POTEG is a prognostic biomarker for ESCC
2 Hepatopancreatobiliary Surgery Department I, Key Laboratory of Carcinogenesis and Translational Rese, Peking University School of Oncology, Beijing Cancer Hospital and Institute, Beijing, China, Email: Xin-YuanGuan@gmail.com
3 State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China, Email: liy6@mail.sysu.edu.cn
Received: 18-Oct-2018 Accepted Date: Nov 15, 2018; Published: 25-Nov-2018
Citation: Li Y, Guan XY, Wang L. POTEG is a Prognostic Biomarker for ESCCJ. J Mol Cancer. 2018;1(3):20-21.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Keywords
Esophageal squamous-cell carcinoma (ESCC); Tumorigenesis; Tumor suppressors.
E sophageal Squamous-cell Carcinoma (ESCC) is a dominant histological subtype of esophageal cancer in China. The high incidence of ESCC in certain ethnic groups and locations is affected by environmental factors (alcohol and tobacco consumption) [1]. Familial aggregation and many studies indicate that genetic susceptibility, such as aberrant activation of oncogenes and inactivation of tumor suppressors, are related with the ESCC tumorigenesis [2]. POTE ankyrin domain family member G (poteg), also known as POTE-14 or ANKRD26-like family C member 2, is located at 14q11.2 [3]. It belongs to POTE family. In human genome, the POTE gene family is composed of at least 13 highly homologous paralogs with preservation of ORFs and splice junctions. The 13 POTE genes are dispersed among eight different chromosomes (2, 8, 13, 14, 15, 18, 21, and 22) and genomic sequence comparison suggests that POTE family genes may evolve from duplicating and remodeling ancestral genes ANKRD26 and ANKRD30A [4,5]. POTE proteins contain three domains: cysteine-rich domains, ankyrin repeat motifs and spectrin-like helices. The NH2-terminal cysteine-rich domain has an extracellular domain without a signal sequence [3,6]. Each ankyrin repeat motif contains a 33-amino acid sequence motif and the structure mediates protein–protein interactions. This protein recognition module is involved in various cellular functions, and consequently, defects in ankyrin repeats are related to a diverse set of human diseases [7]. The spectrin-like helices are similar to the α-helical coiled coil domain of spectrins which play an important role in building up the membrane skeleton [8]. The presence of ankyrin repeat motifs and spectrin-like helices in a single protein, and the observation that these proteins are associated with plasma membrane, suggest that POTE family proteins might facilitate or intercept signal transmission across the plasma membrane [3,9]. The POTE mRNAs were found in a very limited number of human normal tissues including prostate, testis, ovary and placenta [4]. However, POTE family members are expressed in many kinds of human cancers (colon, lung, breast, ovary, and pancreas) [10]. A PCR-based analysis was employed and found that the mRNA levels of POTE-2α, POTE-2β, POTE-2γ, and POTE-22 were predominantly upregulated in cancers compared to normal tissues [10]. A study indicated that deletion of 15q11.1-q11.2, a