Rare case of a five-branched aortic arch exhibiting a retroesophageal right subclavian artery and an accessory left vertebral artery
2 Department of Anatomy, College of Medicine, Howard University, Washington DC, USA
3 Department of Biochemistry and Molecular and Cellular Biology, Georgetown University School of Medicine, Washington DC, USA
Received: 24-Sep-2018 Accepted Date: Oct 08, 2018; Published: 17-Oct-2018
Citation: Granite G, Meshida K, Jones S. Rare case of a five-branched aortic arch exhibiting a retroesophageal right subclavian artery and an accessory left vertebral artery. Int J Anat Var. Dec 2018;11(4):117-122.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Head and neck vascular variations are common in humans, but often go undetected. They are generally asymptomatic. Awareness of such anatomical variations is clinically important for surgeons and interventional radiologists as they may pose risk for iatrogenic complications or even the potential for unanticipated fatalities.
Although aortic arch variations are relatively common, branch variations are observed and/or described variably and fairly infrequently during human cadaveric dissection.
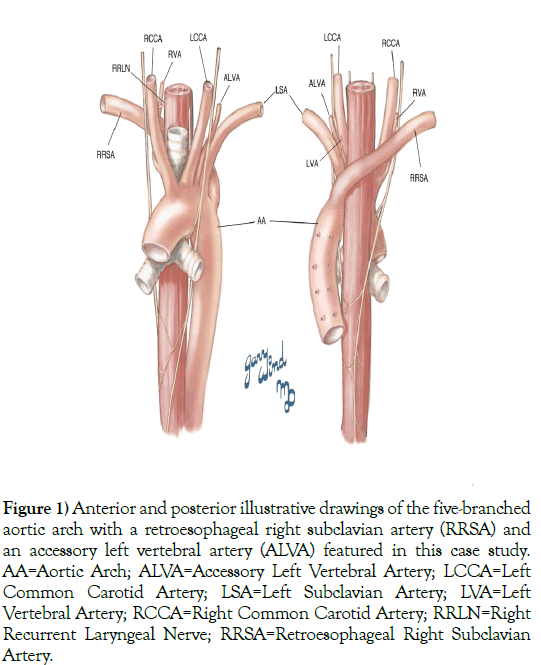
When aberrant subclavian arteries are described in adults, they frequently involve the right side, and are usually retroesophageal with an absent right brachiocephalic trunk. The following four branches of the aortic arch found most commonly then are: a retroesophageal right subclavian artery, left subclavian artery, and right and left common carotid arteries. During cadaveric dissection of a 76-year-old White male, we observed an additional fifth branch: an accessory left vertebral artery. A combination of five such aortic arch vessels may be rare.
Keywords
Retroesophageal right subclavian artery; Aberrant right subclavian artery; Accessory left vertebral artery; Duplicate origin of the left vertebral artery; Vertebral artery variation; Head and neck vascular variations
Introduction
Head and neck vascular variations are common, but are often asymptomatic and undetected. Such anatomical variations become clinically important when performing diagnostic and interventional angiography, thoracic surgeries, neurosurgeries, and cardiac catheterization procedures, since iatrogenic complications or even fatalities may occur [1-8]. Such knowledge is even more essential with the increasing use of carotid and vertebral artery stent placements and new therapeutic options for intracranial interventions [3,9,10].
Approximately 1 to 3% of human fetuses examined critically exhibit aortic arch (AA) variations [11]. The most common form of AA variation is the aberrant subclavian artery, (ASA; 0.1% to 2.5% incidence and more than 100 cases described in the literature [7,8,11-38]. The incidence of an aberrant right subclavian artery (ARSA) is much higher and 80% of these are retroesophageal (RRSA). An aberrant left subclavian artery (ALSA) occurs in one in 1000 people [17,18,21]. In these RRSA cases, the right brachiocephalic trunk (RBT) is absent and the following four arteries emerge as the AA branches: the right common carotid artery (RCCA), left common carotid artery (LCCA), the left subclavian artery (LSA), and the RRSA. In 13.7% of RRSA cases, the right vertebral artery (RVA) originates from the RCCA [19,32,36,39]. Maiti et al. 2016 found that 80% of the 49 reported cases of RVA originating from the RCCA had an RRSA [38]. Other vertebral artery (VA) variants are prevalent in both number and origin, but are documented infrequently in the literature [40-49]. Unilateral duplication of the VA or an accessory VA (AVA) is considered a rare vascular variant as a fourth AA branch (0.295%-0.72%) [1,6,40,50-53].
