Potential of plant flavonoids in pharmaceutics and nutraceutics
2 Bio Sciences Department, Comsats Institute of Information Technology, Islamabad (44000), Pakistan, Email: RumanaKeyani@gmail.com
Received: 03-Nov-2017 Accepted Date: Nov 15, 2017; Published: 23-Nov-2017
Citation: Hayat M, Abbas M, Munir F, et al. Potential of plant flavonoids in pharmaceutics and nutraceutics. J Biomol Biochem November-2017; 1(1): 12-17.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Plant flavonoids represent an important group of secondary metabolites and around 6500 varieties of flavonoids have been reported so far. These are the polyphenolic compounds that are found in almost all plant species. Extensive biological roles of flavonoids have been reported which include antiviral, anti-hepatotoxic, therapeutic, antibacterial, and other roles in nature. The presence of flavonoids in leaves enhances the physiological survival of plants by shielding them from parasitic diseases and UV radiations. Moreover, flavonoids also play role in photosensitisation, photosynthesis, respiration, sex-determination and morphogenesis. Plant flavonoids are generally found in low, variable concentrations and hence, it is challenging to find their consistent supply. Therefore, numerous biotechnological approaches have been explored for the enhanced production of these biologically and pharmacologically beneficial compounds. Flavonoids, mainly flavone-3-ols and proanthocyanins, have been related with decreased risk of cardiovascular disease by increasing the discharge of endothelial nitric oxide and preventing narrowing of the blood vessels. It has been reported that the free radical scavenging properties of these compounds may help lessen the risk of cancer. Flavonoids are also able to chelate (bind) metals, stimulate the immune system and reduce allergic responses and protect against bacteria and viruses. The present review highlights flavonoids as nutraceuticals and pharmaceuticals.
Keywords
Secondary metabolites; Flavonoids; Drugs; Polyphenolic Compound; Antibacterial; Antiallergic; Anti-hepatotoxic; Vasodilatory action; Therapeutic; Photosensitisation
Introduction
The word flavonoid is a Latin derivative of flavus which means ‘yellow’ owing to the colour of these compounds in nature. Flavonoids are phenolic compounds that are commonly found in almost all plant species. They have been referred to as ‘phytonutrients’, because they impart color and taste to numerous fruits and vegetables and protect their essential enzymes and vitamins making them suitable for consumption. Recently, rigorous research has been conducted to study therapeutic properties of flavonoids (Table 1).
| Flavonoids | Plants | Action | References |
|---|---|---|---|
| Apigenin | Matricaria recutica | Relaxant | [52] |
| Kaempferol and quercetin | Tilia sp | Tranquiliser | [54] |
| Quercetin | Calluna vulgaris | Nerve smoothening | [55] |
| Wogonin, Baicalein, and Baicalin | Scutellaria baicalensis | Hyperlipidaemia, Inflammatory diseases, Allergies, and Arteriosclerosis | [42] |
| Geraniol | Daucus carota | Anti-cancerous | [79] |
| Hydroxytyrosol | Olea europaea | Prevention of atherosclerosis | [80] |
| Rutin | Fagopyrum esculentum | Cardioc diseases, Prevent edema, anti-inflammatory property | [81] |
| Resveratrol | Vitis vinifera | Anti-diabetic, anti-oxidant, anti-cancerous | [82] |
Table 1: Table is showing different flavonoids present in different plants and their drug action.
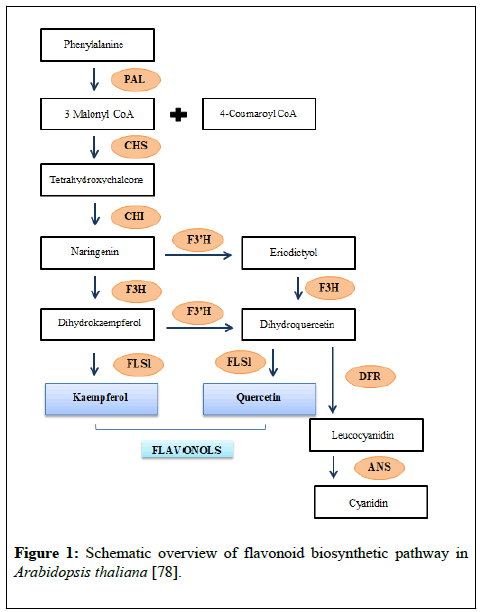
They have been reported beneficial for human health and play a significant role in minimizing effects of various diseases [1]. There are approximately 4000 varieties of the flavonoid compound group that have been identified. Flavonoids are known to be potent metal cheltors and antioxidants. This makes them beneficial chemical compounds that impart various health benefits. Some flavonoids such as the isoflavan called ‘glabridin’, found in Glycyrrhiza glabra, are involved in inhibition of LDL oxidation by scavenging free radicals. Similarly, it has been reported in many studies that flavonoids present in black and green tea are employed for treatment and prevention of cardiovascular diseases [2]. Flavonoids belong to a group of secondary metabolites that are pharmacologically active compounds with roles in plant defence mechanisms [3]. One of the important properties of flavonoids is their low solubility in water which makes them good potential cadidates for drug production. For example aglycones are less soluble in water, so after comsunption they remain in the intestine for less amount of time and have low rate of absorption, thus protenting human body from toxic effects of eating flavonoid rich food [4]. Flavonoids also increase bile discharge, reduce blood cholesterol and lipid levels; and exhibit antimicrobial role against few strains of microorganisms, for example, Staphylococcus aureus [5]. In general, secondary metabolites present in plants are utilized for developmental processes such as photosynthesis etc. These secondary metabolites resulting from phenylalanine and acetyl CoA have roles in plant reproduction, development, and survival [6]. Biosynthesis of flavonoids (Figure 1) starts with production of naringenin chalcone, by condensation of three malonyl-CoA molecules and one p-coumaroyl-CoA molecule in the presence of chalcone synthase (CHS) [7].