region encompassing NBEAP1and POTEB, might be associated with diffuse lymphangiomatosis [11]. Low serum POTEE is a positive prognostic factor for Progression-free Survival (PFS) in Non-small Cell Lung Carcinoma (NSCLC) patients [12]. In glioma patients, POTEH with promoter hypomethylation accounts for POTEH overexpression and poor clinical outcome [13]. And also, our research indicated that POTEG protein level was down-regulated in about 60% ESCC tumor tissues as well as in most ESCC cell lines. The multivariate analysis suggested that POTEG was an independent prognostic marker in ESCC patients [9]. Down-regulation of POTEG is significantly correlated with tumor cell differentiation, lymph node metastasis and advanced clinical staging [9]. Some POTE family members are highly expressed in primary spermatocytes, which are undergoing apoptosis during maturation, suggesting their important roles in inducing programmed cell death [14]. The specific mechanism of its pro-apoptotic function is related to the expression of endogenous POTE-actin fusion protein [15,16]. According to our results, the pro-apoptotic effect of POTEG is also related to activated caspases and decreased anti-apoptotic proteins [9]. Actually, POTEG suppresses ESCC tumor cell growth not only by pro-apoptotic effect but also by blocking G1/S transition. It down-regulates CDK2, CDK6, cyclin D1, and further reduces Rb phosphorylation [9]. As we know, cancer is developed from uncontrolled proliferation of tumor cell with relevant to aberrant activity of specific cell cycle proteins. In the late G1 phase, Rb plays a key role in regulating G1/S transition (a key checkpoint for cell cycle) and following controlled cell progression. Rb, phosphorylated by several cyclins and CDKs, is dissociated from transcription factor E2F, promoting the transcription of S-phase genes which stimulate cell growth [17,18]. POTEG down-regulates Rb phosphorylation and the blocking activity of E2F increases, and consequently ESCC cells growth is inhibited [9,18] (Figure 1). Metastasis is one of the leading causes of cancer-related deaths. A paralog of POTE, POTEF, inhibits metastatic of triple-negative breast cancer cells through regulating Ricinus communis agglutinin I (RCA-I) [19]. Our correlation study also revealed that down-regulation of POTEG was significantly correlated with lymph nodes metastasis in ESCC patients [9]. POTEG orverexpression could inhibit ESCC cells’ motility in vitro and in vivo by inhibiting EMT (Epithelial-Mesenchymal Transition) [9]. EMT is a critical process in embryonic development and now widely reported to play a prominent role in the tumorigenic process by which cancer cells gained motility and invasiveness [20,21]. Taken together, POTEG is a prognostic biomarker for ESCC and plays a critical role in inhibiting tumor cell proliferation and metastasis. Investigating the tumor suppressor gene poteg might shed light on an effective therapeutic strategy for ESCC patients.
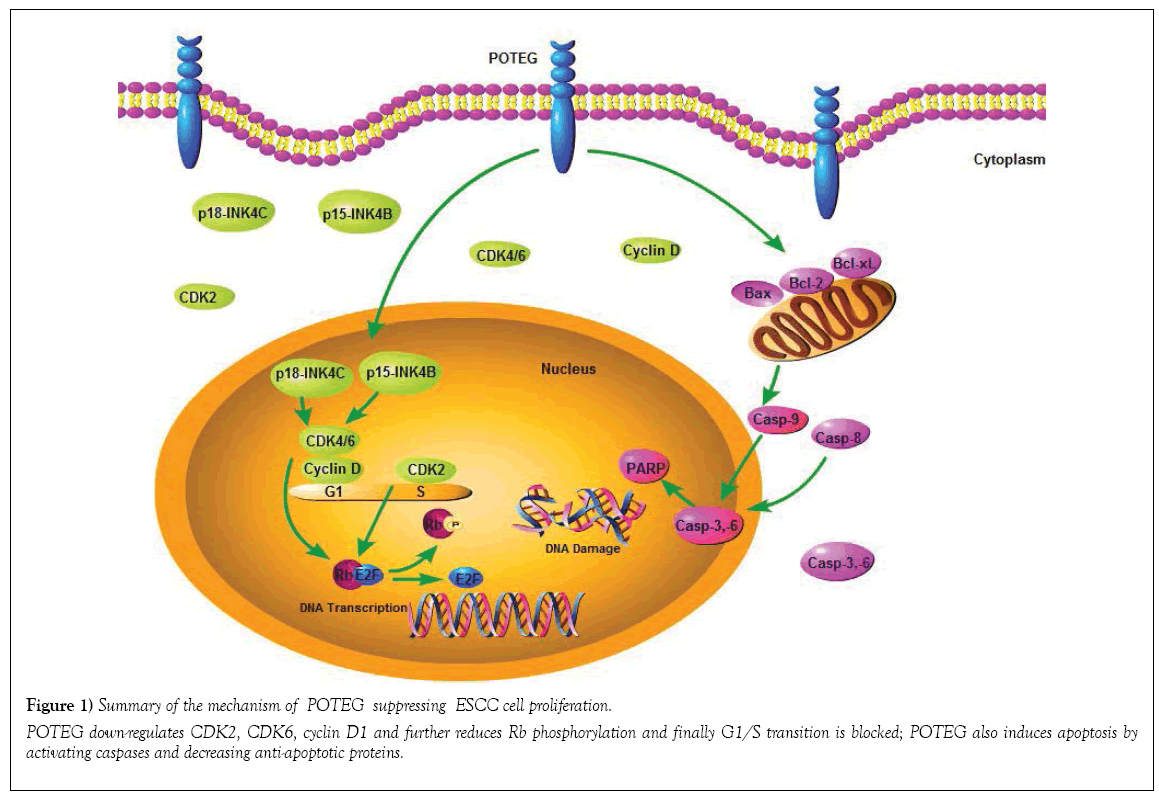
Figure 1: Summary of the mechanism of POTEG suppressing ESCC cell proliferation.
