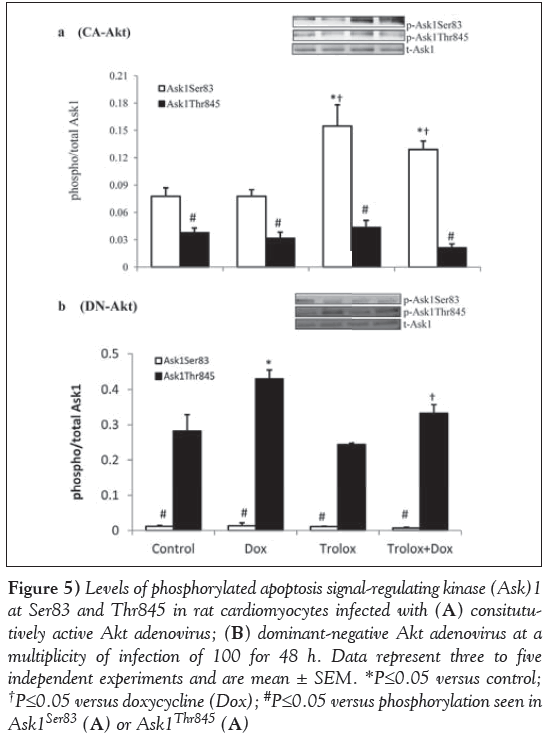
CA-Akt and DN-Akt adenovirus were used as a tool to examine whether Akt regulates Ask1 expression. Ask1 phosphorylation at Ser83 and Thr845 was assessed in the cardiomyocytes treated with CA-Akt and DN-Akt adenovirus (Figure 5). The results showed that in Aktoverexpressed cardiomyocytes (Figure 5A), Ask1Ser83 phosphorylation levels were significantly (P<0.05) upregulated in the Trolox-treated control and Trolox + Dox groups compared with the control and Doxtreated cardiomyocytes. However, cardiomyocytes showed a significantly (P<0.05) lower phosphorylation level of Ask1Thr845 in all groups compared with the phosphorylation at Ser83. On the other hand, in DN-Akt treated cardiomyocytes, Ask1Thr845 levels were upregulated (Figure 5B). The levels were significantly higher in Dox-treated cardiomyocytes compared with the control (P<0.05). The levels of phosphorylation in the Trolox and Trolox+Dox groups were not significantly different from the control (P<0.05). However, there was a small but significant decrease in the phosphorylation of the Thr845 of the Trolox+Dox group compared with the Dox group (P<0.05). DN-Akt almost completely inhibited the Ask1Ser83 phosphorylation compared with phosphorylation at Ask1Thr845 in all four groups of cardiomyocytes (Figure 5B).
Figure 5: Levels of phosphorylated apoptosis signal-regulating kinase (Ask)1 at Ser83 and Thr845 in rat cardiomyocytes infected with (A) consitututively active Akt adenovirus; (B) dominant-negative Akt adenovirus at a multiplicity of infection of 100 for 48 h. Data represent three to five independent experiments and are mean ± SEM. *P≤0.05 versus control; †P≤0.05 versus doxycycline (Dox); #P≤0.05 versus phosphorylation seen in Ask1Ser83 (A) or Ask1Thr845 (A)
Discussion
Using adult rat cardiomyocytes, the present study demonstrated the interplay between Akt and Ask1 in the expression of the effects of Dox mediated through OS, along with the mitigating effects of Trolox. The study reports two major findings: Trolox prevents Dox-induced OS and apoptosis through the upregulation of PI3K/Akt signalling; and Troloxinduced Akt phosphorylation leads to a decrease in Ask1 MAP kinase activity by downregulating phosphorylation at the Thr845 site and upregulating phosphorylation at the Ser83 site. Ask1 phosphorylation at Ser83 is known as a consensus Akt phosphorylation site that promotes cell survival [31].
Dox-induced cardiotoxicity has been extensively reported in the literature [3,4,32,33]; however, its precise mechanism remains unclear. This has hampered the development of an effective clinical treatment for the prevention of Dox-induced cardiotoxicity. The present study demonstrates that Dox leads to a time-dependent increase in cardiomyocyte apoptosis and a decrease in the Akt cell survival signal. This was accompanied by Dox-induced upregulation of Ask1 activity by the phosphorylation at the Thr845 site. Pretreatment with Trolox decreased PARP cleavage, caspase 3/7 activation and the ratio of Bax to Bcl-xL, and rescued the cardiomyocytes from Dox-induced OS as well as apoptosis.
OS is a well-documented mediator of Dox-induced cardiotoxicity [3,4,7,34]. Trolox has been reported to be protective against OSinduced cell damage in various cell types including cardiomyocytes, both in vivo and in cultured cells [26,27,35,36]. We previously reported the protective effect of Trolox in Dox-induced OS and apoptosis [7]. The antioxidant Trolox is readily available to cardiomyocytes under in vitro conditions and, thus, is able to efficiently scavenge free radicals. Furthermore, increased OS has been implicated in the process of apoptosis in the cardiomyocytes under multiple stress conditions [37-40]. In the current study, we report that Trolox induces a decrease in apoptotic proteins (PARP, caspase 3, Bax) and an increase in antiapoptotic protein (Bcl-xL).
Trolox pretreatment increased the phosphorylation of Akt at Ser473 and decreased the phosphorylation of Ask1 at Thr845 in Doxtreated cardiomyocytes. On the other hand, phosphorylation of Ask1 at Ser83 was upregulated by pretreating cardiomyocytes with Trolox. Furthermore, cardiomyocytes pretreated with the PI3K/Akt inhibitor wortmannin showed decreased phosphorylation of Akt at Ser473 in all treatment groups. Akt inhibition led to the downregulation of the phosphorylation levels of Ask1Ser83, whereas the phosphorylation levels of Ask1Thr845 were upregulated. Because Akt inhibition prevented the Trolox-induced upregulation of Ask1Ser83 as well as promoted the upregulation of phosphorylation at Ask1Thr845, it is likely that Akt signalling is essential for Trolox protection in Dox-induced apoptosis in cardiomyocytes in vitro. Furthermore, we propose that Troloxinduced Akt phosphorylation leads to Ask1 inactivation in adult rat cardiomyocytes, thus affecting downstream cell signalling molecules. Trolox-induced protection in other biological systems has also been shown to be due to an increase in Akt phosphorylation [41,42]. A suppression of JNK and p38 activities has also been linked with increased Akt activity [43]. Conversely, inhibition of Akt by PI3K inhibitors has been shown to activate p38 and JNK [23]. Furthermore, a novel functional link between Akt and stress kinases p38 and JNK has been suggested through Ask1 in human embryonic kidney cell lines [23]. It has been reported that Akt phosphorylates its substrate Ask1 at Ser83, which caused a decrease in Ask1 kinase activity that lead to the decrease in p38 and JNK activities [23].