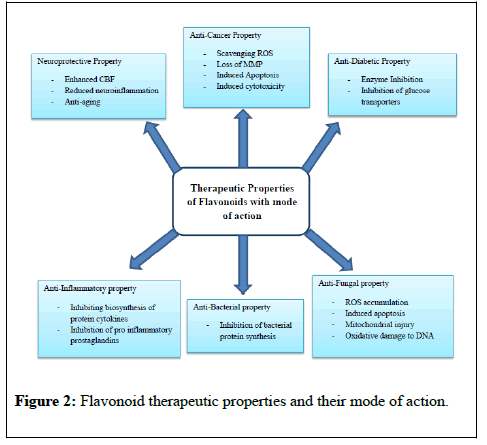
Subsequently, the naringenin chalcone is modified to its isomer naringenin flavanone stereospecifically by the action of chalcone isomerase (also known as chalcone-flavanone isomerase, CHI). Further, the hydroxylation of the naringenin flavanone B ring by flavonoid 3 ׳-hydroxylase (F3 ׳H) or flavonoid 3 ׳ 5׳ -hydroxylase (F3 ׳ 5׳ H) leads to synthesis of eriodictyol or pentahydroxyflavanone, respectively [8]. The synthesis of corresponding dihydroflavonols from three (2S)-flavanones is mediated by the catalyst flavanone 3β- hydroxylase (F3H, also known as FHT). Additionally, dihydrokaempferol, produced from naringenin flavanone directly by enzymatic oxidation, is also the potential substrate for F3 ׳H and F3 ׳ 5׳ H, to produce the corresponding dihydroflavonols, dihydroquercetin. FLS1 catalyses dihydrokaempferol and produces kaempferol (flavonol) while dihydroquercetin catalysed by dihydroflavonol reductase (DRF) to produce leucocyanidin which leads to the formation of proanthocyanidins or it may convert into cyanidin by anthocyanidin synthase (ANS) and forms anthocyanins [9]. Plant natural products have been used by humans as herbal medicine since ancient times. Among numerous plant natural products, flavonoids are phytochemicals used in medicines (Figure 2).
A number of plant species have been utilized as customary medications in European countries. Flavonoids have biological activities including antidepressant [10], cytotoxic, antitumor [11], antioxidant [12] and antiinflammatory activities [5,13]. The current review will reveal pharmaceutical properties of flavonoids which could be helpful for formulating drugs against several afore mentioned diseases.
Flavonoids as potential nutraceuticals
Flavonoids possess innumerable beneficial therapeutic properties. Hence, the concept of utilizing flavonoids as nutraceuticals is gradually emerging and gaining considerable interest. The term nutraceutical, which was coined by Stephen DeFelice, is a combination of two words, ‘nutrition’ and ‘pharmaceutical’. DeFelice described nutraceuticals as ‘a food or part of a food that provides medical or health beneifts, including the prevention and/or treatment of a disease’ [14]. Nutraceuticals may include dietary supplements, herbal extracts, processed food products such as beverages, cereals etc. Today, nutraceuticals are also called functional foods or nutritional supplements. Flavonoids, being ubiquitous in plants and food products of plant origin, constitute a major portion of nutraceuticals. Foods and beverages including berries, beans, nuts, grapes, red wine etc. are a rich source of flavonoids and are consumed worldwide. The several therapeutic and disease prevention properties of flavonoids which can make them an interesting candidate to be used as nutraceuticals have been described in the following sections.
