Prospective randomized control trial to reduce bubbles during colonoscopy with addition of oral simethicone to standard bowel preparation
2 GI Unit, Brandon Regional Health Centre, Brandon, Canada, Email: ShivBhanot@hotmail.com
Received: 28-Nov-2018 Accepted Date: Dec 25, 2018; Published: 31-Dec-2018
Citation: Dhalla SS, Skead C, Bhanot S, et al. Prospective randomized control trial to reduce bubbles during colonoscopy with addition of oral simethicone to standard bowel preparation. J Hepato Gastroenterol. 2018;2(1):56-8.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
BACKGROUND: Colonoscopy plays a vital role in screening and detecting colorectal cancer as well as various other gastrointestinal (GI) conditions. With current standard bowel preparation, bubbles tend to line the mucosa in approximately one third of patients and potentially obscure clear visualization. Simethicone, an over the counter, anti-foaming agent, has been used in GI departments as an irrigating fluid with some success in an effort to reduce bubbles. However, simethicone has been found to be contributing to the formation of a biofilm inside the irrigation channel of the endoscope potentially resulting in scope infection and contributing to transmission of microorganisms. METHODS: A prospective randomized controlled trial was designed to investigate the addition of oral simethicone to bowel preparation on the formation of bubbles during colonoscopy and its impact on visualization. RESULTS: The results of our study demonstrate a clear improvement in mucosal visibility due to the addition of oral simethicone to bowel preparation leading to a significant reduction in bubbles throughout the entire colon. CONCLUSION: Increased visibility during colonoscopies may result in better endoscopy results and a longer endoscope life.
Keywords
Colonoscopy; Bubbles; Simethicone; Endoscopy; Bowel preparation
Introduction
Endoscopy remains critical in detecting and preventing colorectal cancer along with other gastrointestinal (GI) pathologies. With the proliferation of colorectal cancer screening in GI practice, it is vital to have adequate bowel preparation for proper visualization. Adenoma Detection Rate (ADR) is increasingly being used as a metric for endoscopist competency and measurement of GI Unit performance. Visualization can be obscured not only by presence of stool contents but also by bubbles within the lumen of the bowel. [1-4] Despite adequate bowel preparations with minimal residual stool about one third of patients undergoing colonoscopy are found to have air bubbles resulting in limited visualization of the colon mucosa making detection of polyps, early cancer and other flat lesions such as arteriovenous malformations (AVM) difficult [5]. This results in repeated examinations and decreased screening interval translating to increase costs to the system and improper reassurance to the patient. Simethicone has been used especially in paediatrics for burping and routine GI discomfort in clinical practice. It has also been used for abdominal bloating and flatulence in adults. Simethicone is a mixture of polydimethylsiloxane and hydrated silica gel. It works as an anti-foaming agent by reducing surface tension of air bubbles allowing small bubbles to coalesce [6]. It has been mixed with irrigating fluid during colonoscopy in various GI departments to decrease bubbles. With the recent outbreak of contaminated endoscopes, biofilm has been found in the irrigation channels of the endoscopes [7,8]. Some manufacturers are suggesting, such as a letter to practitioners from Olympus in 2009 to discontinue use of simethicone in the irrigating fluid to prevent biofilm and thus reduce contamination of the endoscope. We designed a prospective randomized control trial to examine the effects on the amount of bubbles present during colonoscopy with the addition of oral simethicone taken along with bowel preparation.
