Protective role of L-cysteine against nickel induced hepatotoxicity in Albino Wistar rats
Received: 16-Sep-2018 Accepted Date: Sep 19, 2018; Published: 30-Nov-2018
Citation: Kechrid Z, Bouhalit S. Protective role of L-cysteine against nickel induced hepatotoxicity in Albino Wistar rats. J Pharmacol Med Chem 2018;2(3):32-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
OBJECTIVE: The present study was undertaken to investigate the hepatoprotective effect of L-cysteine on nickel-induced oxidative stress in experimental rats.
METHODS: Male albino (Wistar) rats were divided into four groups of seven each: the first group was used as controls. The second group was given orally L-cysteine at dose of 100 mg/kg b.wt. The third group was administrated intraperitoneally with nickel sulfate at dose of 20 mg/kg and the fourth one given both L-cysteine and nickel for three consecutive weeks. Liver function markers alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), total bilirubin and proteins in serum and hepatic malondialdehyde (MDA) and antioxidants parameters including glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) were measured.
RESULTS: Nickel treatment produced oxidative liver injury characterized by an increase in hepatic markers enzymes (ALT, AST, ALP, LDH) activities and bilirubin level with a reduction in total proteins concentration. Simultaneously, it led to an increase of MDA level with a reduction of GSH concentration, SOD, CAT and GSH-Px activities. These results are also substantiated with obviously changes in hepatohistology. However, the treatment with L-cysteine significantly ameliorated the previous parameters and resulted in an improvement of the histopathological hepatic lesions.
CONCLUSION: Depending on the findings, it can be concluded that L-cysteine possesses a potential antioxidant power effect against nickel hepatotoxicity.
Keywords
Nickel; Rats; L-cysteine; Hepatotoxicity; Antioxidants; Histopathology
Introduction
Nickel (Ni) metal has become an object of great interest because of it’s widely distribution in environmental occurrence. In other words, it is used in a wide variety of applications including metallurgical processes such as catalysis, coinage, foundry plating and electrical components such as batteries [1,2]. High quantity of nickel has been reported to show various toxicities such as pulmonary, renal and cardiovascular effects [3]. Carcinogenic and mutagenic effects of nickel were also reported [4]. The most plausible mechanism that may be operative in Ni toxicity would involve oxidative stress through generation of reactive oxygen species (ROS), which may sustaining lipid peroxidation [5], thereby causing damage to critical macromolecules such as proteins, DNA and cell damage or by inactivation of antioxidant defense system [6,7]. Therefore, depletion of glutathione and other endogenous antioxidants may also contribute mainly for the development of nickel cytotoxicity threat [8]. The oxidative damage may be also attributed to the destruction of thiol groups of amino acids and proteins [9], since thiol compounds are well known for their free radical scavenging property. Compounds rich in free –SH moieties as cysteine and reduced glutathione belong to interesting molecules interacting with heavy metal ions after their entering through cell. In that case, L-cysteine, a sulphur-containing amino acid is known to offer protection to the living system against certain toxicants through its ability to increase the thiol status of tissues [10]. Furthermore, due to the presence of free –SH moieties, this amino acid is a part of peptides and protein directly connected with the protective mechanisms in a cell against adverse effects of metal ions [11]. Hence, these molecules have an important role in participating for the detoxification of heavy metals, because they have an ability to bind heavy metal ions via –SH groups of cysteine units and consequently transport them [12]. Moreover, L-cysteine was found to increase the activity of glutathione related enzymes [13] enhancing the activity of both SOD and CAT and diminish lipid peroxidation [11], alleviating LPO and NO through scavenging free radicals. Thus, the present investigation was undertaken to determine the protective effects of concurrent use of L-cysteine against nickel-induced hepatotoxicity.
