Predictors of spontaneous bacterial peritonitis in patients with cirrhotic ascites
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Spontaneous bacterial peritonitis (SBP) is a serious complication of liver cirrhosis and finding a prognostic model to predict it is needed. Objective: to test the ability of different laboratory tests and the new scoring system consisting of age, CRP and platelet count by Wehmeyer and colleagues to predict it. METHODS: 300 patients admitted at the National Liver Institute, University of Menoufyia, Egypt (2015-2016) with liver cirrhosis and ascites were included in our study. SBP was diagnosed if ascetic neutrophils count ≥ 250/μL with no sign of secondary peritonitis. RESULTS: Age range (29 –81 years), 60% men and primary cause of liver disease were hepatitis C, 91.7%. By univariate analysis: age, total bilirubin, AST, creatinine, INR, MELD score, total leucocytic count, platelet count and CRP were significant. By multivariate analysis independent predictors were age, platelet count and CRP (p=0.004, 0.013 and<0.001, respectively). CRP at a cutoff point ≥ 13.5 mg/L could predict SBP (sensitivity 86.4%and specificity 66.0%). Wehmeyer’s SBP scoring system was able to predict it (p<0.001), only 4% of patients with 0 score developed SBP (CRP cutoff is 30 mg/L), while 92.8% with score of 3 or 4 developed it. By using our modified Wehmeyer score, CRP cutoff value of 13.5 mg/L, no patient with 0 score developed SBP. CONCLUSION: Age, CRP and platelet count are independent predictors for SBP and a scoring system including them could easily predict it. SBP diagnosis could be excluded inpatients with zero score, using CRP cutoff value of 13.5 mg/L.
Keywords
CRP; Predictors; SBP; Cirrhosis; Scoring
Liver disease burden is increasing all over the world and in Egypt is manifesting more due to its high prevalence of HCV [1]. Patients with advanced liver disease are at high risk to develop severe complications, one of them is spontaneous bacterial peritonitis (SBP). Its incidence was reported to reach 25% [2,3] of the cirrhotic patients with ascites, with mortality rate of 20 to 40% [4,5]. There is a real need to find a non-invasive prognostic scoring system to predict patients more liable to develop it, as early treatment could reduce mortality rate [2]. Multiple laboratory tests were introduced as predictors to SBP as C reactive protein CRP [6,7]. Platelet count [6-8]. Impaired prothrombin time [9]. serum creatinine [9], beside liver disease scoring systems like Child-Pugh (CP)8and Model of End stage Liver Disease (MELD) scores [10]. but only contradictory data is available. A new simple SBP prognostic scoring system consisting of age, platelet count and CRP was recently developed by Wehmeyer et al. [7].
Our aim was to study the ability of different clinical and laboratory variables that could predict the development of SBP in Egyptian cirrhotic patients with ascites, and also to test the scoring system consisting of age, CRP and platetets count and its cutoff values.
Methods
Adult patients (>18 years) with liver cirrhosis and ascites admitted for different reasons at the National Liver Institute (NLI) hospital, University of Menoufyia, from March 2015 to December 2016 were included in our study. Data were collected on admission and as SBP in those patients was developed either on admission or very shortly after, i.e., during their hospital stay, we think these laboratory data which include CBC, CRP, liver enzymes and liver function tests represent the patients’ condition when they developed it. We excluded patients with malignancy, hemorrhagic ascites, patients with urinary tract infection, chest infection and evidence of secondary peritonitis or those receiving antibiotic treatment at the time of paracentesis. Our study was ethically approved from the ethical committee, NLI, University of Menoufyia. The diagnostic paracentesis was carried out in all the patients and was evaluated for the polymorphnuclear cells (PMNs) count, total protein, albumin, Gram stain, bacteriological culture, beside pathologic assessment to exclude the presence of atypical cells. SBP was defined according to the EASL Guidelines 2010 (11) as ascetic neutrophil count ≥ 250/μL with or without a positive culture of the ascetic fluid, in the absence of any finding suggestive of secondary peritonitis. scoring system used by Wehmeyer and his colleagues was tested in our patients, on a scale of 0 to 4, where we give one point for age >60 years, one point if platelet count<100,000/ml, one point if CRP is between 30 and 60 mg/L and two points if CRP is above 60 mg/L [7].