Methods
This was an examiner blinded prospective study examining the oral ingestion of simethicone with bowel preparation taken as a split dose. This study was approved by the University of Manitoba Research Ethics Board and registered as a Clinical Trail with clinicaltrial.gov, number NCT03157791. All patients who are referred to undergo colonoscopy are booked centrally in the GI Unit at the Brandon Regional Health Centre (BRHC) and are given instructions regarding bowel preparation and procedure time by the booking department. Prior to the procedure all patients were sent bowel preparation instructions, procedure time and colonoscopy information. A letter explaining the study and requesting participation was also sent prior to the procedure. All patients are instructed to call the GI Unit prior to their procedure to confirm instructions. At this time patients were asked to participate in the study using a standardized script. Patients with no exclusion criteria were asked to participate in the study. If patients agreed to participate, the consent form along with the simethicone ingestion form was placed in an envelope, labeled with the participant number and attached to the patients chart. The participant number was referenced with the master list to determine if the patient was in Group A or Group B. The master list was created using a random generator that randomly sorted participant numbers into two groups (http://stattrek.com/statistics/random-numbergenerator. aspx). Those who were placed in Group A were given instructions regarding simethicone intake followed by standard instructions. Participants in Group B received only the standard instructions. Patients that did not wish to participate in the study also received only standard instructions and their participation status was not made known to the endoscopist. In accordance with the GI Unit protocol, patients were instructed to take bowel preparation in two doses. Timing of the doses varied depending on procedure time and which bowel preparation formulation was prescribed. The intention was to not modify the GI practice of individual endoscopists or ignore patient preference so various bowel preparations were used in the study. Group A received 2 simethicone tablets so that one 180 mg tablet was taken at the same time as each bowel preparation dose. The tablets were available for pick up at the GI Unit at no cost or for purchase as 180 mg tablets at a pharmacy. Participants were advised during their phone instructions for bowel preparation not to speak to the endoscopist at any time about whether they were participant of the study or not. If participants were allotted to Group A but presented on the day of the procedure without having taken simethicone they were excluded from the study. On the day of the procedure patients presented to the GI unit and were treated the same whether or not they were participants in the study. Participants of the study were reminded to not tell any physician or nurse other than the interview nurse of their status. The interviewing nurse asked the patient if they ingested the simethicone. The response was noted on the simethicone ingestion form that was placed in an envelope labeled with the participant number. The interviewing nurse then proceeded with the regular intake form. The endoscopy room had copies of the Boston Bowel Preparation Scale [5] and the Bubble Scale.6 The endoscopist completed these forms as well as the Endoscopy Room Quality Indicators for all patients once the procedure was finished, thus maintaining blinding to participant status. Once completed, a photocopy of the Endoscopy Room Quality Indicators, Bubble Scale and Bowel Preparation Scale was placed with the participant folder in the study envelope by recovery room staff. Bowel preparation score, bubble score, withdrawal time, bowel preparation type, point of the colon reached, whether retroflexion occurred and if polyps were detected was documented. The Boston Bowel Preparation Scale [9] graded bowel preparation in the right colon, transverse colon and left colon with 0 =Unprepared colon segment with mucosa not seen due to solid stool that cannot be cleared, 1=Portion of mucosa of the colon segment seen, but other areas of the colon segment not well seen due to staining, residual stool and/or opaque liquid, 2=Minor amount of residual staining, small fragments of stool and/or opaque liquid, but mucosa of colon segment seen well. The Bubble Scale graded the amount of bubbles in the right colon, transverse colon and left colon with 0=none, 1=minimal-occasional bubbles, must actively look for them, 2=moderate-obviously present, 3=severe-vision obscured [10]. All endoscopists underwent training for the correct use of the Bubble Scale and Bowel Preparation Scale. The scores were confirmed with another endoscopist until there was consistent agreement. The scales were also present on the endoscopy room wall for reference. Subjects: A total of 468 patients participated in the study with 236 participants in Group A and 232 in Group B. Participants were 18 years of age and older, scheduled to undergo colonoscopy. Exclusion criteria included inability to provide informed consent, less than 18 years old, inpatient, pregnancy, known hypersensitivity to simethicone, non residents of Manitoba and/or excessive language barriers.
Statistical analysis
The amount of bubbles was compared between Group A (simethicone) and Group B (no simethicone) using a Mann Whitney test with a statistical significance value of p=0.05. The same was done for bowel cleanliness using the Boston Bowel Preparation Scale scores. Outcome measures: The primary end point was the grade of bubbles present. Secondary end points included bowel cleanliness and polyp detection rate.