We discovered during anatomical cadaveric dissection of a 76-year-old White male, provided by the Maryland State Anatomy Board, an additional vessel. This individual exhibited an AA with five branches: RCCA, LCCA, LSA, ARSA (RRSA), and an accessory left vertebral artery (ALVA) (Figure 1). The presence of a fifth AA branch accompanying the aforementioned variations, an ALVA, has not, based on these authors’ literature search, been described previously, and thus, may be a rare variant.
Figure 1) Anterior and posterior illustrative drawings of the five-branched aortic arch with a retroesophageal right subclavian artery (RRSA) and an accessory left vertebral artery (ALVA) featured in this case study. AA=Aortic Arch; ALVA=Accessory Left Vertebral Artery; LCCA=Left Common Carotid Artery; LSA=Left Subclavian Artery; LVA=Left Vertebral Artery; RCCA=Right Common Carotid Artery; RRLN=Right Recurrent Laryngeal Nerve; RRSA=Retroesophageal Right Subclavian Artery.
Case Report
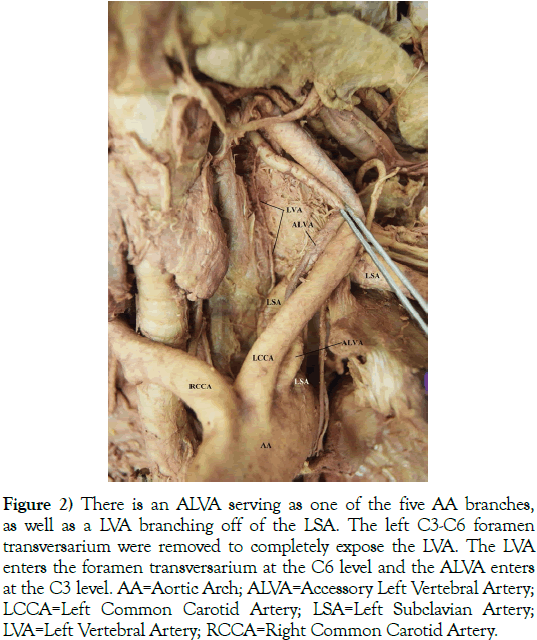
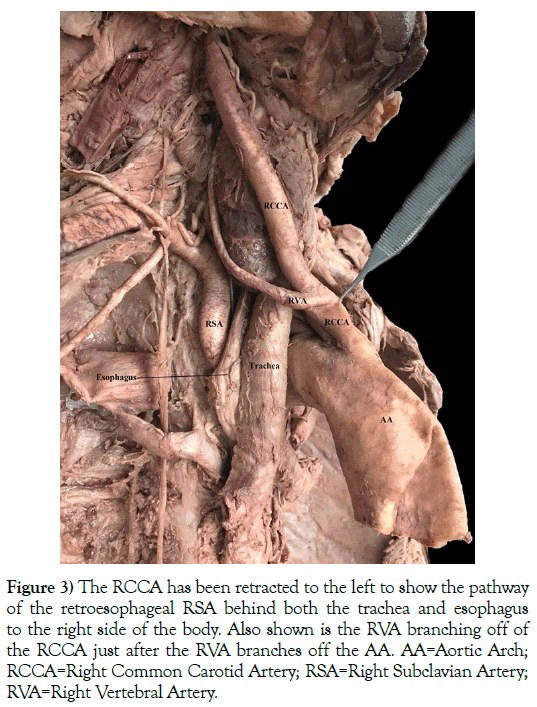
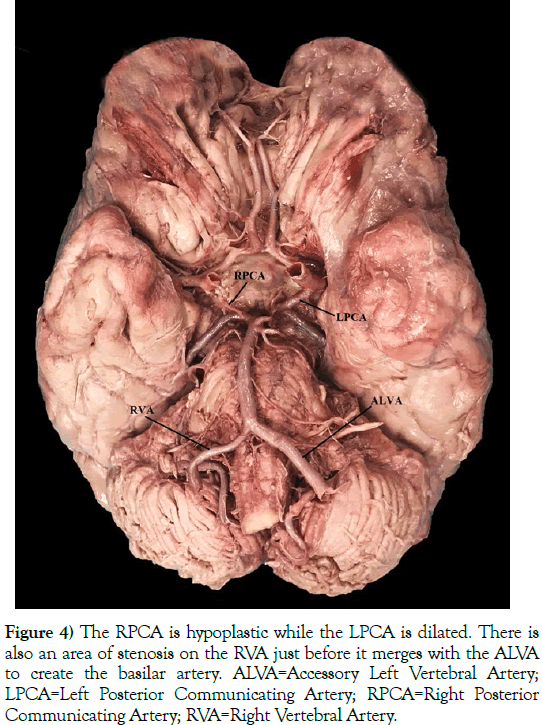
This 76-year-old White male (listed cause of death of acute kidney failure) presented with a five-branched AA. The branches included a RCCA, LCCA, LSA, ARSA (an RRSA), and an ALVA (Figures 2 and 3). A left vertebral artery (LVA) was present as the first branch off of the LSA. Both the RVA and ALVA entered the foramen transversarium at the C4 level, while the LVA entered at the C6 level. The RVA had a diameter of 3.5 mm. The LVA was hypoplastic, with a diameter of 1 mm. As the LVA traveled cranially and prior to merging with the ALVA, it gave off two small radiculomedullary (RMA) branches. The first RMA traveled medially and entered between the C5 and C6 vertebral bodies, and the second RMA entered between C4 and C5. The length of the LVA was 5.4 cm before uniting with the ALVA within the C4 foramen transversarium. As the ALVA ascended cranially, it exhibited stenosis with a diameter ranging between 4 and 5 mm. The circle of Willis also presented with several variations: the right posterior communicating artery (RPCA) was slightly hypoplastic while the left posterior communicating artery (LPCA) was markedly dilated, and the RVA had an area of stenosis just before it joined the ALVA to become the basilar artery (Figure 4). There were no variations detected with the cerebellar or basilar arteries.