Materials and Methods
Chemicals
Nickel sulfate, L-cysteine, 2-thiobarbituric acid (TBA), butylated hydroxytoluene (BHT), 5,5’-dithiobis-2-nitrobenzoic acid (DTNB), trichloroacetic acid (TCA), nitrobluetetrazolium (NBT), 1-chloro-2,4- dinitrobenzene (CDNB) were obtained from Sigma Chemical Co. (St. Louis, France) and all other chemicals were of analytical grade.
Experiment design
Male albino (Wistar) rats (180–220 g) were maintained under standard conditions of temperature and humidity with 12 h light/dark cycle and fed standard pellet diet and water ad-libitum for two weeks as an adaptation period. The study protocol was approved by the Ethical Committee of our institution. Then, animals randomly divided into four groups of seven rats each: group I, rats was served as controls. Group II, rats orally administered with L-cysteine (100 mg/kg b.wt) dissolved in distilled water [14]. Group III received intraperitoneally nickel sulfate (20 mg/kg b.wt.) [15]. Group IV, rats treated also in the same way with both nickel sulfate and L-cysteine simultaneously. The experiment period was lasted for three weeks and at the end animals were sacrificed by cervical decapitation after overnight fasting. Serum was separated by centrifugation for 10 minutes at 3000 rpm and stored at -20°C for the biochemical analysis. Liver was removed immediately, rinsed in ice cold saline 0.9%. Then, one part was homogenized in 2 ml ice cold TBS (50 mM Tris, 150 mM NaCl, pH 7.4). The homogenate was centrifuged at 10.000 g for 15 min at 4°C and the resultant supernatant was frozen at -20°C for oxidative parameters determination. The other part was fixed in 10% neutral formalin and used for histological examination.
Analytical Methods
Determination of biochemical parameters
Glucose, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total proteins, total bilirubin in serum were assessed using Spinreact Laboratory Spain diagnostic kits and spectrophotometer (Jenway 6505, Jenway LTD, Essex, UK). The references kits were as follow: glucose-41011, AST-1001161, ALT-1001171, ALP-1001131, LDH-1001260, bilirubin-1001044, total proteins-100129.
Lipid peroxidation level
The lipid peroxidation level in liver homogenate was estimated as malondialdehyde (MDA), which is the end product of lipid peroxidation, it reacts with thiobarbituric acid (TBA) as a TBA reactive substance (TBARS) to produce a red colored complex that has peak absorbance at 530 nm according to Buege and Aust [16]. 125 μl of supernatants were mixed with 50 μl of TBS and 125 μl of TCA-BHT (trichloroacetic acid–butylhydroxytoluene) in order to precipitate proteins and then was centrifuged (1000 × g, 10 min and 4°C). Then, 200 μl of the new supernatants were mixed with 40 μl of HCl (0.6 M) and 160 μl of TBA dissolved in 26 Mm Tris, and the mixture was heated at 80°C for 10 min. The absorbance of the resulting supernatants was measured at 532 nm. The amount of MDA was calculated using a molar extinction coefficient of 1.56 × 105 M/cm.
Reduced glutathione concentration
Liver GSH content was estimated using a colorimetric technique, as mentioned by Jollow et al. [17], based on the development of yellow color when (DTNB) is added to compounds containing sulfhydryl groups. In brief, 0.8 ml of homogenate supernatant was added to 0.3 ml of 0.25% sulfosalycylic acid, and then tubes were centrifuged at 2500 x g for 15 min. Supernatant (0.5 ml) was mixed with 0.025 ml of 0.01 M DTNB and 1 ml phosphate buffer (0.1 M, pH 7.4). Finally, the absorbance was recorded at 412 nm. Total GSH content was expressed as nmol GSH/mg protein.
Antioxidant enzymes assays
Superoxide dismutase (SOD) activity was determined by measuring of its ability to inhibit the photoreduction of NBT [18]. Catalase (CAT) activity was assayed spectro photometrically as described by Aebi [19]; the H2O2 decomposition rate was followed by monitoring absorption at 240 nm. Glutathione peroxidase activity (GSH-Px) was assayed by the method based on the reaction between glutathione remaining after the action of GSHPx and 5,5-Dithio-bis (2-nitrobenzoic acid) to form a complex that absorbs maximally at 412 nm [20].