Our data regarding the pharmacological inhibitor was further supported by studies in which cardiomyocytes were infected with virus containing the DN-Akt gene. There was insignificant Ser83 phosphorylation and a significant increase in Thr845 phosphorylation when cardiomyocytes were incubated with adenovirus constructs containing DN-Akt mutants. This increase was further substantiated by Dox treatment, which was significantly different from Trolox-treated cardiomyocytes. Furthermore, when the Akt gene was overexpressed in cardiomyocytes, the phosphorylation levels of Ser83 were not affected by Dox treatment and, in fact, Ser83 phosphorylation levels were upregulated in cardiomyocytes pretreated with Trolox, confirming that Trolox activates the Akt survival signal. Akt gene transfer in the heart has also been shown to ameliorate Dox-induced contractile dysfunction (38). Akt-induced Ask1 inhibition has also been supported by the studies of heat shock proteins [21,40]. Similar to Trolox, selenite-induced suppression of Ask1/JNK through the activation of PI3K/Akt pathway during reperfusion has also been reported [44].
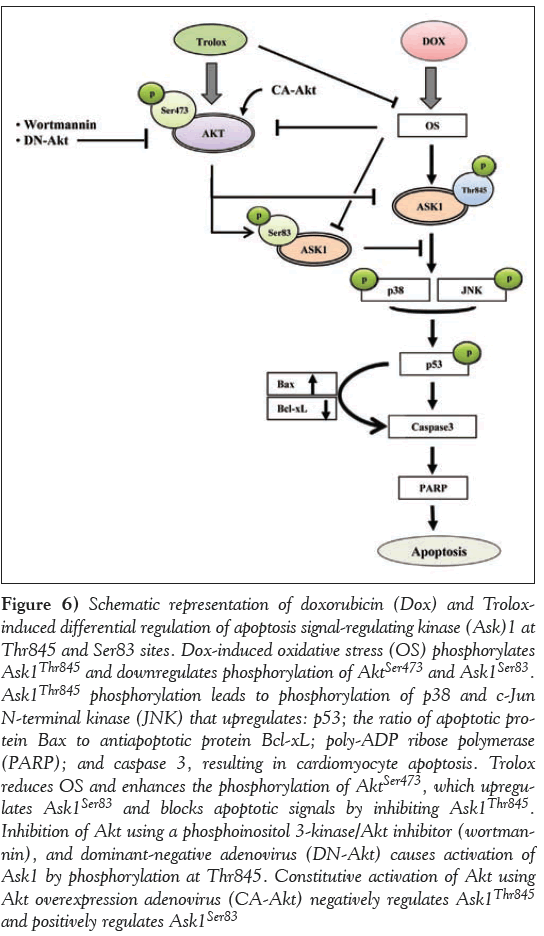
The mechanism of action of Trolox against Dox-induced OS and apoptosis in cardiomyocytes as supported by the data in the present study is presented schematically in Figure 6. Dox impaired cardiac cell survival through increased OS followed by the upregulation of Ask1Thr845 phosphorylation that further activated JNK, p38 and p53. This ultimately lead to mitochondrial dependent cell apoptosis by activating PARP, caspase 3 and Bax, and downregulating Bcl-xL. Trolox treatment ameliorated nearly all these changes caused by Dox via the activation of PI3K/Akt that lead to the activation of Akt consensus site at Ask1Ser83 and inactivating Ask1Thr845 and its downstream signalling molecules, as shown in the proposed scheme (Figure 6). The key finding of a switch of Akt regulation from Ask1Ser83 to Ask1Thr845 or vice versa determines the fate of the cardiomyocytes vis-à-vis cell survival or cell apoptosis. Inhibition of the prosurvival protein Akt using a PI3K/Akt inhibitor (wortmannin) and DN-Akt adenovirus caused the activation of apoptosis-inducing protein Ask1, and CA-Akt adenovirus caused the inactivation of Ask1 and promoted phosphorylation at Ask1Ser83. These impressive benefits of Trolox through the interplay between Akt and Ask1 may provide better molecular targets for the management of Dox-induced cardiomyopathy.
Figure 6: Schematic representation of doxorubicin (Dox) and Troloxinduced differential regulation of apoptosis signal-regulating kinase (Ask)1 at Thr845 and Ser83 sites. Dox-induced oxidative stress (OS) phosphorylates Ask1Thr845 and downregulates phosphorylation of AktSer473 and Ask1Ser83. Ask1Thr845 phosphorylation leads to phosphorylation of p38 and c-Jun N-terminal kinase (JNK) that upregulates: p53; the ratio of apoptotic protein Bax to antiapoptotic protein Bcl-xL; poly-ADP ribose polymerase (PARP); and caspase 3, resulting in cardiomyocyte apoptosis. Trolox reduces OS and enhances the phosphorylation of AktSer473, which upregulates Ask1Ser83 and blocks apoptotic signals by inhibiting Ask1Thr845. Inhibition of Akt using a phosphoinositol 3-kinase/Akt inhibitor (wortmannin), and dominant-negative adenovirus (DN-Akt) causes activation of Ask1 by phosphorylation at Thr845. Constitutive activation of Akt using Akt overexpression adenovirus (CA-Akt) negatively regulates Ask1Thr845 and positively regulates Ask1Ser83
Acknowledgements
The study was supported by an operating grant from CIHR. Dr Pawan Singal is the holder of the Dr Naranjan S Dhalla Chair in Cardiovascular Research supported by the St Boniface Hospital & Research Foundation.
References
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: The good, the bad and the ugly effect. Curr Med Chem 2009;16:3267-85.
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302-14.
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998;339:900-5.
- Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: Pathophysiology and prevention. FASEB J 1997;11:931-6.
- Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 1998;40:426-32.
- Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicininduced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435-45.
- Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann N Y Acad Sci 1999; 874:156-68.
- Simunek T, Sterba M, Popelova O, et al. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 2009;61:154-71.
- Ludke A, Sharma AK, Bagchi AK, Singal PK. Subcellular basis of vitamin C protection against doxorubicin-induced changes in rat cardiomyocytes. Mol Cell Biochem 2012;360:215-24.
- Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2007;293:H3524-31.
- Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal 2006; 8:2111-24.
- Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 1998;336(Pt 1):241-6.
- Liu AX, Testa JR, Hamilton TC, et al. AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res 1998;58:2973-7.
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002;296:1655-7.
- Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: The role of PI 3-kinase and Akt. J Mol Cell Cardiol 2005;38:63-71.
- Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther 2008;324:160-9.
- Negoro S, Oh H, Tone E, et al. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/ caspase-3 interaction. Circulation 2001;103:555-61.
- Taniyama Y, Walsh K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J Mol Cell Cardiol 2002;34:1241-7.
- Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 2008;1780:1325-36.
- Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001;2:222-8.
- Zhang R, Luo D, Miao R, et al. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 2005;24:3954-63.
- Ichijo H. From receptors to stress-activated MAP kinases. Oncogene 1999;18:6087-93.
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 2001;21:893-901.
- Wang Q, Zhang QG, Wu DN, Yin XH, Zhang GY. Neuroprotection of selenite against ischemic brain injury through negatively regulating early activation of ASK1/JNK cascade via activation of PI3K/AKT pathway. Acta Pharmacol Sin 2007;28:19-27.
- Mickle DA, Li RK, Weisel RD, Tumiati LC, Wu TW. Water-soluble antioxidant specificity against free radical injury using cultured human ventricular myocytes and fibroblasts and saphenous vein endothelial cells. J Mol Cell Cardiol 1990;22:1297-304.
- Dean RT, Hunt JV, Grant AJ, Yamamoto Y, Niki E. Free radical damage to proteins: the influence of the relative localization of radical generation, antioxidants, and target proteins. Free Radic Biol Med 1991;11:161-8.
- Rubinstein JD, Lesnefsky EJ, Byler RM, Fennessey PV, Horwitz LD. Trolox C. A lipid-soluble membrane protective agent, attenuates myocardial injury from ischemia and reperfusion. Free Radic Biol Med 1992;13:627-34.
- Hempel G, Flege S, Wurthwein G, Boos J. Peak plasma concentrations of doxorubicin in children with acute lymphoblasticleukemia or non-Hodgkin lymphoma. Cancer Chemother Pharmacol 2002;49:133-41.
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemiareperfusion injury in mouse heart. Circulation 2000;101:660-7.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1199;96:857-68.
- Lou H, Kaur K, Sharma AK, Singal PK. Adriamycin-induced oxidative stress, activation of MAP kinases and apoptosis in isolated cardiomyocytes. Pathophysiology 2006;13:103-9.
- Lv X, Yu X, Wang Y, et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One 2012;7:e47351.
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther 1990;47:219-31.
- Mickle DA, Li RK, Weisel RD, et al. Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg 1989; 47:553-7.
- Zeng LH, Wu J, Carey D, Wu TW. Trolox and ascorbate: Are they synergistic in protecting liver cells in vitro and in vivo? Biochem Cell Biol 1991;69:198-01.
- Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000;60:1789-92.
- Ichihara S, Yamada Y, Kawai Y, et al. Roles of oxidative stress and Akt signaling in doxorubicin cardiotoxicity. Biochem Biophys Res Commun 2007;359:27-33.
- Sharma AK, Dhingra S, Khaper N, Singal PK. Activation of apoptotic processes during transition from hypertrophy to heart failure in guinea pigs. Am J Physiol Heart Circ Physiol 2007;293:H1384-90.
- Fan GC, Zhou X, Wang X, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res 2008;103:1270-9.
- da Frota Junior ML, Pires AS, Zeidan-Chulia F, et al. In vitro optimization of retinoic acid-induced neuritogenesis and TH endogenous expression in human SH-SY5Y neuroblastoma cells by the antioxidant Trolox. Mol Cell Biochem 2011;358:325-34.
- Sun C, Wang D, Zheng W. Hydrogen peroxide attenuates the prosurvival signaling of insulin-like growth factor-1 through two pathways. Neuroreport 2012;23:768-73.
- Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 1998;273:10792-7.
- Yoon SO, Kim MM, Park SJ, et al. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J 2002;16:111-3.
- *Corresponding Author:
- Dr Pawan K Singal
Institute of Cardiovascular Sciences, St Boniface General Hospital Research Centre, 351 Tache Avenue, Room R3022, Winnipeg, Manitoba R2H 2A6.
Telephone: 204-235-3887
Fax 204-233-6723
E-mail psingal@sbrc.ca
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
OBjECTIvE: To examine the modulatory effects of the antioxidant Trolox in the interplay of Akt and apoptosis signal-regulating kinase (Ask) 1 in doxorubicin (Dox)-induced oxidative stress (OS).
METHODS: Rat cardiomyocytes were treated with Dox (5 μM) or Trolox (20 μM) for 24 h, or were pretreated with Trolox (20 μM) for 4 h before treatment with Dox (5 μM) for 24 h, and were compared with cardiomyocytes without any treatment. For the Akt inhibition study, cardiomyocytes were pretreated overnight with the phosphoinositol 3-kinase/Akt inhibitor wortmannin (1 μM) before treatment with Dox, Trolox or Trolox + Dox. The gain and loss of Akt gene function with HA-tagged constitutively active Akt1 or dominant-negative mutant Akt1 (T308A; S473A) was also studied in the cardiomyocytes under all treatment conditions. Cells exposed to various treatment conditions were analyzed for OS, apoptotic and antiapoptotic proteins, and phosphorylation of Ask1Ser83, Ask1Thr845 and AktSer473.
-Keywords
Apoptosis signal-regulating kinase 1; Cell survival signalling; Oxidative stress; Reactive oxygen species
Doxorubicin (Dox), an anthracycline antibiotic, is one of the most effective chemotherapeutic agents and is used to treat a wide range of malignancies [1-4]. However, cardiotoxic side effects of its use remain a major concern [2,4-6]. Among different pathological mechanisms, oxidative stress (OS) is widely considered to be the subcellular basis of this cardiotoxicity [4,7,8]. At the molecular level, OS-induced apoptosis has been reported to be influenced by the regulation of protein kinases such as phosphatidylinositol 3-kinase (PI3K), protein kinase B (Akt) and mitogen-activated protein (MAP) kinases including apoptosis signal-regulating kinase 1 (Ask1), p38 and c-jun N-terminal kinase (JNK) [9-11].