Statistical Analysis
Patients were categorized in two groups according to the presence or absence of SBP. Data was statistically analyzed using SPSS version 20 for windows and a p-value<0.05 was considered statistically significant for all the analysis. Data were shown as mean, range or value. Independent samples t-test was used to examine the difference between the two groups for continuously-distributed variables, while chi-square test was used for categorical variables. Significant factors were tested in a univariate binary logistic regression analysis, and then only significant variables were entered in a stepwise multivariate logistic regression analysis to identify the independent predictors for the occurrence of SBP.
Results
Our study included 300 patients with liver cirrhosis and ascites, range of age (29 –81 years) and 60% were males (180 male and 120 female). The primary cause of liver disease was chronic hepatitis C, 275 patients (91.7%), hepatitis B, 20 patients (6.6%) and cryptogenic cause, 5 patients (1.7%). Diagnostic paracentesis revealed that 59 patients (19.6%) were diagnosed with SBP. Patients’ characteristics are shown in Tables 1A and 1B.
| Variables | SBP | |||
|---|---|---|---|---|
|  Positive | Negative | |||
| No. | % | No. | % | |
| Male | 34 | 57.6% | 146 | 60.6% |
| Female | 25 | 42.4% | 95 | 39.4% |
| Diabetic | 27 | 45.8% | 86 | 35.7% |
| Child-Paugh | ||||
| Child A Child B Child C | 0 | 0.0% | 2 | 0.8% |
| 23 | 39.0% | 99 | 41.1% | |
| 36 | 61.0% | 140 | 58.1% | |
Table 1a: Patients’ characteristics
| Variables | SBP | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | |||||
| Mean ± SD | Range | Median | Mean ± SD | Range | Median | |
| Age | 54.5 ± 8.6 | 29-81 | 56 | 58.9 ± 8.3 | 33-70 | 60 |
| Bilirubin (mg/dL) | 2.8 ± 2.7 | 0.5-26 | 2 | 5.1 ± 4.7 | 0.5-26 | 2 |
| Albumin (gm/dL) | 2.7 ± 0.5 | 1.2-4.1 | 3 | 2.6 ± 0.5 | 1.3-3.5 | 3 |
| INR | 1.8 ± 0.5 | 1.1-6.2 | 1.7 | 2.2 ± 0.8 | 1.3-5.9 | 2 |
| MELD | 18.7 ± 6.7 | 7-46 | 17 | 25.1 ± 7 | 12-49 | 23 |
| Creatinine (mg/dL) | 1.5 ± 0.9 | 0.5-6.1 | 1 | 2.1 ± 1.7 | 0.5-8.9 | 2 |
| TLC  (/mL) |
8,167.7 ± 3,858 | 580 – 28,400 | 7,500 | 10,005.1 ± 5,350.7 | 3,700 – 35,000 | 8900 |
| Platelet (/mL) | 113,257.3 ± 46,652.4 | 20,000 – 303,000 | 110,000 | 73,678 ± 31,110.7 | 21,000 – 174,000 | 67,000 |
| CRP (mg/L) | 12.6 ± 6.6 | 5-44 | 11 | 29.1 ± 13.6 | 8-64 | 27 |
| ALT (U/L) | 53.8 ± 153.6 | 10 – 2,004 | 33 | 85.4 ± 105.9 | 13 - 611 | 46 |
| AST (U/L) | 62.5 ± 104.7 | 16 – 1,390 | 42 | 153.7 ± 242.7 | 24 -1,374 | 79 |
SD: Standard Deviation; INR: International Normalized Ratio; MELD: Model for End-Stage Liver Disease; TLC: Total Leucocytic Count; CRP=C-reactive-protein; ALT: Alanine Transaminase; AST: Aspartate Transaminase
Table 1b: Laboratory tests values
Our results showed that age had a highly statistically significant difference between patients with or without SBP (p=0.001) while sex or the presence or absence of DM had no statistical significant difference (p=0.678 and 0.152, respectively).