Anti-oxidant properties: Plant flavonoids are a great source of antioxidant substances, equipped for minimizing the risk of cancer, aging and damaging free superoxide radicals. The adverse effects of oxidative processes on organic molecules like carbohydrates, lipids, DNA and proteins in biological systems are reduced by a wide range of substances found in flavonoids. Flavonoids are present in fruits and vegetables as phytonutrients, containing flavones and catechins, which are important sources of antioxidants. Antioxidant activity of flavonoids exhibit double action by scavenging ROS and by inhibiting oxidases. One of the important antioxidants is quercetin, which scavenges highly reactive species such as peroxynitrite and the hydroxyl radicals. Genistein is an isoflavonone, which is also reported as an important antioxidant [15]. The iron chelation activity of quercetin works to reduce oxidative injury induced in the erythrocyte membranes. This injury is induced by a number of oxidizing agents such as phenylhydrazine and acrolein [16]. One of the properties of some flavonoids is chelation with metal ions like copper and iron to reduce free radical development. Different diseases can be prevented by intake of antioxidants that are present in our food in the form of flavonoids in fruits and vegetables. They can help to reduce risk of cancer, liver disorder, heart diseases and diabetes. Interaction of these biomolecules with antioxidants in food enhances the activation of certain enzymes and inhibition of others [17]. Different plants have antioxidant flavonoids, for example Hibiscus sabdariffa extracts, which have potential to scavenge free radicals by conjugating with glucuronic acid and glutathione with increased uridine diphosphate glucuronosyltransferase (UDPGT) activity and glutathione S-transferase, respectively. Hibiscus sabdariffa infusions are useful in treating cancer because of their action as antioxidants and their enhancing of drug detoxification [18].
Antimicrobial properties: Research data indicates that plant flavonoids are the key area for anti-infective investigations, as these natural plant products depict antifungal, antiviral and antibacterial activities. Furthermore, studies have revealed interaction between flavonoids and chemotherapeutics [19]. In several pharmaceuticals, plant parts and their extracts are used to improve human immune system against diseases [20]. The properties of different wine constituents such as quercetin, rutin, vanillic acid, gallic acid and caffeic acid have been examined against pathogenic microorganisms [21]. Additionally, the antimicrobial activity of naringin and quercetin has also been reported [22]. Plants having higher amounts of carotinoids, flavonoids and vitamin C are found to be more effective and useful for improving immune system. Uncaria torments, Allium sativum, Echinacea are the plants rich in immunostimulatory molecules. Such plants can further help in production of lymphocyte, macrophages and natural killer cells. They also increase phagocytosis and interferon synthesis [23]. The consequences and effects of medicinal plants and oils extracted from such plants as immune stimulators, particularly the effects at microscopic level, are not well known [24]. Another research finding indicates that oil infusions from medicinal herbs help improve immune system [24]. Plants from different species rich in flavonoid are found exhibiting enhanced antibacterial activity [25]. Numerous flavonoids such as apigenin, galangin, glycosides, flavones, isoflavones, chalcones, flavanones, flavonol have shown effective antibacterial activity [26,27]. Flavonoids that act as antibacterial agents may possess different cell targets, as opposed to one particular site of activity. It has been reported that they have one unique molecular activity through which they are able to form non-specific bonds such as hydrophobic, hydrogen and covalent bonds, with proteins. It has also been investigated that flavonoids which have lipophilic nature may also disturb microbial membranes [27]. Thus, such antimicrobial functions can be correlated to minimizing microbial adhesions, intracellular transport proteins etc. The world is facing a serious problem of antibiotic resistant which makes several infections harder to treat. It is a threat to global health and development. A study has depicted the use of flavonoids to fight antibiotic resistant bacteria. In their study, Xu and Lee report that the flavonoid ‘myricetin’ inhibited growth of MDR (multi drug resistant) bacteria Burkholderia cepacia and the mode of action was reported to be the inhibition of protein synthesis by B. cepacia [28]. Similar results were observed when the effect of flavonoids were studied on the replication and infectivity of human viruses such as herpes simplex virus type 1 (HSV-1), respiratory syncytial virus (RSV) etc. The result of the study concluded that quercetin caused negative effects on the infectivity and replication of these viruses [29].
Role in cardiovascular diseases: Studies have reported that flavonoids help decline blood cholesterol and glucose levels in humans. Flavonoids present in Camellia sinensis (tea plant) are known to prevent cardiovascular diseases. Both green and black tea is a rich source of flavonoids. Sufficient intake of flavonoids is related to reduce effect of coronary heart disease [30]. Tea contains flavonoids which reduce levels of cholesterol in blood, damage caused by oxidative stress, lower blood pressure and inflammation. Many studies suggest that flavonoids of tea also enhance functions of the endothelial [31]. Similarly, bioactive compounds of flavonoids such as non-caloric, non-nutrient secondary metabolites, polyphenolic are found mainly in cocoa, wine, tea, vegetables, nuts and fruits. These flavonoids may reduce LDL cholesterol and regulate anti-inflammatory and antioxidant activities [32]. A good source of quercetin is cranberries, which can help lower the blood pressure [33]. Heart diseases are a major cause of mortality worldwide and polyphenols reduce the possibility of cardiovascular diseases. A study investigated that specific hydroxyl (-OH) groups on the isoflavone structure were critical for inhibition of the phosphodiesterase isoenzymes [34]. This may also explain the therapeutic effects of flavonoids on platelet aggregability and blood pressure.