Figure 2) There is an ALVA serving as one of the five AA branches, as well as a LVA branching off of the LSA. The left C3-C6 foramen transversarium were removed to completely expose the LVA. The LVA enters the foramen transversarium at the C6 level and the ALVA enters at the C3 level. AA=Aortic Arch; ALVA=Accessory Left Vertebral Artery; LCCA=Left Common Carotid Artery; LSA=Left Subclavian Artery; LVA=Left Vertebral Artery; RCCA=Right Common Carotid Artery.
Figure 3) The RCCA has been retracted to the left to show the pathway of the retroesophageal RSA behind both the trachea and esophagus to the right side of the body. Also shown is the RVA branching off of the RCCA just after the RVA branches off the AA. AA=Aortic Arch; RCCA=Right Common Carotid Artery; RSA=Right Subclavian Artery; RVA=Right Vertebral Artery.
Figure 4) The RPCA is hypoplastic while the LPCA is dilated. There is also an area of stenosis on the RVA just before it merges with the ALVA to create the basilar artery. ALVA=Accessory Left Vertebral Artery; LPCA=Left Posterior Communicating Artery; RPCA=Right Posterior Communicating Artery; RVA=Right Vertebral Artery.
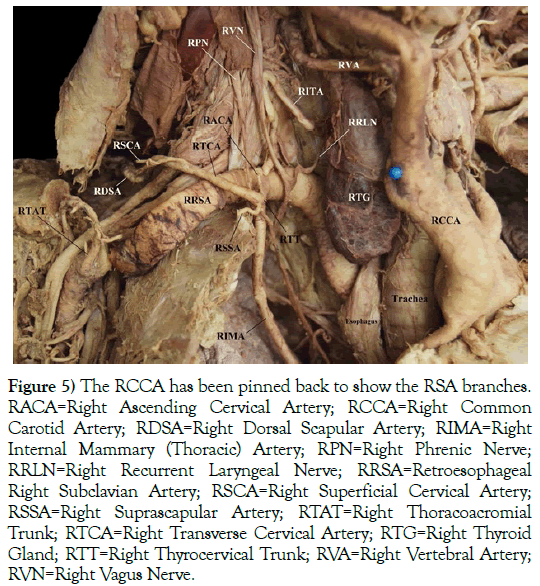
Several of the RRSA branches were damaged during student dissection of the root of the neck, including the right costocervical trunk. Assessment of the branches by further careful dissection revealed that the right thyrocervical trunk (RTT) arose from a common trunk with the right internal mammary (thoracic) artery (RIMA). The RTT was the origin of a large number of the RRSA branches (Figure 5). These branches included the right transverse cervical artery (RTCA), right ascending cervical artery (RACA), and the right suprascapular artery (RSSA). The right inferior thyroid artery (RITA) was its own branch from the RRSA. The RVA originated from the RCCA instead of the RRSA. Figure 4 also highlights that the right recurrent laryngeal nerve (RRLN) did not loop around the RRSA.