Results
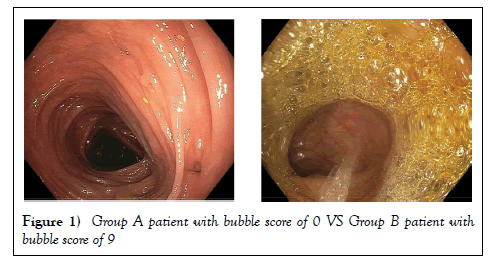
A total of 468 individuals participated in the study with 236 participants in Group A and 232 in Group B. Both groups have five different types of bowel preparation according to patient and endoscopist preference. These were Golytely, Peglyte, Bipeglyte, Picosalax and Colyte. As shown in Table 1, the average of participants was 60.1 years with 51.9% female and 48.1% male. Due to the non-parametric distribution of data a Mann Whitney test was performed to assess for any significant differences in bowel preparation and bubble scores between the two groups. A significant difference was found in bubble score between Group A and Group B (p=0.0000096) with Group A having an average total bubble score of 0.84 compared to 1.86 for Group B (Table 2). When a section of bowel in Group A was compared to the bubble score in the corresponding bowel section in Group B, the significant difference remained for each section (RC, TC, and LC (Table 3)). The highest bubble score was 9 out of 9 (6 patients in Group B) with the lowest being 0 (Group A) (Figure 1).
| Demographics | Group A | Group B |
|---|---|---|
| Female | 118 | 125 |
| Male | 118 | 107 |
| Average Age | 59.6 (22-90) | 60.7 (20-90) |
Table 1: Patient demographics
| Average | Group A | Group B |
|---|---|---|
| Avg Total Bowel Prep | 7.14 | 7.33 |
| Avg Total Bubble | 0.84 | 1.86 |
Table 2: Average bowel preparation and bubble scores by group.
| comparison | p value |
|---|---|
| Total Bowel Prep A Vs B | 0.192 |
| Total Bubble A vs B | 0.0000096 |
| Bowel prep scores A vs. B | |
| RC | 0.323 |
| TC | 0.295 |
| LC | 0.28 |
| Bubble scores A vs. B | |
| RC | 0.00001 |
| TC | 0.00029 |
| LC | 0.00039 |
Table 3: p Values comparing total and bowel section bowel preparation/bubble score
Although scores of 0, meaning minimal bubbles in all three sections of the colon were found in both groups, Group A had 163 participants with scores of 0 compared to only 116 participants in Group B. Group A also had no score of 9 out of 9 and only one participant with a score of 8, followed by 5 patients with a score of 6 and median of 0. Group B had a median of 1. No significant difference was found in average bowel preparation score (p=0.192). The average bowel preparation score for Group A was 7.14 compared to an average total of 7.33 for Group B.
Discussion
Different methods have been employed to reduce bubbles in colonoscopy. These include flushing with water during the procedure or flushing with simethicone, found to result in retained fluid droplets on the endoscope even with proper reprocessing involving pre-cleaning, manual cleaning and high level disinfection, thereby possibly increasing infection rates.8 Yet these methods, in particular the use of flushing, results in increased procedure time, discomfort for the patient and longer anesthesia. A quick and harmless way to reduce bubbles while also increasing visualization is of great benefit to endoscopists and patients. The results of this study demonstrate a substantial benefit with addition of oral simethicone to bowel preparation resulting in a decreased amount of bubbles present during colonoscopy. The amount of bubbles using the Bubble Scale were significantly less throughout the entire colon in Group A when compared to Group B. Interestingly, the average bubble score increased from the LC to TC to the RC in both groups. The right colon is known to be a difficult area in terms of visualization [11]. Bowel preparation scores were not significantly different between the groups. The lack of difference is not unexpected, as the use of simethicone is not known to affect the presence of stool in the colon [12]. Much effort has gone into optimizing bowel preparations to increase visibility and decrease the need for flushing during colonoscopy. Split dose bowel preparations have been found to be the most effective [13]. Yet despite this, bubbles have remained a problem for endoscopists. In our study some patients had no bubbles yet a poor bowel preparation score and vice versa, demonstrating that the two are separate entities and the next phase in optimizing colon visibility is to reduce bubbles. While not assessed in this study, other studies have reported increased patient tolerance in terms of decreased bloating and discomfort when simethicone was used prior or