Hepatic proteins content
Protein was measured by the method of Bradford [21], using bovine serum albumin as a standard.
Liver histopathology examination
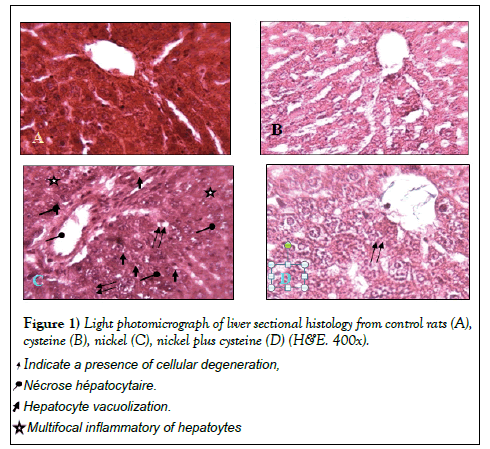
Histological evaluation was performed on a lobe of the liver and portion of specimen fixed in 10% formalin and embedded in paraffin wax. Then sections were cut at 4 µm in thickness, stained with hematoxylin and eosin and viewed under light microscope for histological examination [22].
Statistical analysis
Data are shown as means ± SEM. Statistical significance of the results obtained for various comparisons was estimated by applying one way analysis of variance (ANOVA) followed by Student’s t-test and the level of significance was set at p<0.05
Results
Effect of treatment on body, absolute and relative liver weights
The administration of nickel led to a decrease of body weight with an increase of both absolute and relative liver weights. However, the administration of L-cysteine raised body weight and decreased liver weight (Table 1).
| Parameters |  Experimental groups | |||
|---|---|---|---|---|
| Control | Cys | Ni | Ni+Cys | |
| Initial body weight (g) | 201.33 ± 6.5 | 203.83 ± 6.3 | 202.33 ± 6.9 | 202.3 ± 4.9 |
| Final body weight (g) | 250. 3 ± 6.8 | 227.3 ± 8.0 | 181.7 ± 11.3a1 | 216.3 ± 7.6b |
| Absolute liver weight (g) | 7.39 ± 0.38 | 7.2 ± 0.3 | 8.94 ± 0.46 | 6.72 ± 0.43b |
| Relative liver weight (g/100 g bw) | 2.84 ± 0.16 | 5.21 ± 0.21 | 5.21 ± 0.36a | 2.94 ± 0.18b |
Values are given as mean ± SEM of seven rats each group. Statistically significantly differences from control: ap<0.05, a1 p<0.01; from Ni: bp<0.05
Effects of treatments on biochemical parameters
As seen from Table 2, treatment with nickel caused an augmentation of AST, ALT, LDH, ALP activities and total bilirubin concentration. Meanwhile, the level of serum total protein was diminished. Whereas, the supplementation of L-cysteine resulted a decrease in the above mentioned biochemical parameters (AST, ALT, LDH, ALP, total bilirubin) and an increase of total protein.
| Parameters |  Experimental groups | |||
|---|---|---|---|---|
| Control | Cys | Ni | Ni+Cys | |
| Initial body weight (g) | 201.33 ± 6.5 | 203.83 ± 6.3 | 202.33 ± 6.9 | 202.3 ± 4.9 |
| Final body weight (g) | 250. 3 ± 6.8 | 227.3 ± 8.0 | 181.7 ± 11.3a1 | 216.3 ± 7.6b |
| Absolute liver weight (g) | 7.39 ± 0.38 | 7.2 ± 0.3 | 8.94 ± 0.46 | 6.72 ± 0.43b |
| Relative liver weight (g/100 g bw) | 2.84 ± 0.16 | 5.21 ± 0.21 | 5.21 ± 0.36a | 2.94 ± 0.18b |
Values are given as mean ± SEM of seven rats each group. Statistically differences from control: ap<0.05, a1 p<0.01, a2 p<0.001; from Ni: bp<0.05, b1 p<0.01.