Table 2 shows the different variables statistical significant difference between patients with and without SBP. Variables like serum total bilirubin, AST, creatinine, INR, TLC, platelet count and CRP showed a statistically significant difference between the two groups (p=0.001, 0.006, 0.006,<0.001, 0.003,<0.001 and<0.001). On the other hand variables like serum albumin and ALT had no statistical significant difference (p=0.198 and 0.136). MELD score showed a highly statistically significant difference (p<0.001) with positive correlation between the patients’ MELD scores and the development of SBP, while Child-Paugh score failed to show such relation (p=0.737). So by univariate analysis, [9]. variables had a p value of<0.05 as predictive factors of an episode of SBP (age, total bilirubin, AST, creatinine, INR, MELD score, TLC, platelet count and CRP). These variables were entered in a stepwise multivariate analysis which showed that only age, platelet count and CRP (p=0.004,0.013 and<0.001, respectively) were independently correlated with the risk of developing SBP (Table 3).
| Independent sample t test | P value | Chi square | P-value | ||
|---|---|---|---|---|---|
| Age | 3.51 | 0.001 | Sex | 0.17 | 0.67 |
| Bilirubin | 3.58 | 0.001 | DM | 2.05 | 0.15 |
| INR | 4.15 | <0.001 | Child-Paugh | 0.61 | 0.73 |
| Albumin | -1.29 | 0.19 | SBP scoring system | 79.46 | <0.001 |
| Creatinine | 2.82 | 0.006 | |||
| ALT | 1.49 | 0.13 | |||
| AST | 2.85 | 0.006 | |||
| TLC | 3.02 | 0.003 | |||
| Platelets | -7.84 | <0.001 | |||
| CRP | 9.11 | <0.001 | |||
| MELD | 6.59 | <0.001 |
INR: International Normalized Ratio; ALT: Alanine transaminase; AST: Aspartate
transaminase; TLC: Total Leucocytic Count; CRP=C-reactive protein; MELD: Model
for End-stage Liver Disease; DM: Diabetes Mellitus (p-value significant if<0.05).
Table 2: Different variables statistical significant difference between patients with and without SBP
| Variables | B | S.E. | Wald | Sig. | Exp (B) |
|---|---|---|---|---|---|
| Age | 0.082 | 0.029 | 8.241 | .004 | 1.086 |
| Bilirubin | 0.010 | 0.072 | 0.020 | .89 | 1.010 |
| INR | 0.165 | 0.585 | 0.080 | .78 | 1.180 |
| MELD | 0.100 | 0.090 | 1.233 | .27 | 1.105 |
| Creatinine | 0.045 | 0.325 | 0.019 | .89 | 1.046 |
| TLC | 0.000 | 0.000 | 0.944 | .33 | 1.000 |
| Platelet count | 0.000 | 0.000 | 6.110 | .013 | 1.000 |
| CRP | 0.205 | 0.033 | 37.989 | <0.001 | 1.228 |
| AST | 0.002 | 0.001 | 1.936 | .16 | 1.002 |
INR: International Normalized Ratio; MELD: Model for End-stage Liver Disease;
TLC: Total Leucocytic Count; CRP=C-reactive protein; AST: Aspartate transaminase
Table 3: Logistic regression analysis displaying independent predictors of the occurrence of SBP.