Figure 5) The RCCA has been pinned back to show the RSA branches. RACA=Right Ascending Cervical Artery; RCCA=Right Common Carotid Artery; RDSA=Right Dorsal Scapular Artery; RIMA=Right Internal Mammary (Thoracic) Artery; RPN=Right Phrenic Nerve; RRLN=Right Recurrent Laryngeal Nerve; RRSA=Retroesophageal Right Subclavian Artery; RSCA=Right Superficial Cervical Artery; RSSA=Right Suprascapular Artery; RTAT=Right Thoracoacromial Trunk; RTCA=Right Transverse Cervical Artery; RTG=Right Thyroid Gland; RTT=Right Thyrocervical Trunk; RVA=Right Vertebral Artery; RVN=Right Vagus Nerve.
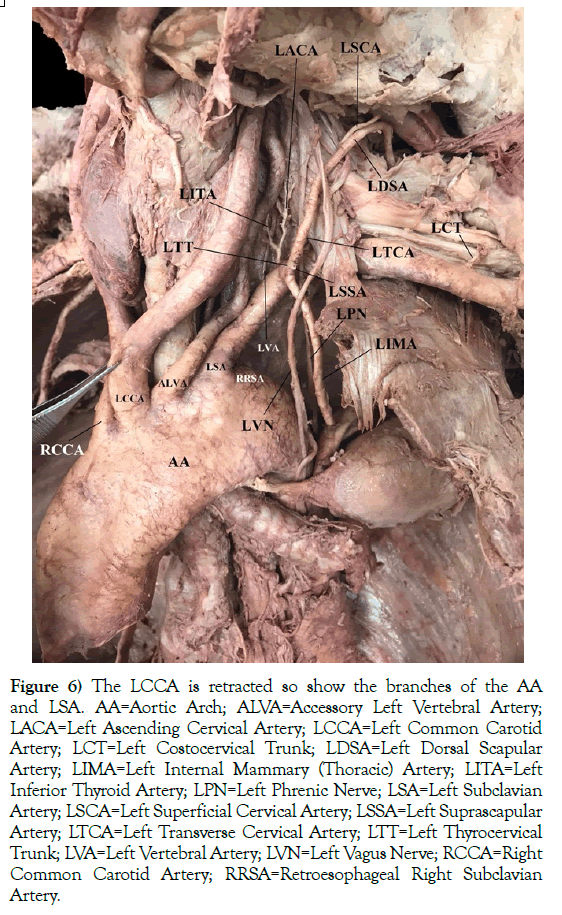
The LSA branched slightly differently from the RRSA, along with other branching variations (Figure 6). As commonly seen, the LVA is the first branch off of the LSA. Here, the left inferior thyroid artery (LITA) was an independent branch, similar to the RRSA, but it originated from a common trunk with the left ascending cervical artery (LACA). Like the RRSA, the left thyrocervical trunk (LTT) arose from a common trunk with the left internal mammary (thoracic) artery (LIMA). The branches of the LTT included the left transverse cervical artery (LTCA) and the left suprascapular artery (LSSA). Only a small portion of the LSSA was present since it was damaged during student dissection of the root of the neck. However, the left costocervical trunk (LCT) was not damaged during student dissection.
Figure 6) The LCCA is retracted so show the branches of the AA and LSA. AA=Aortic Arch; ALVA=Accessory Left Vertebral Artery; LACA=Left Ascending Cervical Artery; LCCA=Left Common Carotid Artery; LCT=Left Costocervical Trunk; LDSA=Left Dorsal Scapular Artery; LIMA=Left Internal Mammary (Thoracic) Artery; LITA=Left Inferior Thyroid Artery; LPN=Left Phrenic Nerve; LSA=Left Subclavian Artery; LSCA=Left Superficial Cervical Artery; LSSA=Left Suprascapular Artery; LTCA=Left Transverse Cervical Artery; LTT=Left Thyrocervical Trunk; LVA=Left Vertebral Artery; LVN=Left Vagus Nerve; RCCA=Right Common Carotid Artery; RRSA=Retroesophageal Right Subclavian Artery.