Table 2: Hepatic biochemical parameters in serum of control and experimental rats after three weeks of treatment.
Effects of treatments on hepatic oxidative stress parameters
The exposure to nickel produced an increase in MDA level accompanied by a reduction in hepatic GSH concentration and hepatic antioxidant enzymatic system (SOD, CAT, GSH-Px) activities. Conversely the coadministration of L-cysteine produced a reduction in MDA with an increase of GSH and the hepatic antioxidant enzymes activities (Table 3).
| Parameters | Experimental groups | |||
|---|---|---|---|---|
| Control | Cys |  Ni | Ni+Cys | |
| MDA (nmol/mg protein) | 0.48 ± 0.02 | 0.47 ± 0.02 | 0.96 ± 0.05a2 | 0.46 ± 0.02b2 |
| GSH (nmol/mg protein) | 108.7 ± 2,9 | 111.3 ± 4.05 | 82.35 ± 3.34a2 | 110.4 ± 3.84b1 |
| GSH-Px (nmol GSH/mg prot) | 0.68 ± 0.03 | 0.63 ± 0.02 | 0.58 ± 0.02a | 0.68 ± 0.02b1 |
| CAT (µmol H2O2/min/mgprotein) | 157.55 ± 3 | 144.9 ± 4.5 | 122.7 ± 4.95a2 | 141.87 ± 3.12ab |
| SOD (U/mg protein) | 49.95 ± 2.6 | 46.52 ± 1.9 | 29.4 ± 1.74a2 | 39.13 ± 2.65b |
Values are given as mean ± SEM of seven rats each group. Significantly differences from control: ap<0.05, a2p<0.001; from Ni: bp< 0.05, b1p<0.01, b2p<0.001.
Table 3: MDA, GSH, GSH-Px, CAT and SOD in liver of control rats, treated with cysteine, nickel and nickel plus cysteine after three weeks of treatment
Histopathological results
Figure 1 demonstrates the histopathological examination of the liver sections of controls and the experimental rats. The liver sections of control animals showed normal architecture with no damage in the central vein and no changes in sinusoids and hepatocytes architecture (Figure 1A). Almost, the same histology structure was shown in L-cysteine group (Figure 1B). In toxic nickel group, the liver sections indicated hepatic cell necrosis along; a few of the hepatocytes were vacuolated with severe damage associated with central vein (Figure 1C). In nickel group co-administrated with L-cysteine showed nearly normal appearance of binuclei with small size, and nearly restoration of some distingerated cell contents (Figure 1D).
Discussion
Nickel is a heavy metal regarded as a potent real human toxicant, having the ability to influence cellular antioxidant defense system, which can be a hepatotoxic agent [23]. The increased level of Ni accumulation in the liver could be discussed by his great affinity for SH-containing molecules. Hence, SH group is involved in the function of many enzymes; the Ni-SH complex possibly disturbs many functions of cell mainly mitochondrial dysfunction. The treatment of this toxicity can include chelation or antioxidant administration to remove nickel and scavenge generated ROS. There are many appreciated studies have demonstrated the potential use of cysteine as ROS scavenger, possesses potent antioxidant activity on the basis of its sulfhydryl group within its structure [24]. Therefore, this study devoted to assess and examine the possibility of this amino acid to prevent the alterations induced by nickel sulfate in kidney tissues of male albino Wistar rats. The body weight change is used as a good index of the overall health status. So in our study, changes in rats’ body weight along with liver weights can act as an essential indication of nickel toxicity. The decreased body weight gain of rats observed in this study could be due to low food consumption [25], or as a result of the overall increased degeneration of lipids and proteins [26]. The findings indicated also an increase of absolute and relative liver weight. This might be as a result of hypertrophy and the selective accumulation of nickel in the liver [27] or nickel can lead to cell death by apoptosis of certain cell lines, due to the accumulation of toxic lipid derivatives such as ceramides. The later induce cellular hypertrophy of the target organ [28]. However, the co-administration of L-cysteine with nickel to animals improved body and liver weights. Many studies have reported that L-cysteine showed significant protective effect against damage induced by heavy metals such as arsenic and cadmium [14,29]. A decline in serum proteins