Anti-diabetic properties: Diabetes mellitus (DM) is a chronic metabolic disorder characterized by defective insulin secretion or function, or both. Diabetes mellitus is prevalent worldwide and has been reported in all age groups. It was estimated that its prevalence in all age groups may reach upto 4.4% globally by 2030. There are two types of diabetes Type 1 and Type 2. The Type 1 diabetes is caused by degradation of pancreatic β- cells, which results in lack of insulin in the body. Type 2 diabetes occurs when body becomes resistant to effects of insulin (insulin resistance) or the pancreas loses the ability to produce enough insulin. Diabetes can be cured by natural flavonoids present in plants such as shamimin, diadzein, epicatechin, myricetin, epigallocatechin, hesperidin, naringenin, hesperitin, chrysin, apigenin, genistein, kaempferol, luteolin, quercetin, etc [35].
Flavonoids can restrain catalyst aldose reductase that converts sugars to sugar alcohols and are involved in diabetic intricacies, for example, neuropathy, cardiac disorder and retinopathy [36]. Another study has proved the role of flavonoids in inhibiting α-glucosidase and α-amylase, the key enzymes involved in carbohydrate digestion [37]. This inhibition leads to suppression of carbohydrate digestion and consequently, the glucose absorption hence imparting hypoglycemic effects. Another mechanism, by which flavonoids are known to help reduce hyperglycemia, is by interrupting absorption of glucose from the intestine. Catechin, a flavan-3-ol, is a known inhibitor of the sodium-dependent glucose transporter, SGLT1 [38].
Anti-cancer agents: Consumption of more flavonoid rich foods has been reported to lower the risk of cancer. There are various mechanisms of action reported in this regard including cell cycle arrest, inhibiting proliferation, antioxidation, induction of apoptosis etc. A very recent study conducted on Andrographis glandulosa, discovered rich sources of flavonoids present in the plant. HeLa cells treated with flavonoid extracts from Andrographis glandulosa, exhibited loss in their mitochondrial membrane potential (MMP) and induced apoptosis. This cytotoxic activity of the flavonoid extracts makes them promising candidates for production of anticancer drugs [39].
Flavonoids have the potential to treat cancer at any stage [40], so it is necessary to recognize harmless anticancer constituents from plants as crucial for treatment. Since the herbal infusions are complex mixture, distinctive constituents may have contradicting actions [41], which may decrease the useful effects of plant extracts [42]. Hence, separation of effective components from the plant infusions is essential for producing anticancer medicines. Scutellaria baicalensis infusions have the potential to be utilized as chemoprotective against many types of cancers. These infusions have been reported to cause growth inhibiton in several cancer cell lines such as breast cancer, colon cancer, hepatocellular carcinoma, squamous cell carcinoma etc [43]. Another study concerning the bioactive flavonol ‘fisetin’, showed that treatment of human melanoma cells with fistein caused reduced cell viability by moderating G1 phase arrest [44].
Anti-inflammatory agents: Flavonoids are present in various plant parts and possess anti-inflammatory properties [45]. Fisetin, lutoelin and apigenin are some of the flavonoids reported to have good antiinflammatory properties. The anti-inflammatory property of fisetin has shown to diminish effects of asthma, a disease caused by airway inflammation [46]. In China and Japan, most of the herbs used in medicines and infusions are obtained from the roots of Scutellaria baicalensis. These roots have several groups of flavonoids for example wogonin, baicalein, and baicalin, that have medicinal properties. Scutellaria baicalensis infusions have been used for hyperlipidemia, inflammatory diseases, allergies, and arteriosclerosis [47].
The Desmodium plant, which is an important member of Papilionaceae (Fabaceae) family, consists of nearly 350 different types of species. It is predominantly spread worldwide in tropical and subtropical areas. Among these, approximately 28 types of species are found in China only [48]. The species of this category have been used to isolate nearly 200 compounds including steroids, flavonoids, terpinoids, alkaloids, and phenylpropanoids, whereas, the biological activities have been verified in few plants. Alkaloids and flavonoids are considered as chief components which play major role for such activities undertaken by flora of this genus [49]. Besides, in vivo and in vitro experiments undertaken have proved that the Desmodium plant extracts have a wide-range of pharmacological properties. These improve the cardiovascular and cerebrovascular function besides improving immunity system, imparting anti-inflammatory, nootropic, antimicrobial, anti-diabetic, antifungal, and cyto-toxic functions [22].