Akt is a serine/threonine protein kinase and is activated in a PI3Kdependent manner by a variety of stimuli [12,13]. PI3K/Akt is now regarded as a major cell survival signalling pathway in modulating cardiomyocyte apoptosis [14-16]. It has been demonstrated that Dox suppresses PI3K/Akt signalling in isolated neonatal cardiomyocytes [17]. It has also been reported that adenovirus-mediated intracoronary delivery of a constitutively active Akt1 gene protects the myocardium against Dox-induced cardiomyopathy [18].
Ask1, a serine/threonine MAP3 kinase, is sensitive to OS and is known to be phosphorylated at several sites, which either upregulates or downregulates its activity [19]. In this regard, in response to cellular damage due to OS, Ask1 is phosphorylated at Thr845 and dephosphorylated at Ser83, resulting in an increase in its activity [20,21]. Phosphorylated Ask1 activates downstream MAP2 kinase 4/7 and 3/6, which further induce phosphorylation of JNK and p38, respectively [22], resulting in cell apoptosis. In contrast, Akt-induced phosphorylation of Ask1 at Ser83 has been reported to inactivate this kinase and promotes cell survival [23]. Similarly, selenite-induced phosphorylation of PI3K/Akt has also been shown to negatively regulate Ask1 by phosphorylation at Ser83 [24]. Furthermore, Ask1 is an intermediate step between PI3K/Akt and proapoptotic kinases such as p38 and JNK [23].
Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) is a water-soluble derivative of alpha-tocopherol with a carboxylic group that enables it to permeate cell membrane [25]. This antioxidant has been reported to protect against OS-induced cell damage in various cell types, including cardiomyocytes, both in vivo and in isolated cells [7,26,27]. In the present study, we examined the crosstalk among AktSer473, Ask1Ser83 and Ask1Th845 in adult rat cardiomyocytes exposed to Dox as well as Trolox.
Methods
All experimental protocols were approved by the University of Manitoba (Winnipeg, Manitoba) Animal Care Committee, following the guidelines established by the Canadian Council on Animal Care.
Isolation and treatment of adult rat cardiomyocytes
Primary ventricular cardiomyocytes were isolated from male Sprague Dawley rats weighing 250±10 g as previously described [10], with slight modifications. Briefly, hearts were perfused with a calcium-free Krebs buffer containing 110 mM NaCl, 2.6 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3 and 11 mM glucose (pH 7.4) for 5 min. The perfusion was then switched to a recirculating mode with buffer containing 20 μM calcium, 0.1% w/v collagenase and 0.1% w/v bovine serum albumin for 30 min. The collagenase-digested ventricles (both left and right) were gently processed to obtain the final isolated cardiomyocyte preparation in M199 medium supplemented with antibiotics (streptomycin-penicillin, 100 mg/mL) containing 1.8 mM CaCl2. After initial culture of cardiomyocytes on laminin-coated dishes (20 μg/mL) for 2 h to remove any dead cells, cardiomyocytes (1×106 per dish) were further incubated overnight in serum-free M199 medium at 37°C in a 5% CO2 and 95% O2 atmosphere. The viability of cardiomyocytes was assessed using 0.4% Trypan blue (1:1).
Viable cardiomyocytes (>95%) were treated with Dox (5 μM) for 24 h; Trolox (20 μM) for 24 h; or Trolox (20 μM) for 4 h followed by Dox (5 μM) for 24 h; cardiomyocytes without any treatment served as controls. The treatment duration as well as concentrations were based on the authors’ preliminary studies as well as on the reports in the literature [28], as described in the results section. At the end of the various treatment protocols, the cells were harvested and stored at −80°C for biochemical analysis, and were processed fresh for the measurement of reactive oxygen species (ROS) and activity of the apoptotic protein caspase 3/7. Frozen cardiomyocytes were analyzed for phosphorylation of Ask1Ser83, Ask1Thr845 and AktSer473, as well as apoptotic (poly-ADP ribose polymerase [PARP], Bax) and antiapoptotic (Bcl-xL) proteins.
For the time-course studies, phosphorylated proteins (AktSer473, p38, JNK and p53) were detected using a phosphoprotein immunoassay kit according to the manufacturer’s protocol (Bio-Rad Laboratories, USA). Data acquisition and analysis were performed using Bio-Plex Manager software version 4.1.1. Apoptotic (PARP, cleaved caspase 3, Bax) and antiapoptotic (Bcl-xL) proteins were also analyzed.
Akt inhibition studies
Wortmannin treatment: Isolated cardiomyocytes were pretreated overnight with and without the PI3K/Akt inhibitor wortmannin (1 μM), before treating with Dox, Trolox and Trolox + Dox.
Akt Adenovirus culture: Cardiomyocytes were infected with adenovirus constructs according to previously described methods [29], with some modifications. Virus containing enhanced green fluorescent protein (Ad.eGFP) was used as a control as well as to optimize viral infection conditions. The cardiomyocytes were cultured in M199 medium containing 10% fetal calf serum overnight. Ad.eGFP was added to the cardiomyocytes in the medium containing 2% fetal calf serum at different multiplicities of infection (MOI) (20, 50 and 100) for up to 48 h. Transfection efficiency of Ad.eGFP was evaluated at 24 h and 48 h by measuring GFP expression by fluorescence microscopy. Adenovirus vectors containing HA-tagged constitutively active Akt1 (CA-Akt1) or dominant-negative mutant Akt1 (DN-Akt1) (Vector Biolabs, USA) were used at the MOIs and time points indicated in Figure 5. The viral suspension was removed and the cardiomyocytes were subjected to different treatments as described above. At the end of the treatment period, cells were harvested and stored (−80°C) for further analysis of Ask1Ser83, Ask1Thr845 and AktSer473 phosphorylation by Western blotting.
Protein isolation: Cardiomyocytes from different treatment groups were homogenized in RIPA buffer containing protease inhibitor cocktail (Sigma Aldrich, USA) and phosphatase inhibitor (Santa Cruz Biotechnology, USA). The lysates were centrifuged at 14,000 rpm for 10 min at 4°C. The upper layer containing protein fraction was frozen in liquid nitrogen and stored at −80°C. Total protein concentration was determined using bovine serum albumin as the standard according to the modified Bio-Rad (Bio-Rad, USA) microassay procedure [30].