level and an increase of bilirubin were recorded in nickel group. The decrease in proteins concentration of Ni-treated rats probably due to changes in protein synthesis and/or metabolism [30]. The observed hyperbilirubinemia might be due to excessive heme destruction and blockage of biliary tract in nickel-treated rats. This obstruction might have resulted to mass inhibition of conjugation reaction and release of unconjugated bilirubin from damaged and dead hepatocytes [31]. The activities of serum transaminases, alkaline phosphatase and lactate dehydrogenase were also significantly increased. This it could be attributed to the hepatic damage resulting a release and leakage out of these enzymes from the liver cytosol into the blood stream, which gives an indication on the hepatotoxic effect of this metal [32]. Interestingly, the biochemical perturbations seem to be correlated with the liver histological alterations such as the presence of cellular debris within a central vein, a cytoplasmic vacuolization, plasma membrane destruction and cellular hypertrophy. Simultaneously the co-treatments with L-cysteine increased protein concentration and reduced bilirubin level, AST, ALT, ALP and LDH activities, suggesting that these compound offered a considerable level of hepatoprotection through a contributory hepatoprotective mechanism, which accelerates the regeneration process and the production of liver cells [33]. It was reported that L-cysteine attenuate selected drug and chemicalinduced hepatotoxicities. In other words, the thiol group and L-cysteine has a high affinity for heavy metals, and a supplement can be used to remove them from the body and allow these metals to be excreted in the usual fashion [14,34]. Nickel is well known to produce oxidative damage in liver by enhancing lipid peroxidation [35]. Lipid peroxidation is supposed to cause the destruction and damage to cell membranes, lead to changes in membrane permeability and fluidity and enhance the protein degradation [36]. Corroborate with the findings of this investigation, the administration of nickel resulted in a significant increase in LPO as indicated by the significant increase of MDA. It has been generally reported that treatment with nickel causes an accumulation of iron, which in turn generate ROS via Haber–Weiss and Fenton’s reaction. The obviously decrease GSH in nickel group was in accordance with previous reports [8,37]. Moreover, the results showed also that nickel administration induced a signiflcant decrease SOD, CAT and GSH-Px activities, which confirms the work of Misra et al. [33], Hfaïedh et al. [38] and Boulila et al. [39]. This might be due to their increased utilization in scavenging free radicals induced by the metal, thus causing irreversible inhibition in their activities or due to direct binding of the metal to the active sites of these enzymes [5,40]. In other words, SOD was inhibited by hydrogen peroxide, while GSH-Px and catalase were inhibited by an excess of superoxide radical [41]. The results observed in the present study highlight the fact that L-cysteine protects against nickel toxicity evaluated through the reduction of MDA and increase of the antioxidant defenses system including GSH level and SOD, CAT, GSH-Px activities. Similarly protective effects of L-cysteine against oxidative damages induced by various toxins were recently reported [11,42]. So the protective action of L-cysteine might be due to enhance glutathione production by providing more substrate for reactive intermediates that promote detoxification mechanisms and increasing the antioxidant enzymes activities as well as their anti-oxidant and free radical scavenging effects, preventing oxidative degradation of the biological membranes [43].
Conclusion
The co-administration of L-cysteine alleviated nickel oxidative damage effects by inhibiting ROS generation. The histological studies also supported the beneficial role of L-cysteine against Ni-induced hepatic damages. Therefore, it is suggested that L-cysteine could protect hepatic tissues against Ni-induced oxidative stress probably through its antioxidant properties.