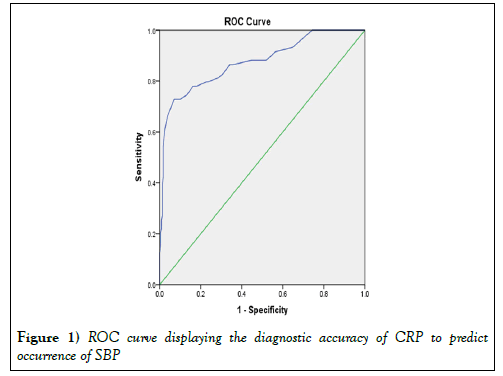
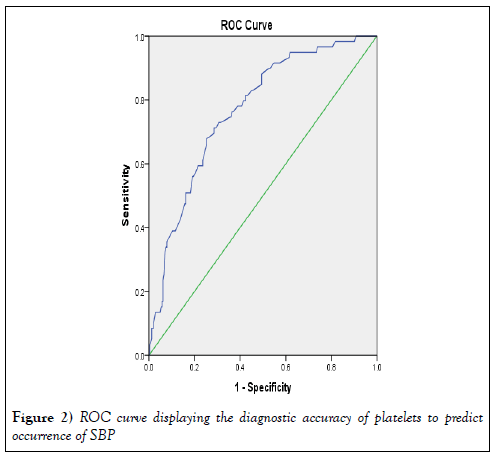
Table 3 shows the logistic regression analysis results of the independent predictors SBP. Our results showed that CRP and platelet count could significantly differentiate between patients groups at a cutoff point ≥ 13.5 mg/Land ≤ 82,500/ml, respectively (sensitivity 86.4% and 71.2%; specificity 66.0% and 71.4%, respectively) (Tables 4 and 5).
| Area under the curve | Std. Errora | Asymptotic Sig.b | Asymptotic 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| CRP | 0.875 | 0.029 | <0.001 | 0.819 | 0.931 |
| Platelets | 0.767 | 0.032 | <0.001 | 0.703 | 0.831 |
a. Under the non-parametric assumption
b. Null hypothesis: true area=0.5
Table 4: Diagnostic accuracy of CRP and platelets to predict occurrence of SBP in the studied patients.
| Coordinates of the Curve | |||
|---|---|---|---|
| Positive if Greater Than or Equal Toa | Sensitivity | Specificity | |
| CRP | 13.5000 | 0.864 | 0.340 |
| Platelets | 82500.0000 | 0.712 | 0.286 |
a. Under the non-parametric assumption
Table 5: Best cut off points of CRP and platelets to predict occurrence of SBP in the studied patients
Figure 1 shows the diagnostic accuracy and the best cut off values and the ROC curves.
Figure 2 shows the diagnostic accuracy of CRP and platelet count in predicting SBP. When we tested our scoring system, CRP cutoff value of 13.5 mg/L, we found that no patient with zero score developed SBP.
Table 6 showed our modified Wehmeyer’s SBP scoring system and the incidence of SBP, where we gave one point if CRP value is between 13.5 mg/L and 30 mg/L, two points if between 30 mg/L and 60 mg/L and three points if more than 60 mg/L).
| SBP | Modified Wehmeyer et al. SBP scoring system (n) | Total | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Negative | 58 (100%) | 120 (94.5%) | 55 (72.4%) | 6 (25%) | 2 (15.4%) | 0 (0%) | 241 (80.3%) |
| Positive | 0 (0%) | 7 (5.5%) | 21 (27.6%) | 18 (75%) | 11 (84.6%) | 2 (100%) | 59 (19.7%) |
| Total | 58 | 127 | 76 | 24 | 13 | 2 | 300 |
Chi-square test<0.001
Table 6: Our Modified Wehmeyer’s SBP scoring system and the incidence of SBP.
Discussion
Cirrhosis increases the risk of developing bacterial infections and those patients are more vulnerable to die from sepsis than normal population [12,13]. Mortality may reach 70% due to the development of shock or multiorgan failure [14].