Analysis of cell signalling proteins: The protein samples were subjected to one-dimensional 8% to 12% sodium dodecylsulfate polyacrylamide gel electrophoresis in a discontinuous system. Equal loading of protein was confirmed using Coomassie blue staining and antiactin antibody as an internal control (Santa Cruz Biotechnology, USA). Separated proteins were transferred to 0.45 μm polyvinylidene fluoride membranes and incubated overnight with phospho- or total rabbit antibodies to the cell signalling molecules AktSer473, Ask1Ser83 and Ask1Thr845 (Cell Signaling, USA). Primary antibodies were detected using a goat antirabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibody (Bio-Rad, USA) using the BM Chemiluminiscence (POD) kit (Roche Diagnostics, Canada). The protein bands were visualized using Flour S-Multi- Imager MAX system (Bio-Rad, USA) or x-ray films (Thermo Scientific, USA) and quantified using image-analysis software (Quantity One, Bio-Rad, USA).
Apoptosis: Apoptosis was estimated by analyzing the cleavage of PARP and caspase 3, activity of caspase 3/7 and the ratio of Bax to Bcl-xL. PARP, caspase 3, Bax and Bcl-xL were analyzed using Western blotting. Caspase 3/7 activity was measured using Apo-ONE Homogeneous Caspase-3/7 Assay kit (Promega, USA) following the instructions provided. Briefly, Apo-ONE Caspase-3/7 reagent was added to the wells containing samples, blank and control. Fluorescence of each well was measured at 521 nm after incubation of the samples for 18 h at room temperature. The amount of fluorescence is proportional to the amount of caspase 3/7 activity.
Measurement of ROS: OS in isolated cardiomyocytes was assessed by quantifying the endogenous production of ROS by incubating the cardiomyocytes with 10 μM of 5-(6)-chloromethyl-2’7’-dihydroflourescein diacetate probe (CM-H2 DCFDA) (Molecular Probes, USA) for 30 min at 37°C in a humidified chamber [30]. The study was performed in triplicate in small dishes (35 mm2 × 10 mm) for each treatment group. Fluorescence of 100 cells from multiple fields per dish was recorded using an Olympus BX51 fluorescence microscope (Olympus Corporation, Japan). Fluorescence intensity, which is proportional to the level of ROS, was measured using a digital imaging processing software (Image Pro Plus). An excitation wavelength of 485 nm and emission wavelength of 530 nm were used. Quantitation was performed by normalizing the fluorescence intensity per unit area to that of the control group.
Statistical analysis: Data are expressed as mean ± SEM. Groups were compared using one-way ANOVA, or two-way ANOVA when more than one variable was analyzed for different groups. Bonferroni’s test was performed to identify differences between groups; P<0.05 was considered to be statistically significant. All statistical analyses were performed using Origin version 6 (OriginLab, USA).
Results
Time-course of Dox effects
The concentration of 5 μM Dox was selected for the present study because this concentration has been shown to reproduce plasma peak values achieved in patients receiving standard infusion [28]. To establish an optimal time of exposure to Dox, a time-course study of the effects of Dox was also conducted. Cardiomyocytes showed timedependent changes due to Dox (5 μM) treatment in the phosphorylation of AktSer473, p53, p38 and JNK as well as in the levels of cleaved caspase 3 and the ratio of Bax to Bcl-xL. Because the maximum change in all of the parameters studied was observed at 24 h (data not shown), the remainder of the study was performed at this time point.
Effect of Trolox on Dox-induced OS
OS in the cardiomyocytes was analyzed by measuring ROS fluorescence of DCF at 24 h (Figure 1A). The fluorescence intensity was quantified and the data are presented as mean fluorescence intensity per unit area (Figure 1B). Dox-treated cardiomyocytes showed a significant (P<0.05) increase in the fluorescence intensity per unit area over the control cardiomyocytes at 24 h of treatment (Figures 1A and 1B). Trolox was used as an antioxidant to study its protective role in Dox-induced OS. ROS production in Trolox treated cardiomyocytes was comparable with those of control cardiomyocytes, and pretreatment with Trolox for 4 h significantly (P<0.05) reduced Dox-induced ROS production in the Trolox + Dox group.
Figure 1: Effects of Trolox on doxorubicin (Dox)-induced oxidative stress as measured by dihydrofluorescein diacetate in rat cardiomyocytes at 24 h after treatment. A Representative fluorescence microscopic images of cardiomyocytes. B Fluorescence intensity expressed as mean fluorescence intensity (MFI)/unit area. Data represent three to five independent experiments and are presented as mean ± SEM; *P<0.05 versus control; †P<0.05 versus Dox
Effect of Trolox on Dox-induced apoptotic and antiapoptotic proteins
To study the modulation of Dox-induced apoptosis by Trolox, the cellular extracts were analyzed for PARP cleavage, activity of caspase 3/7, and Bax and Bcl-xL protein levels (Figure 2). The ratio of cleaved PARP (Figure 2A) was significantly higher in Dox-treated cardiomyocytes (P<0.05) and significantly lower in Trolox-treated cardiomyocytes (P<0.05) compared with the control. When compared with Dox-only treated cardiomyocytes, Trolox treatment significantly lowered the cleaved PARP in Trolox + Dox cardiomyocytes (P<0.05). Caspase 3/7 activity (Figure 2B) was significantly higher in Dox-treated cardiomyocytes (P<0.05). Trolox pretreatment showed a significant reduction in caspase 3/7 activity in Dox-treated cardiomyocytes (P<0.05). However, the ratio in the Trolox+Dox group was still significantly higher than the control group (P<0.05). The ratio of Bax to Bcl-xL was also significantly higher in Dox-treated cardiomyocytes (P<0.05), and Trolox treatment reduced this ratio significantly compared with Dox treatment (P<0.05) (Figure 2C).
Figure 2: Effects of doxorubicin (Dox) and Trolox on (A) poly-ADP ribose polymerase (PARP), (B) caspase 3/7 activity and (C) ratio of Bax to Bcl-xL in rat cardiomyocytes 24 h after treatment. Data represent three to five independent experiments and are presented as mean ± SEM. *P≤0.05 versus control; †P≤0.05 versus Dox
Effect of Trolox and interplay of signalling proteins (AktSer473, Ask1Thr845 and Ask1Ser83)
Inhibition of PI3K/Akt: To elucidate the molecular mechanism of protection against Dox-induced changes by Trolox, its effects on the phosphorylation of prosurvival protein Akt at Ser473, and redox sensor Ask1 at Thr845 and Ser83 sites with or without the PI3K/Akt inhibitor wortmannin were studied (Figure 3).