Acknowledgements
Authors thank Pasteur institute for providing rats and Tichati Lazhar for technical assistance. Authors declare also that no conflicts of interest.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Vidotti M, Salvador RP, Cordoba de Torresi. SI Synthesis and characterization of stable Co and Cd doped nickel hydroxide nanoparticles for electrochemical applications. Ultrason Sonochem 2009;16:35-40.
- Siddiqui MA, Ahamed M, Ahmad J, et al. Nickel oxide nanoparticles induce cytotoxicity, oxidative stress and apoptosis in cultured human cells that are abrogated by the dietary antioxidant curcumin. Food Chem Toxicol 2012;50:641-47.
- Kang GS, Gillespie PA, Gunnison A, et al. Long-term inhalation exposure to nickel nanoparticle sex acerbated atherosclerosis in susceptible mouse model. Environ Health Presp 2011;119:176-81.
- Maha AF, Nagwa HH, Farouk R, et al. Studies on the genotoxic effect of nickel chloride in mice and the possible protective role of soybean seeds extracts. Global J Pharmacol 2014;8:625-34.
- Sun H, Wu W, Guo J, et al. Effects of nickel exposure on testicular function, oxidative stress and male reproductive dysfunction in Spodopteralitura Fabriciu. Chemosphere 2016;148:178-87.
- Jia J, Chen J. Chronic nickel-induced DNA damage and cell death: the protection role of ascorbic acid. Environ Toxicol 2008; 23:401-06.
- Pan J, Chang Q, Wang X, et al. Reactive oxygen species-activated Akt/ASK1/p38 signaling pathway in nickel compound-induced apoptosis in BEAS 2B cells. Chem Res Toxicol 2010; 23:568-77.
- Chen CY, Wang YF, Lin YH, et al. Nickel-induced oxidative stress and effect of antioxidants in human lymphocytes. Arch Toxicol 2003;77:123-30.
- Saravanan N, Senthil D, Varalakshmi P. Effect of L-cysteine on lipid peroxidation in experimental urolithiatic rats. Pharmaco Res 1995; 32:165-69.
- Ahmed EA, Omar MO, Abdelghaffar SK, et al. The antioxidant activity of vitaminC, DPPD and l-cysteineagainst cisplatin-induced testicular oxidative damage in rats. Food Chem Toxicol 2011;49:1115-21.
- Omar HM, Abdelghaffar SK, Ahmed EA, et al. L-cysteine ameliorated testicular toxicity induced by acrylamide in rats. Eur J Bio Res 2015;5:1-8.
- Cobbett CS. Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 2000;3:211-16.
- Skrzydlewska E, Farbiszewski R. Protective effect of N-acetylcysteine on reduced glutathione, reduced glutathione-related enzymes and lipid peroxidation in methanol intoxication. Drug and Alcohol Dependence 1999; 57:61-67.
- Manal MS, Khaled MAH, Waleed S. Protective effects of thymoquinone and l-cysteine on cadmium-induced reproductive toxicity in rats. Toxicology Reports 2014;1:612-20.
- Bordes E, Papillion VV. Myocardial change induced by nickel and in association with cadmium. Rev Ig Bacteriol Virusal. Parazitol Epidemol Pneumotizol 1983;32:51-56.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Method Enzymol 1984;105:302-10.
- Jollow DL, Mitchell JR, Zampaglione Z, et al. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacol 1974;11:151-69.
- Asada K, Takahashi M, Nagate M. Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 1974;38:471-73.
- Aebi, H. Catalase in vitro. In: Packer, L (Ed) Methods in Enzymology. Academic Press, Orlando 1984;105:121-126.
- Flohé L, Günzler WA. Analysis of glutathione peroxidase. Methods Enzymol 1984;105: 114-20.
- Bradford M. A rapid and sensitive method for the quantitiesofmicrogram quantities of protein utilizing the principle of protein binding. Anal Biochem 1976;72:248-54.
- Hould R. Techniques d’histopathologie et de cytopathologie. Ed Maloine 1984;19:225-27.
- Sidhu P, Garg ML, Dhawan DK. Protective role of zinc in nickel-induced hepatotoxicity in rats. Chem Biol Interact 2004;150:199-209.