It has also been found that flavonoids impart their anti-inflammatory action on the biosynthesis of protein cytokines that moderate attachment of circulating leukocytes to the location of injury. Few flavonoids are potent inhibitors of synthesis of dominant proinflammatory molecules called ‘prostaglandins’ [50]. Several flavonoids are involved in platelet adhesion, aggregation, and secretion significantly at 1-10 mM concentration [51]. The effects of flavonoid on platelets have been related to the carbon monoxide prohibition of arachidonic acid metabolism [52].
Neuroprotective activity: Flavonoids and phenolic compounds have biological activities such as, antidepressant [10], cytotoxic and antitumor [11], antioxidant [12], and anti-inflammatory [13,14] activities. Flowers obtained from Camomile (Matricaria recutica) have been been employed for their nerve-relaxing propoerties, due to presence of apigenin which belongs to the flavone class [53]. Tanacetum parthenium commonly known as Feverfew has also been utilized as a prophylactic solution for treatment of headaches [54]. Tilia sp. such as Linden blooms have been employed globally as tranquilizer and it has been demonstrated that kaempferol as well as quercetin flavonoids have some soothing impact [55]. Heather, (Calluna vulgaris) which is extinct now, had been utilized as a nerve soothing agent and was thought to have MAO-A diminishing action, through quercetin being the dynamic part [56]. Evidence suggests that inflammation of the neuron cells and oxidative stress are major causes of decline of the cognitive function. Cocoa flavonoid nutraceuticals can be great in this regard due to their neuroprotective activities. One of such activities is increased cerebral blood flow. Human trials conducted in this regard reported that a 900 mg/day treatment with cocoa for a week increased CBF in grey matter [57]. A meta-analysis of three individual studies with 114, 009 participants reported reduction of risk of stroke by 29% in high chocolate consumers compared with consumers who had low intake [58].
Antifungal activity: Antifungal activities have been reported in flavonoids such as neohesperidin, hesperidin, naringin and some of the derivatives isolated from the citrus plants. These are modified enzymatically and are being studied on four fungi that usually contaminate food. These contaminating fungi are Fusarium semitectum, Aspergillus parasiticus, Penicillium expansum, Aspergillus flavus. Similarly mycelia growth of the Penicillium expansum is inhibited by flavonoid ‘hesperetin’. Growth of the Fusarium semitectum, Aspergillus parasiticus, Aspergillus flavus can also be inhibited by another flavonoid called ‘prunin decanoate’ [59].
Various studies have suggested the antifungal action of flavonoids. This has been reported for Aspergillus flavus, as its growth is affected by flavones extracted from the Artemisia giraldii [60]. Similarly, Eysenhardita texana have some prenylated flavanones that work against Candida albicans [61]. Antifungal activity has also been reported in flavonoids ectracted from citrus fruits after processesing in industries and bergamot peel, which is used to stop the activity of Saccharomyces cervisiae [62]. One of the flavanol commonly present in propolis, a chemical produced by bees, is suggested to be used against moulds [63]. Grapes are rich source of flavonoids and their pomaces are used to stop the growth of Zygosaccharomyces bailii and Zygosaccharomyces rouxii [64] and Chilean grape pomace extract is reported to have antifungal activity against Botrytus cinerea [65]. The growth of common causative agents of diseases in human Candida albicans, can be stopped by extracts from Brazilian grapes which contains flavonoids [66]. A study conducted in this regard exhibited that flavonoids enhanced antifungal action of fluconazole (an antifungal drug) when both were used as a cotreatment [67]. The mode of actions studied for the antifungal activity was induction of apoptosis, ROS accumulation, DNA fragmentation, mitochondrial damage etc.
Biotechnological approaches towards enhanced production of flavonoids in plants
Numerous chemicals have been extracted from plants and are being employed for the formulation of drugs. In spite of the enormous developments made in unravelling the flavonoid biosynthetic pathway, the biosynthesis of target flavonoids from plant for drug discovery encounters innumerable defies. The utilization of plants as bioreactors for the sufficient production of a target flavonoid has been limited due to presence of low amount of a specific metabolite and restrictions to diversify basic chemistry of the compound, which ultimately restricts its application in basic research, development, and in clinical trials [68,69]. Biotechnological approaches such as metabolic engineering and plant cell and tissue culture techniques are the potential targets for higher production of secondary metabolites in plants for drugs production [70].