Figure 3: Effects of doxorubicin (Dox) and Trolox on levels of phosphorylated (A) AktSer473, (B) apoptosis signal-regulating kinase (Ask)1Ser83 and (C) Ask1Thr845 in rat cardiomyocytes at 24 h after treatment with and without wortmannin (a phosphoinositol 3-kinase/Akt inhibitor) pretreatment. Data represent three to five independent experiments and are presented as mean ± SEM. *P≤0.05 versus respective control; †P≤0.05 versus Dox; #P≤0.05 versus no inhibitor
Akt phosphorylation was significantly (P<0.05) reduced by Dox (Figure 3A). A small but significant (P<0.05) increase in Akt phosphorylation was recorded in Trolox-treated control cardiomyocytes. Furthermore, pretreatment with Trolox also prevented Dox-induced decline in Akt phosphorylation (Figure 3A). Treatment with wortmannin significantly inhibited the activation of Akt in all the treatment groups (P<0.05), and the inhibition was highest in Dox-treated cardiomyocytes (Figure 3A).
Phosphorylation of Ask1Ser83 in Dox-only treated cardiomyocytes declined significantly (P<0.05). Trolox-treated control cardiomyocytes exhibited a significantly higher level of Ser83 phosphorylation (P<0.05). Trolox also prevented Dox-induced decline in Ask1Ser83 phosphorylation, such that these values were no longer different from the control. However, the level was significantly higher compared with Dox-only treated cardiomyocytes (P<0.05) (Figure 3B). Phosphorylation at Ask1Ser83, which is a consensus site for activated Akt, was significantly inhibited by wortmannin in all the treatment groups (P<0.05) (Figure 3B). Phosphorylation of Ask1Thr845 (Figure 3C) was significantly higher in Dox-only treated cardiomyocytes (P<0.05). Phosphorylation in Trolox-treated control and Troloxpretreated Dox cardiomyocytes were comparable (P>0.05) with those of control cardiomyocytes. However, these values were significantly lower compared with Dox-only treated cardiomyocytes (P<0.05) (Figure 3C). Phosphorylation levels of Ask1Thr845 (Figure 3C) were upregulated significantly in the presence of the PI3K inhibitor in all four groups of cardiomyocytes (P<0.05); Dox-treated cardiomyocytes were affected to the greatest extent.
Loss or gain of Akt function
To further substantiate the role of Akt, the gain and loss of Akt gene function with CA-Akt or DN-Akt (T308A; S473A) mutant adenoviruses was also tested in the cardiomyocytes with all treatments. In a dose- (20 MOI, 50 MOI, 100 MOI) as well as time-dependent (24 h or 48 h) study of virus transfection using Ad.eGFP, a maximum expression of GFP was observed at 100 MOI and 48 h (Figure 4A).
Whether the conditions optimized with Ad.eGFP were able to overexpress or block Akt phosphorylation levels was confirmed using immunoblotting with anti-phospho-Akt antibody. Cardiomyocytes infected with CA-Akt showed phosphorylation in all four treatments (Figure 4B). AktSer473 phosphorylation in CA-Akt control cardiomyocytes was normalized to one to compare with all other treatment groups (Dox, Trolox and Dox + Trolox). Akt phosphorylation levels were significantly higher in the Trolox group (P<0.05), but the Trolox + Dox group was not significantly different from the Trolox group (P>0.05). On the other hand, cardiomyocytes infected with DN-Akt gene did not exhibit any phosphorylation at AktSer473 (Figure 4B), although control cardiomyocytes infected with DN-Akt showed some basal-level total Akt protein expression using Akt antibody (data not shown).
Figure 4: A Representative green fluorescent protein fluorescence microscopic images for rat cardiomyocytes infected with virus containing enhanced green fluorescent protein (Ad.eGFP) at three different multiplicities of infection (MOI) (20, 50 and 100) at 24 h and 48 h after virus infection; B Phosphorylation levels of Akt in rat cardiomyocytes infected with consititutively active Akt and dominant-negative Akt adenovirus at a multiplicity of infection of 100 for 48 h. Data represent three to five independent experiments and are mean ± SEM. *P≤0.05 versus control. Dox Doxycycline
CA-Akt and DN-Akt adenovirus were used as a tool to examine whether Akt regulates Ask1 expression. Ask1 phosphorylation at Ser83 and Thr845 was assessed in the cardiomyocytes treated with CA-Akt and DN-Akt adenovirus (Figure 5). The results showed that in Aktoverexpressed cardiomyocytes (Figure 5A), Ask1Ser83 phosphorylation levels were significantly (P<0.05) upregulated in the Trolox-treated control and Trolox + Dox groups compared with the control and Doxtreated cardiomyocytes. However, cardiomyocytes showed a significantly (P<0.05) lower phosphorylation level of Ask1Thr845 in all groups compared with the phosphorylation at Ser83. On the other hand, in DN-Akt treated cardiomyocytes, Ask1Thr845 levels were upregulated (Figure 5B). The levels were significantly higher in Dox-treated cardiomyocytes compared with the control (P<0.05). The levels of phosphorylation in the Trolox and Trolox+Dox groups were not significantly different from the control (P<0.05). However, there was a small but significant decrease in the phosphorylation of the Thr845 of the Trolox+Dox group compared with the Dox group (P<0.05). DN-Akt almost completely inhibited the Ask1Ser83 phosphorylation compared with phosphorylation at Ask1Thr845 in all four groups of cardiomyocytes (Figure 5B).
Figure 5: Levels of phosphorylated apoptosis signal-regulating kinase (Ask)1 at Ser83 and Thr845 in rat cardiomyocytes infected with (A) consitututively active Akt adenovirus; (B) dominant-negative Akt adenovirus at a multiplicity of infection of 100 for 48 h. Data represent three to five independent experiments and are mean ± SEM. *P≤0.05 versus control; †P≤0.05 versus doxycycline (Dox); #P≤0.05 versus phosphorylation seen in Ask1Ser83 (A) or Ask1Thr845 (A)
Discussion
Using adult rat cardiomyocytes, the present study demonstrated the interplay between Akt and Ask1 in the expression of the effects of Dox mediated through OS, along with the mitigating effects of Trolox. The study reports two major findings: Trolox prevents Dox-induced OS and apoptosis through the upregulation of PI3K/Akt signalling; and Troloxinduced Akt phosphorylation leads to a decrease in Ask1 MAP kinase activity by downregulating phosphorylation at the Thr845 site and upregulating phosphorylation at the Ser83 site. Ask1 phosphorylation at Ser83 is known as a consensus Akt phosphorylation site that promotes cell survival [31].