Finding the prognostic factors which could evaluate the clinical condition of the hepatic patients and predict the occurrence of severe complications like SBP or even mortality is important for patients’ allocation in the liver transplantation waiting list. The most widely used prognostic scoring systems for the general condition of such patients are Child Turcot Pugh and MELD scores, but still there is no agreement on specific prognostic scoring system for the development of each severe complication. SBP is an example, a low ascetic fluid protein was associated with high risk of its development but the search was for non-invasive scoring system. The new SBP prognostic scoring system developed by Wehmeyer et al. [7]. which depended on three parameters found to be related to SBP occurrence, age, platelet count and CPR, is a simple and easy to use scoring system as it depends on a routinely assessed parameters for admitted patients. In our study which included Egyptian patients suffering advanced liver disease, mostly secondary to hepatitis C infection, the incidence of SBP was 19.6% which shows how much it is common to face this problem in this group of patients. We tested different variables, including the variables used in Wehmeyer’s SBP scoring system, to see their relation to SBP prediction. The results showed that, although 9 parameters showed a statistical difference to SBP occurrence (age, total bilirubin, AST, creatinine, INR, TLC, platelet count, CRP and MELD score), only age, platelet count and CRP could independently predict SBP. Regarding age, there is an association between increasing age and the susceptibility to infections due to impaired immunity with aging [15,16]. Also, aging is an adverse prognostic factor in most liver diseases with increasing morbidity and mortality, compared to young patients [17,18]. Low platelet count is common in chronic liver disease, due to splenic platelet sequestration, increase in its breakdown or decrease of production. It was used as an indirect indicator of portal hypertension and liver disease severity [19-22]. Also, as infections especially sepsis produce thrombocytopenia [23]. it was used as a predictor of SBP with low ascetic fluid protein. CRP is a well-known parameter to detect inflammation or infection in general population [24,25], yet it was used to have a weak predictive value for infection in advanced liver disease because its basal level is common to be higher than normal in cirrhotics, owed to the underlying liver disease, and as it is produced mainly by hepatocytes, it doesn’t increase so much with infection in patients with advanced cirrhosis [26,27]. In spite of this, CRP was found to indicate a serious infection in cirrhotics if it is significantly high [28]. Also, Guler et al. [29]. found significant high CRP values in the SBP and the bacteriascites groups compared to non-infected patients (68.4, 68 .3, and 6.5 mg/L, respectively). Wehmeyer et al. [7] found the same results and included it in their three parameters scoring system by giving it one point if its level is more than 30 mg/L and two points if more than 60 mg/L. In our validation of the Wehmeyer’s SBP scoring system we found higher scores (scores of 3 and 4) to be correlated significantly with the occurrence of SBP (p<0.001). Zero score was able to rule out SBP correctly in most cases (only 4 patients out of 100 patients with 0 score developed SBP). According to our modification to the scoring system (one point was given if CRP value is between 13.5 and 30 mg/L, two points if between 30 and 60 mg/L and three points if more than 60 mg/L) the results showed that, scores of 4 or 5 are good predictors of SBP (only two patients with score of 4, out of 13 patients didn’t have SBP, while both patients with score of 5 had SBP). On the other hand scores of 0 or 1 could be used to exclude SBP diagnosis (only 7 patients with score of 1, out of 127 developed SBP, while no patient with score of zero, 58 patients, developed it). Based on these results, we conclude that SBP as a serious complication could be diagnosed by a simple scoring system depends on age, platelet count and CRP. Patients with score of zero are unlikely to have SBP, while we should start treatment once they have a score of 4 or 5. Ascetic sample testing and culture could be saved for patients with scores of 1,2 or 3. Our results still needs further work to validate it in larger groups of patients.
Conclusion
Age, CRP and platelet count are independent predictors for SBP and a scoring system including them could easily predict it. SBP diagnosis could be excluded in patients with zero score, using CRP cutoff value of 13.5 mg/L.
Acknowledgements
The presentation of the manuscript in The 27th Asian Pacific Association for the Study of the Liver (APASL 2018). 14th International conference on Gastro Education 2018
REFERENCES
- Kandeel A, Genedy M, El-Refai S, et al. The prevalence of hepatitis C virus infection in Egypt:implications for future policy on prevention and treatment. Liver Int 2017;(37):45-53.
- Lutz P, Nischalke HD, Strassburg CP, et al. Spontaneous bacterial peritonitis:The clinical challenge of a leaky gut and a cirrhotic liver. World J. Hepatol 2015;(7):304-314.
- Kaymakoglu S, Eraksoy H, Okten A, et al. Spontaneous ascitic infection in different cirrhotic groups:prevalence, risk factors and the efficacy of cefotaxime therapy. Eur. J. Gastroenterol. Hepatol 1997;(9):71-76.