Metabolic engineering is an emerging technique for enhanced production of a specific secondary metabolite. Several methods are being used for altering the genes, enzymes and proteins involved in synthesis of a metabolite. Similarly competitive pathways can be blocked by antisense genes which result in higher production of desired secondary metabolites [71]. For plants to be engineered for the elevated production of plant secondary metabolites such as flavonoids, information about their biosynthetic pathway is required. Many approaches can be applied such as over-expression of regulatory genes, reducing the competitive pathway, overcoming rate limiting steps etc. Utilizing micro-organisms for over expression of a plant gene is another method, which is useful bioconversion of precursors into desired chemicals [72]. Cell and tissue culture represent another approach for the extraction of desired chemical compounds for drugs production. However, there are certain issues associated with plant cell culture which include genetic instabilities, slow and variable culture growth, increased susceptibility to stress and aggregation [73]. The problem has been addressed by the initiation of culture using undifferentiated cambial meristematic cells (CMC), which are multipotent with stem-cell-like properties instead of dedifferentiated cells (DDC) [74]. The utilization CMCs can provide a strong foundation for the future metabolic engineering strategies, and may ultimately enhance flavonoid biosynthesis.
Another novel approach that may evade the limited production of plant flavonoids is the biochemical synthesis in microorganisms [69]. Both yeast and bacteria have been used as model organisms for bioreactorbased flavonoid production [75]. Gut bacteria Escherichia coli and the bread and wine yeast Saccharomyces cereviciae [76] have been utilized for the biosynthesis of different flavonoids. This has been made possible through the simultaneous co-expression of several downstream enzymatic activities in a flavonone biosynthetic pathway [77].
Conclusion
Today, a growing demand exists for nutraceuticals globally. They possess superiority over other therapeutic agents because they are natural in origin, safe to consume and are easy to access. But, there are still many challenges ahead of establishing nutraceutical industries. These challenges include the need to accurately test the efficacy and safety of such chemical compounds, identify their exact mode of action, evaluate their bioavailability and study possible interactions with various body organs and systems [78-82]. Research has proven the health benefits of flavonoids against a number of public health concerns such as heart diseases, diabetes, cancer etc. This strongly indicates that flavonoids are worthy of more research as potent nutraceuticals. Therefore, concerns with their use as nutraceuticals should be addressed so that their remarkable array of pharmacological actions can be benefited from.
REFERENCES
- Mishra J, Dash AK, Dash DK. Medicinal And Therapeutic Potentialities Of Green Tea (Camellia Sinensis)- A Review. World J Pharm and Pharmaceutical Sciences. 2013;2: 4745-4763.
- Jiang J, Zhang X, True AD, et al. Inhibition of Lipid Oxidation and Rancidity in Precooked Pork Patties by Radical‐Scavenging Licorice (Glycyrrhiza glabra) Extract. J food sci. 2013;78:C1686-94.
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food chemistry. 2003;81:321-26.
- Asha MK, Debraj D, Prashanth D et al. Gastro Protective and Anti-Helicobacter Pylori Effects of a Flavonoid rich Fraction obtained from Achyrocline Satureoides (lam) dc. InteR J Pharm and Pharmaceutical Sciences. 2014;6:177.
- Debeaujon I, Nesi N, Perez P, et al. Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell. 2003;15:2514-31.
- Sun W, Meng X, Liang L, et al. Molecular and biochemical analysis of chalcone synthase from Freesia hybrid in flavonoid biosynthetic pathway. PLoS One. 2015;10:e0119054.
- Castellarin SD, Pfeiffer A, Sivilotti P, et al. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Cell & Envir. 2007;30:1381-99.
- He F, Mu L, Yan GL, et al. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules. 2010;15:9057-91.
- Paulke A, Noldner M, Zsilavecz MS, et al. St. John's wort flavonoids and their metabolites show antidepressant activity and accumulate in brain after multiple oral doses. J Pharm Sci. 2008;63:296-302.
- Murakami T, Ajima k, Miyawaki J, et al. Drug-loaded carbon nanohorns: adsorption and release of dexamethasone in vitro. Mol Pharm. 2004;1:399-405.
- Ghasemzadeh A, Ghasemzadeh N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J Med Plant Res. 2011;5:6697-703.
- Lee JH, Park KH, Lee MH. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food chemistry. 2013;136:843-52.
- Araujo C, Leon L. Biological activities of Curcuma longa L. Memórias do Instituto Oswaldo Cruz. 2001;96:723-28.
- DeFelice SL. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci Technol. 1995;6:59-61.
- Unnikrishnan MK. Antidiabetic, antihyperlipidemic and antioxidant effects of the flavonoids. Polyphenols in Human Health and Disease. 2014;1:143-61.
- Prochazkova D, Bousova I, Wilhelmova N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82:513-23.