Dox-induced cardiotoxicity has been extensively reported in the literature [3,4,32,33]; however, its precise mechanism remains unclear. This has hampered the development of an effective clinical treatment for the prevention of Dox-induced cardiotoxicity. The present study demonstrates that Dox leads to a time-dependent increase in cardiomyocyte apoptosis and a decrease in the Akt cell survival signal. This was accompanied by Dox-induced upregulation of Ask1 activity by the phosphorylation at the Thr845 site. Pretreatment with Trolox decreased PARP cleavage, caspase 3/7 activation and the ratio of Bax to Bcl-xL, and rescued the cardiomyocytes from Dox-induced OS as well as apoptosis.
OS is a well-documented mediator of Dox-induced cardiotoxicity [3,4,7,34]. Trolox has been reported to be protective against OSinduced cell damage in various cell types including cardiomyocytes, both in vivo and in cultured cells [26,27,35,36]. We previously reported the protective effect of Trolox in Dox-induced OS and apoptosis [7]. The antioxidant Trolox is readily available to cardiomyocytes under in vitro conditions and, thus, is able to efficiently scavenge free radicals. Furthermore, increased OS has been implicated in the process of apoptosis in the cardiomyocytes under multiple stress conditions [37-40]. In the current study, we report that Trolox induces a decrease in apoptotic proteins (PARP, caspase 3, Bax) and an increase in antiapoptotic protein (Bcl-xL).
Trolox pretreatment increased the phosphorylation of Akt at Ser473 and decreased the phosphorylation of Ask1 at Thr845 in Doxtreated cardiomyocytes. On the other hand, phosphorylation of Ask1 at Ser83 was upregulated by pretreating cardiomyocytes with Trolox. Furthermore, cardiomyocytes pretreated with the PI3K/Akt inhibitor wortmannin showed decreased phosphorylation of Akt at Ser473 in all treatment groups. Akt inhibition led to the downregulation of the phosphorylation levels of Ask1Ser83, whereas the phosphorylation levels of Ask1Thr845 were upregulated. Because Akt inhibition prevented the Trolox-induced upregulation of Ask1Ser83 as well as promoted the upregulation of phosphorylation at Ask1Thr845, it is likely that Akt signalling is essential for Trolox protection in Dox-induced apoptosis in cardiomyocytes in vitro. Furthermore, we propose that Troloxinduced Akt phosphorylation leads to Ask1 inactivation in adult rat cardiomyocytes, thus affecting downstream cell signalling molecules. Trolox-induced protection in other biological systems has also been shown to be due to an increase in Akt phosphorylation [41,42]. A suppression of JNK and p38 activities has also been linked with increased Akt activity [43]. Conversely, inhibition of Akt by PI3K inhibitors has been shown to activate p38 and JNK [23]. Furthermore, a novel functional link between Akt and stress kinases p38 and JNK has been suggested through Ask1 in human embryonic kidney cell lines [23]. It has been reported that Akt phosphorylates its substrate Ask1 at Ser83, which caused a decrease in Ask1 kinase activity that lead to the decrease in p38 and JNK activities [23].
Our data regarding the pharmacological inhibitor was further supported by studies in which cardiomyocytes were infected with virus containing the DN-Akt gene. There was insignificant Ser83 phosphorylation and a significant increase in Thr845 phosphorylation when cardiomyocytes were incubated with adenovirus constructs containing DN-Akt mutants. This increase was further substantiated by Dox treatment, which was significantly different from Trolox-treated cardiomyocytes. Furthermore, when the Akt gene was overexpressed in cardiomyocytes, the phosphorylation levels of Ser83 were not affected by Dox treatment and, in fact, Ser83 phosphorylation levels were upregulated in cardiomyocytes pretreated with Trolox, confirming that Trolox activates the Akt survival signal. Akt gene transfer in the heart has also been shown to ameliorate Dox-induced contractile dysfunction (38). Akt-induced Ask1 inhibition has also been supported by the studies of heat shock proteins [21,40]. Similar to Trolox, selenite-induced suppression of Ask1/JNK through the activation of PI3K/Akt pathway during reperfusion has also been reported [44].
The mechanism of action of Trolox against Dox-induced OS and apoptosis in cardiomyocytes as supported by the data in the present study is presented schematically in Figure 6. Dox impaired cardiac cell survival through increased OS followed by the upregulation of Ask1Thr845 phosphorylation that further activated JNK, p38 and p53. This ultimately lead to mitochondrial dependent cell apoptosis by activating PARP, caspase 3 and Bax, and downregulating Bcl-xL. Trolox treatment ameliorated nearly all these changes caused by Dox via the activation of PI3K/Akt that lead to the activation of Akt consensus site at Ask1Ser83 and inactivating Ask1Thr845 and its downstream signalling molecules, as shown in the proposed scheme (Figure 6). The key finding of a switch of Akt regulation from Ask1Ser83 to Ask1Thr845 or vice versa determines the fate of the cardiomyocytes vis-à-vis cell survival or cell apoptosis. Inhibition of the prosurvival protein Akt using a PI3K/Akt inhibitor (wortmannin) and DN-Akt adenovirus caused the activation of apoptosis-inducing protein Ask1, and CA-Akt adenovirus caused the inactivation of Ask1 and promoted phosphorylation at Ask1Ser83. These impressive benefits of Trolox through the interplay between Akt and Ask1 may provide better molecular targets for the management of Dox-induced cardiomyopathy.
Figure 6: Schematic representation of doxorubicin (Dox) and Troloxinduced differential regulation of apoptosis signal-regulating kinase (Ask)1 at Thr845 and Ser83 sites. Dox-induced oxidative stress (OS) phosphorylates Ask1Thr845 and downregulates phosphorylation of AktSer473 and Ask1Ser83. Ask1Thr845 phosphorylation leads to phosphorylation of p38 and c-Jun N-terminal kinase (JNK) that upregulates: p53; the ratio of apoptotic protein Bax to antiapoptotic protein Bcl-xL; poly-ADP ribose polymerase (PARP); and caspase 3, resulting in cardiomyocyte apoptosis. Trolox reduces OS and enhances the phosphorylation of AktSer473, which upregulates Ask1Ser83 and blocks apoptotic signals by inhibiting Ask1Thr845. Inhibition of Akt using a phosphoinositol 3-kinase/Akt inhibitor (wortmannin), and dominant-negative adenovirus (DN-Akt) causes activation of Ask1 by phosphorylation at Thr845. Constitutive activation of Akt using Akt overexpression adenovirus (CA-Akt) negatively regulates Ask1Thr845 and positively regulates Ask1Ser83
Acknowledgements
The study was supported by an operating grant from CIHR. Dr Pawan Singal is the holder of the Dr Naranjan S Dhalla Chair in Cardiovascular Research supported by the St Boniface Hospital & Research Foundation.