POTEG down-regulates CDK2, CDK6, cyclin D1 and further reduces Rb phosphorylation and finally G1/S transition is blocked; POTEG also induces apoptosis by
activating caspases and decreasing anti-apoptotic proteins.
REFERENCES
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400-12.
- Yen CC, Chen YJ, Chen JT, et al. Comparative genomic hybridization of esophageal squamous cell carcinoma: correlations between chromosomal aberrations and disease progression/prognosis. Cancer. 2001;92:2769-77.
- Bera TK, Huynh N, Maeda H, et al. Five POTE paralogs and their splice variants are expressed in human prostate and encode proteins of different lengths. Gene. 2004;337:45-53.
- Bera TK, Zimonjic DB, Popescu NC, et al. POTE, a highly homologous gene family located on numerous chromosomes and expressed in prostate, ovary, testis, placenta, and prostate cancer. PNAS. 2002;99:16975-80.
- Redfield SM, Mao J, Zhu H, et al. The C-terminal common to group 3 POTES (CtG3P): a newly discovered nucleolar marker associated with malignant progression and metastasis. Ame J Cancer Res. 2013;3:278-89.
- Hahn Y, Bera TK, Pastan IH, et al. Duplication and extensive remodeling shaped POTE family genes encoding proteins containing ankyrin repeat and coiled coil domains. Gene. 2006;366:238-45.
- Mosavi LK, Cammett TJ, Desrosiers DC, et al. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435-48.
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353-92.
- Wang L, Li M, Zhan Y, et al. Down-regulation of POTEG predicts poor prognosis in esophageal squamous cell carcinoma patients. Mol Carcinogen. 2018;57:886-95.
- Bera TK, Saint Fleur A, Lee Y, et al. POTE paralogs are induced and differentially expressed in many cancers. Cancer Res. 2006;66:52-6.
- Chen CP, Wang KG, Huang HK, et al. Detection of mosaic 15q11.1-q11.2 deletion encompassing NBEAP1 and POTEB in a fetus with diffuse lymphangiomatosis. Taiwan J Obstet Gyne. 2017;56:230-3.
- Wang Q, Li X, Ren S, et al. Serum levels of the cancer-testis antigen POTEE and its clinical significance in non-small-cell lung cancer. PloS one. 2015;10:e0122792.
- Liu X, Tang H, Zhang Z, et al. POTEH hypomethylation, a new epigenetic biomarker for glioma prognosis. Brain Res. 2011;1391:125-31.
- Bera TK, Walker DA, Sherins RJ, et al. POTE protein, a cancer-testis antigen, is highly expressed in spermatids in human testis and is associated with apoptotic cells. Biochem Bioph Res Co. 2012;417:1271-4.
- Liu XF, Bera TK, Liu LJ, et al. A primate-specific POTE-actin fusion protein plays a role in apoptosis. Apoptosis. 2009;14:1237-44.
- Lee Y, Ise T, Ha D, et al. Evolution and expression of chimeric POTE-actin genes in the human genome. PNAS. 2006;103:17885-90.
- Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93-115.
- Dick FA, Rubin SM. Molecular mechanisms underlying RB protein function. Nat Reviews Mol Cell Bio. 2013;14:297-306.
- Zhou SM, Cheng L, Guo SJ, et al. Lectin RCA-I specifically binds to metastasis-associated cell surface glycans in triple-negative breast cancer. Breast Cancer Research. 2015;17:36.
- Brabletz T, Kalluri R, Nieto MA, et al. EMT in cancer. Nat Rev Cancer. 2018;18:128-34.
- Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol. 2018;13:395-412.