- Ajiboye TO, Salau AK, Yakubu MT, et al. Acetaminophen perturbed redox homeostasis in Wistar rat liver: protective role of aqueous Pterocarpus osun leaf extract. Drug Chem Toxicol. 2010;33:77-87.
- Ajiboye TO, Salawu NS, MT Yakubu, et al. Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem Toxicol. 2011;34:109-15.
- Atoui AK, Mansouri A, Boskou G, et al. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food chemistry. 2005;89:27-36.
- Vaquero MR, Alberto M, de Nadra MM. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18:93-101.
- Ma X, Zheng C, Hu C, et al. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J Ethnopharmacol. 2011;138:314-32.
- El-Moez SIA, Abdelmonem MA (2013) Effect of Sterilization on Microbial Contaminants in Medicinal Herbs and their Antimicrobial Activities against Animal Foodborne Pathogens. in Proceedings of the 6th Scientific Conference of Animal Wealth Research in the Middle East and North Africa, Hurghada,. Massive Conferences and Trade Fairs Egypt.
- Aghsaghali MA. Importance of medical herbs in animal feeding: A review. Ann Biol Res. 2012;3:918-23.
- Mishra A, Kumar S, Pandey AK. Scientific validation of the medicinal efficacy of Tinospora cordifolia. The Scientific World Journal. 2013;13; 8.
- Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26:343-56.
- Mishra AK, Mishra A, Kehri HK, et al. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann Clin Microbiol Antimicrob. 2009;8:9.
- Xu H, Lee SF. Activity of plant flavonoids against antibiotic-resistant bacteria. Phytother Res.2001;15:39-43.
- Tej NK, Middleton EO, Pearay L. Antiviral effect of flavonoids on human vriuses. J Med Virol.1985;15:71-9.
- van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol. 2013;24:25-33.
- Bøhn SK, Ward NC, Hodgsona JM et al. Effects of tea and coffee on cardiovascular disease risk. Food & function. 2012;3: 575-91.
- McCullough ML, Peterson JJ, Patel R, et al. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454-64.
- Novotny JA, Baer DJ2, Khoo C, et al. Cranberry Juice Consumption Lowers Markers of Cardiometabolic Risk, Including Blood Pressure and Circulating C-Reactive Protein, Triglyceride, and Glucose Concentrations in Adults. J Nutr. 2015;56:203190.
- Nichols MR, Morimoto BH. Differential inhibition of multiple cAMP phosphodiesterase isozymes by isoflavones and tyrphostins. Mol Pharmacol.2000;57:738-45
- Mohan V, Pradeepa R, Anjana RM. Epidemiology and Its Application to Clinical Care in Diabetes. Improving Diabetes Care in the Clinic. 2014:31.
- Kaul C, P Ramarao. The role of aldose reductase inhibitors in diabetic complications: recent trends. Methods Find Exp Clin Pharmacol. 2001;23:465.
- Tadera K, Minami Y, Takamatsu K, et al. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol. 2006;52:149-53.
- Kobayashi Y, Suzuki M, Satsu H, et al. Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem. 2000;48:5618-23.
- Cherukupalli N. Phytochemical profiling and In vitro anticancer activity of purified flavonoids of Andrographis glandulosa. Planta Med. 2017;4:e24-34
- Majewska WM, Czeczot H. Anticancer activity of flavonoids. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego, 2012;33:364-69.
- Sengupta S, Toh SA, Sellers LA, et al. Modulating angiogenesis the Yin and the Yang in ginseng. Circulation. 2004;110:1219-25.
- Matthias A, Banbury L, Bone KM, et al. Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia. 2008;79:53-8.
- Ye F, Xui L, Yi J, et al. Anticancer activity of Scutellaria baicalensis and its potential mechanism. J Altern Complement Med. 2002;8:567-72.
- Syed DN, Afaq F, Maddodi N, et al. Inhibition of human melanoma cell growth by the dietary flavonoid fisetin is associated with disruption of Wnt/beta-catenin signaling and decreased Mitf levels. J Invest Dermatol. 2011;131:1291-99.
- Funakoshi-Tago M, Nakamura K, Tago K, et al. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int Immunopharmacol. 2011;11:1150-59.
- Tanaka T, Takahashi R. Flavonoids and asthma. Nutrients.2013;5:2128-43.
- Wang CZ. Botanical flavonoids on coronary heart disease. Am J Chem Med. 2011;39:661-71.
- Houghton PJ, Ren Y, Howes MJ. Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep. 2006;23:181-99.
- Dzoyem JP, Hamamoto H, Ngameni B et al. Antimicrobial action mechanism of flavonoids from Dorstenia species. Drug discoveries & therapeutics. 2013;7:66-72.
- Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000;7:S29-34.