References
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: The good, the bad and the ugly effect. Curr Med Chem 2009;16:3267-85.
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302-14.
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 1998;339:900-5.
- Singal PK, Iliskovic N, Li T, Kumar D. Adriamycin cardiomyopathy: Pathophysiology and prevention. FASEB J 1997;11:931-6.
- Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 1998;40:426-32.
- Zhang YW, Shi J, Li YJ, Wei L. Cardiomyocyte death in doxorubicininduced cardiotoxicity. Arch Immunol Ther Exp (Warsz) 2009;57:435-45.
- Kumar D, Kirshenbaum L, Li T, Danelisen I, Singal P. Apoptosis in isolated adult cardiomyocytes exposed to adriamycin. Ann N Y Acad Sci 1999; 874:156-68.
- Simunek T, Sterba M, Popelova O, et al. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep 2009;61:154-71.
- Ludke A, Sharma AK, Bagchi AK, Singal PK. Subcellular basis of vitamin C protection against doxorubicin-induced changes in rat cardiomyocytes. Mol Cell Biochem 2012;360:215-24.
- Dhingra S, Sharma AK, Singla DK, Singal PK. p38 and ERK1/2 MAPKs mediate the interplay of TNF-alpha and IL-10 in regulating oxidative stress and cardiac myocyte apoptosis. Am J Physiol Heart Circ Physiol 2007;293:H3524-31.
- Sugden PH, Clerk A. Oxidative stress and growth-regulating intracellular signaling pathways in cardiac myocytes. Antioxid Redox Signal 2006; 8:2111-24.
- Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J 1998;336(Pt 1):241-6.
- Liu AX, Testa JR, Hamilton TC, et al. AKT2, a member of the protein kinase B family, is activated by growth factors, v-Ha-ras, and v-src through phosphatidylinositol 3-kinase in human ovarian epithelial cancer cells. Cancer Res 1998;58:2973-7.
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002;296:1655-7.
- Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: The role of PI 3-kinase and Akt. J Mol Cell Cardiol 2005;38:63-71.
- Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther 2008;324:160-9.
- Negoro S, Oh H, Tone E, et al. Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/ caspase-3 interaction. Circulation 2001;103:555-61.
- Taniyama Y, Walsh K. Elevated myocardial Akt signaling ameliorates doxorubicin-induced congestive heart failure and promotes heart growth. J Mol Cell Cardiol 2002;34:1241-7.
- Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta 2008;1780:1325-36.
- Tobiume K, Matsuzawa A, Takahashi T, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2001;2:222-8.
- Zhang R, Luo D, Miao R, et al. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene 2005;24:3954-63.
- Ichijo H. From receptors to stress-activated MAP kinases. Oncogene 1999;18:6087-93.
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol 2001;21:893-901.
- Wang Q, Zhang QG, Wu DN, Yin XH, Zhang GY. Neuroprotection of selenite against ischemic brain injury through negatively regulating early activation of ASK1/JNK cascade via activation of PI3K/AKT pathway. Acta Pharmacol Sin 2007;28:19-27.
- Mickle DA, Li RK, Weisel RD, Tumiati LC, Wu TW. Water-soluble antioxidant specificity against free radical injury using cultured human ventricular myocytes and fibroblasts and saphenous vein endothelial cells. J Mol Cell Cardiol 1990;22:1297-304.
- Dean RT, Hunt JV, Grant AJ, Yamamoto Y, Niki E. Free radical damage to proteins: the influence of the relative localization of radical generation, antioxidants, and target proteins. Free Radic Biol Med 1991;11:161-8.
- Rubinstein JD, Lesnefsky EJ, Byler RM, Fennessey PV, Horwitz LD. Trolox C. A lipid-soluble membrane protective agent, attenuates myocardial injury from ischemia and reperfusion. Free Radic Biol Med 1992;13:627-34.
- Hempel G, Flege S, Wurthwein G, Boos J. Peak plasma concentrations of doxorubicin in children with acute lymphoblasticleukemia or non-Hodgkin lymphoma. Cancer Chemother Pharmacol 2002;49:133-41.
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemiareperfusion injury in mouse heart. Circulation 2000;101:660-7.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1199;96:857-68.
- Lou H, Kaur K, Sharma AK, Singal PK. Adriamycin-induced oxidative stress, activation of MAP kinases and apoptosis in isolated cardiomyocytes. Pathophysiology 2006;13:103-9.
- Lv X, Yu X, Wang Y, et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One 2012;7:e47351.
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther 1990;47:219-31.
- Mickle DA, Li RK, Weisel RD, et al. Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg 1989; 47:553-7.
- Zeng LH, Wu J, Carey D, Wu TW. Trolox and ascorbate: Are they synergistic in protecting liver cells in vitro and in vivo? Biochem Cell Biol 1991;69:198-01.
- Arola OJ, Saraste A, Pulkki K, et al. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res 2000;60:1789-92.
- Ichihara S, Yamada Y, Kawai Y, et al. Roles of oxidative stress and Akt signaling in doxorubicin cardiotoxicity. Biochem Biophys Res Commun 2007;359:27-33.
- Sharma AK, Dhingra S, Khaper N, Singal PK. Activation of apoptotic processes during transition from hypertrophy to heart failure in guinea pigs. Am J Physiol Heart Circ Physiol 2007;293:H1384-90.
- Fan GC, Zhou X, Wang X, et al. Heat shock protein 20 interacting with phosphorylated Akt reduces doxorubicin-triggered oxidative stress and cardiotoxicity. Circ Res 2008;103:1270-9.
- da Frota Junior ML, Pires AS, Zeidan-Chulia F, et al. In vitro optimization of retinoic acid-induced neuritogenesis and TH endogenous expression in human SH-SY5Y neuroblastoma cells by the antioxidant Trolox. Mol Cell Biochem 2011;358:325-34.
- Sun C, Wang D, Zheng W. Hydrogen peroxide attenuates the prosurvival signaling of insulin-like growth factor-1 through two pathways. Neuroreport 2012;23:768-73.
- Berra E, Diaz-Meco MT, Moscat J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J Biol Chem 1998;273:10792-7.
- Yoon SO, Kim MM, Park SJ, et al. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J 2002;16:111-3.