- Cheong HS, Kang CI, Lee JA, et al. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am 2009;(48):1230-1236.
- Tandon P and Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin. Liver Dis 2008;(28):26-42.
- Lata J, Fejfar T, Krechler T, et al. Spontaneous bacterial peritonitis in the Czech Republic:prevalence and aetiology. Eur. J. Gastroenterol. Hepatol 2003;(15):739-743.
- Wehmeyer MH, Krohm S, Kastein F, et al. Prediction of spontaneous bacterial peritonitis in cirrhotic ascites by a simple scoring system. Scand. J. Gastroenterol 2014;(49):595-603.
- Guarner C, SolàR, Soriano G, et al. Risk of a first community-acquired spontaneous bacterial peritonitis in cirrhotics with low ascitic fluid protein levels. Gastroenterology 1999;(117):414-419
- Shi KQ, Fan YC, Ying L, et al. Risk stratification of spontaneous bacterial peritonitis in cirrhosis with ascites based on classification and regression tree analysis. Mol. Biol. Rep 2012;(39):6161-6169.
- Obstein KL, Campbell MS, Reddy KR, et al. Association between model for end-stage liver disease and spontaneous bacterial peritonitis. Am. J. Gastroenterol 2007;(102):2732-2736.
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol 2010;(53):397-417.
- Arvaniti V, D'Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010;(139):1246-1256, 1256.e1-5.
- Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death:analysis of the National Hospital Discharge Survey. Chest 2003;(124):1016-1020.
- Plessier A, Denninger MH, Consigny Y, et al. Coagulation disorders in patients with cirrhosis and severe sepsis. Liver Int. Off. J. Int. Assoc. Study Liver 2003;(23):440-448.
- Schneider EL. Infectious diseases in the elderly. Ann. Intern. Med 1983;(98):395-400.
- Yoshikawa TT, Norman DC, Grahn D. Infections in the aging population. J. Am. Geriatr. Soc 1985;(33):496-503.
- Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr. Opin. Gastroenterol 2015;(31):184-191.
- Floreani A. Liver diseases in the elderly:An update. Dig. Dis. Basel Switz 2007;(25):38-143.
- Qamar AA, Grace ND, Groszmann RJ, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol 2009;(7):689-695.
- Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with non-alcoholic chronic liver disease. Am. J. Gastroenterol 2000;(95):2936-2939.
- Poynard T, Bedossa P. Age and platelet count:A simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. Metavir and Clinivir Cooperative Study Groups. J. Viral Hepat 1997;(4):199-208.
- Sheikh MY, Raoufi R, Atla PR, et al. Prevalence of cirrhosis in patients with thrombocytopenia who receive bone marrow biopsy. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc 2012;(18);257-262.
- Venkata C, Kashyap R, Farmer JC, et al. Thrombocytopenia in adult patients with sepsis:incidence, risk factors, and its association with clinical outcome. J. Intensive. Care. 2013;(9):1-9.
- Eklund CM. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem 2009;(48):111-136.
- Castelli GP, Pognani C, Meisner M, et al. Procalcitonin and C-reactive protein during systemic inflammatory response syndrome, sepsis and organ dysfunction. Crit. Care Lond. Engl 2004;(8):R234-242.
- Le Moine O, Devière J, Devaster JM, et al. Interleukin-6:an early marker of bacterial infection in decompensated cirrhosis. J. Hepatol 1994;(20):819-824.
- Park WB, Lee KD, Lee CS, et al. Production of C-reactive protein in Escherichia coli-infected patients with liver dysfunction due to liver cirrhosis. Diagn. Microbiol. Infect. Dis 2005;(51):227-230.
- Preto-Zamperlini M, Farhat SC, Perondi MB, et al. Elevated C-reactive protein and spontaneous bacterial peritonitis in children with chronic liver disease and ascites. J. Pediatr. Gastroenterol. Nutr 2014;(58):96-98.
- Guler K, Vatansever S, Kayacan SM, et al. High sensitivity C-reactive protein in spontaneous bacterial peritonitis with non-neutrocytic ascites. Hepatogastroenterology 2009;(56):452-455.