- Beretz A, Cazenave JP. The effect of flavonoids on blood-vessel wall interactions. Prog Clin Biol Res. 1988;280:187.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;6:176
- Sarris J, McIntyre E, Camfield DA. Plant-based medicines for anxiety disorders, part 2: a review of clinical studies with supporting preclinical evidence. CNS drugs. 2013;27:301-19.
- Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471-85.
- Hernández EA, González-Trujano ME, Martínez AL, et al. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J Ethnopharmacol. 2010;127:91-97.
- Saaby L, Rasmussen HB, Jager AK. MAO-A inhibitory activity of quercetin from Calluna vulgaris Hull. J Ethnopharmacol. 2009;121:178-81.
- Fisher ND, Sorond FA, Hollenberg NK. Cocoa flavanols and brain perfusion. J Cardiovasc Pharmacol. 2006; 47:S210-14.
- Lopez AB, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488.
- Salas MP, Celiz G, Geronazzo H, et al. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011;124:1411-15.
- Zheng WF, Tan RX, Yang L, et al. Two flavones from Artemisia giraldii and their antimicrobial activity. Planta medica. 1996;62:160-62.
- Cushnie T, Lamb AJ. Antibacterial and antifungal flavanones from Eysenhardtia texana. Phytochemistry. 1999;52:1469-71.
- Mandalari G. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J Appl Microbiol. 2007;103: 2056-64.
- Afolayan AJ, Meyer JJM. The antimicrobial activity of 3, 5, 7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J Ethnopharmacol. 1997;57:177-81.
- Sagdic O. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011;126:1749-58.
- Mendoza L, Yañez K, Vivanco M, et al. Characterization of extracts from winery by-products with antifungal activity against Botrytis cinerea. Ind Crops Prod. 2013;43:360-64.
- Han Y. Synergic effect of grape seed extract with amphotericin B against disseminated candidiasis due to Candida albicans Phytomedi. 2007;14:733-38.
- Silva CR, Andrade Neto JB, Campos R, et al. Synergistic effect of the flavonoid catechin, quercetin, or epigallocatechin gallate with fluconazole induces apoptosis in Candida tropicalis resistant to fluconazole. Antimicrob Agents Chemother. 2014;58:1468-78.
- Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67:2141-53.
- Leonard E, Lim KH, Saw PN, et al. Engineering central metabolic pathways for high-level flavonoid production in Escherichia coli. Appl Environ Microbiol. 2007;73:3877-86.
- Chandra S. Natural plant genetic engineer Agrobacterium rhizogenes: role of T-DNA in plant secondary metabolism. Biotechnology letters. 2012;34:407-15.
- Verpoorte R, Memelink J. Engineering secondary metabolite production in plants. Current opinion in biotechnology. 2002;13: 181-87.
- Verpoorte R Heijden RV, Memelink J. Engineering the plant cell factory for secondary metabolite production. Transgenic research. 2000;9:323-43.
- Howat S, Park B, Oh IS, et al. Paclitaxel: biosynthesis, production and future prospects. N Biotechnol. 2014;31:242-45.
- Lee EK, Jin YW, Park JH, et al. Cultured cambial meristematic cells as a source of plant natural products. Nat Biotech. 2010;28:1213-17.
- Zhang Y, Li SZ, Li J, et al. Using unnatural protein fusions to engineer resveratrol biosynthesis in yeast and mammalian cellsJ Am Chem Soc. 2006;128:13030-31.
- Chemler JA, Yan Y, Koffas MAG, Biosynthesis of isoprenoids, polyunsaturated fatty acids and flavonoids in Saccharomyces cerevisiae. Microb Cell Fact. 2006;5:20.
- Beekwilder J, Wolswinkel R, Jonker H, et al. Production of resveratrol in recombinant microorganisms. Appl Environ Microbiol. 2006;72:5670-72.
- Sut S, Baldan V, Faggian M, et al. Nutraceuticals, a New Challenge for Medicinal Chemistry. Curr Med Chem 2016;23:1-26.
- Owens DK, Alerding AB, Crosby KC, et al. Functional analysis of a predicted flavonol synthase gene family in Arabidopsis. Plant Physio. 2008;147:1046-61.
- Kai G, Wu C, Gen L, et al. Biosynthesis and biotechnological production of anti-cancer drug Camptothecin. Phytochem Rev. 2015;14:525-39.
- Zrelli H, Kusunoki M, Miyazaki H. Role of Hydroxytyrosol dependent Regulation of HO Expression in Promoting Wound Healing of Vascular Endothelial Cells via Nrf De Novo Synthesis and Stabilization. Phytothera Res. 2015;6;109.
- Lee SJ. Rutin Attenuates lipopolysaccharide-induced nitric oxide production in macrophage cells. J Food Nutr Res. 2015;3:202-05.
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health-a comprehensive review of human clinical trials. Molr nutri & food res. 2011;55:1129-41.