during colonoscopy [14]. Oral simethicone prior to colonoscopy is an easy and practical way to reduce bubbles and increase visibility without damaging effects to the endoscope. It may be argued that simethicone is not necessary as only 1/3 of patients are affected by bubbles and the majority are not.6 However, the lack of harm in using oral simethicone and our inability to the predict possible presence of bubbles during the procedure further supports the use of oral simethicone taken with bowel preparation.5 Our study has clearly demonstrated the benefit of the addition of oral simethicone to bowel preparations in improving visualization during colonoscopy. Hopefully, this will increase detection of pathology which may have been obscured otherwise. Furthermore, it has the potential to decreased damage and contamination of the endoscope. Future areas of research include analysis of various bowel preparations compared to bubble score. As well as secondary analysis of cancer detection rates comparing Group A and Group B. Limitations of the study include possible variation in grading of scales by individual endoscopists, although all endoscopists received training on using the scales and were blinded as to whether the patient was a study participant. Patients may not have ingested proper dosage of simethicone although instructed to take two 180 mg tablets. A selection bias may be present in terms of those willing to participate in the study, as they may be more likely to be motivated and properly prepare with the bowel preparation and follow instructions.
Conclusion
The use of oral simethicone with bowel preparation significantly reduced the amount of bubbles throughout the entire colon. The results demonstrate a clear improvement in mucosal visibility due to the addition of oral simethicone to bowel preparation. Increased visibility during colonoscopy may result in better endoscopy results and hopefully longer endoscope life.
REFERENCES
- Sherer EA, Imler TD, Imperiale TF. The effect of colonoscopy preparation quality on adenoma detection rates. Gastrointest Endosc. 2012;75(3):545-53.
- Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European panel of appropriateness of gastrointestinal endoscopy European multicenter study. Gastrointest Endosc. 2005;61(3):378-84.
- Chokshi RV, Hovis CE, Hollander T, et al. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75(6):1197-203.
- Oh CH, Lee CK, Kim JW, et al. Suboptimal bowel preparation significantly impairs colonoscopic detection of non-polypoid colorectal neoplasms. Digestive Diseases and Sciences. 2015;60(8):2294-303.
- Matro R, Tupchong K, Daskalakis C, et al. The effect on colon visualization during colonoscopy of the addition of simethicone to polyethylene glycol-electrolyte solution: A randomized single-blind study. Clin Transl Gastroenterol. 2012;3(11):e26.
- Parikh VA, Khanduja KS. Use of simethicone during colonoscopy. Dis Colon Rectum. 1995;38(9):1007-8.
- Ofstead CL, Wetzler HP, Johnson EA, et al. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016;44(11):1237-40.
- Barakat MT, Huang RJ, Banerjee S. Simethicone is retained in endoscopes despite reprocessing: impact of its use on working channel fluid retention and adenosine triphosphate bioluminescence values (with video). Gastrointest Endosc. 2018.
- Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69(3):620-25.
- Sudduth RH, Deangelis S, Sherman KE, et al. The effectiveness of simethicone in improving visibility during colonoscopy when given with a sodium phosphate solution : A double-blind randomized study. Gastrointest Endosc. 1995;42(5):413-5.
- Hewett DG, Rex DK. Miss rate of right-sided colon examination during colonoscopy defined by retroflexion: An observational study. Gastrointest Endosc. 2011;74(2):246-52.
- Shaver WA, Storms P, Peterson WL. Improvement of oral colonic lavage with supplemental simethicone. Dig Dis Sci. 1988;33(2):185-8.
- Martel M, Barkun AN, Menard C, et al. Split-dose preparations are superior to day-before bowel cleansing regimens: A meta-analysis. Gastroenterology. 2015;149(1).
- Lazzaroni M, Petrillo M, Desideri S, et al. Efficacy and tolerability of polyethylene glycol-electrolyte lavage solution with and without simethicone in the preparation of patients with inflammatory bowel disease for colonoscopy. Aliment Pharmacol Ther. 2007;7(6):655-9.