- Kamal EM, Shalahmetova T, Deraz S, et al. Combined effect of vanadium and nickel on lipid peroxidation and selected parameters of antioxidant system in liver and kidney of male rat. Afr J Biotech 2011;10:18319-25.
- Cempel M, Janicka K. Distribution of nickel, zinc and copper in rat organs after oral administration of nickel (II) chloride. Biol Trace Elem Res 2002;90:215-26.
- Lee YI, Lim SS, Baek BJ, et al. Nickel (II)-induced nasal epithelial toxicity and oxidative mitochondrial damage. Environ Toxicol Pharmacol 2016;42:76-84.
- Al-Humadi HW. The neuroprotective effect of L-cysteine towards cadmium or nickel neurotoxicity on adult rat brain antioxidant status and acetylcholine esterase activity.Karbala J Med 2015;8:2043-48.
- Chinoy NJ, Memon MR. Beneficial effects of some vitamins and calcium on fluoride and aluminium toxicity on gastrocnemius muscle and liver of male mice. Fluoride 2001;34:21-23.
- Sabiu S, Sunmonu TO, Ajani EO, et al. Combined administration of silymarin and vitamin C stalls acetaminophen-mediated hepatic oxidative insults in Wistar rats. Revista Brasileira de Farmacognosia 2015;25:29-34.
- Kechrid Z, Dahdouh F, Djabar RM, et al. Combined effect of water contamination with cobalt and nickel on metabolism of albino (Wistar) rats. Iranian Environ Health Sci Eng2006;3:65-69.
- Awang D. Milk thistle. Can Pharm J 1993;23:749-54.
- Upadhyay G, Kumar A, Singh MP. Effect of silymarin on pyrogallol and rifampicin-induced hepatotoxicity in mouse. Eur J Pharmacol 2007;565:190-201.
- Misra M, Rodriguez RE, Kasprzak KS. Nickel induced lipid peroxidation in the rat: correlation with nickel effect on antioxidant defense systems. J Toxicol 1990;64:1-17.
- El-Megharbel SM, Hamza RZ, Refat MS. Preparation, spectroscopic, thermal, antihepatotoxicity, hematological parameters and liver antioxidant capacity characterizations of Cd (II), Hg (II), and Pb (II) mononuclear complexes of paracetamol anti-inflammatory drug. Spectrochim Acta A Mol Biomol Spectrosc 2014;131:534-44.
- Stohs SJ, Bagchi D. Oxidative mechanism in the toxicity of metalions. Free Radic Biol Med 1995;18:321-36.
- Cempel M, Nikel G. Nickel: A review of its sources and environmental toxicology. PolJ Environ Stud 2006;15:375-82.
- Pari L, Prasath A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chem Biol Interact 2008;173:77-83.
- Hfaïedh N, Allaqui MS, Hfaïedh M, et al. Protective effect of cactus (Opuntiaficusindica) cladode extract upon nickel-induced toxicity in rats. Food Chem Toxicol 2008;46:3759-63.
- Boulila S, El-Feki A, Oudadesse H, et al. Detoxification of rats subjected to nickel chloride by a biomaterial-based carbonated orthophosphate. Pharma 2014;345:15.
- Ihechiluru NB, Henry AN, Taiwo IE. Heavy metal bioaccumulation and oxidative stress in Austroaeschnainermis (Dragon fly) of the Lagos Urban ecosystem. Journal of Environmental Chemistry and Ecotoxicololy 2015;7:11-19.
- Reham ZH, Mohammad SA. Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver with silymarin and extract supplements. Asian Pac J Trop Biomed 2015;5:521-31.
- Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology 2005;211: 26-35.
- Omar MO, Ahmed EA, Abdelghaffar SK, et al. Hepatoprotective effects of vitamin C, DPPD and L-cysteine against cisplatin-induced oxidative stress in male rats. J Biol Earth Sci 2012;2:28-36.