Discussion
Aberrant right subclavian artery
Aberrant right subclavian artery (ARSA) or arteria lusoria is a rare congenital vascular variation that is usually asymptomatic or symptoms appear later in life [7,12,13,17-22,24,27,29,31,35,37,54-56]. Symptomatic patients usually exhibit dysphagia (termed dysphagia lusoria), dyspnea, cough, pneumonia, stridor, and/or thoracic pain. These symptoms are usually associated with compression of the adjacent structure (esophagus and/or trachea). Adults most typically present with dysphagia, while infants more often present with respiratory distress, since adults are more resistant to tracheal compression [7,8,11-14,16-19,21,23-25,27,30-35,37,39,55-60].
François Joseph Hunauld first reported the occurrence of ARSA in 1735 [61]. In 1794, Dr. David Bayford described ARSA in a 62-year-old woman who died from starvation after years of dysphagia. Bayford coined the term ‘dysphagia lusoria’ meaning ‘difficulty swallowing due to a quirk of nature’ [62]. Dr. Burckhard Friedrich Kommerell described the radiological findings of ARSA in 1936 in relation to an aortic diverticulum (Kommerell’s diverticulum) [63]. A diverticulum of Kommerell is a pouch-like aneurysmal dilatation that occurs rarely in conjunction with an ASA aneurysm, found in 0.5% of the population. This pseudodiverticulum may be present at the origin of the ARSA and may represent a remnant of the right AA. It is present in 60% of ARSA cases. Aneurysmal dilatations can cause right arm ischemia, thoracic outlet syndrome, and esophageal fistula formation and rupture. There is a marked propensity for an acute rupture and subsequent death with ARSA aneurysms (approaching 50%) [12,14,16-21,23-25,27,30-33,35,37,54-56,59,62-66].
Other complications associated with an ARSA include atherosclerosis, abdominal aortic aneurysms (10%-20% of cases), stenosis, and dissections [23,24,65]. ARSA also associates with other anatomical variations, such as a non-recurrent right inferior laryngeal nerve, a common origin of the CCAs (truncus bicaroticus) (20.6%-29% of cases), ipsilateral VA arising from the CCA, an anomalous point of entrance of the VA into the cervical spine, a replaced right or left VA, coarctation of the aorta, right-sided AA, and an aberrant right thoracic duct [8,13,14,16-19,30-33,56,57].
Most human embryos studied develop six pairs of AAs that arise from the distal dilatation of the truncus arteriosus, known as the aortic sac. Each pair of AAs connects to the dorsal aorta of the corresponding side. The right fourth AA usually becomes the most proximal segment of the right subclavian artery (RSA). The distal part of the RSA forms from the union of a portion of right dorsal aorta and the seventh intersegmental artery. The portion of the right arch distal to the origin of the RSA regresses with the RCCA and RCA, thereby merging to form the RBT. The LSA is not derived from a pharyngeal arch artery; it forms from the left seventh intersegmental artery. As the development proceeds, differential growth shifts the origin of LSA cranially, resulting in its final placement close to the origin of LCCA. The left fourth arch forms the AA that continues and descends on the left side of the spine as the descending aorta [7,11,15,18,21,33,35,38]. In 64.9- 94.3% of cases, the AA has three great vessels as its branches from right to left: RBT, LCCA, and LSA. The RBT branches into the RSA and the RCCA. Such a pattern is considered typical [8,19,25,26,28,30,33,55].
In rare cases, the right fourth aortic arch involutes or disappears; an abnormal situation leaving the seventh intersegmental artery attached to the descending aorta. As a result, the RSA develops from the right seventh intersegmental artery and part of the right dorsal aorta caudal to the intersegmental artery [7,11,15-20,24,26-30,32,39,59,64,65]. When the right sixth dorsal segmental artery persists, the RVA arises from the RCCA. In addition, persistent attachment of the first or second dorsal intersegmental arteries to the fourth left aortic arch, associates with the origin of the LVA from the aortic arch [25,39]. To reach the right upper extremity, the ARSA crosses obliquely from left to right in one of three ways: posterior to the esophagus (80% of cases), between the esophagus and the trachea (10% to 15%), or anterior to the trachea (4.2-5%) [8,14,17,18,23,25,27,29-32,34,37,55,57,58,64,65]. Under these circumstances, the AA has four branches: the RCCA, LCCA, LSA, and the ARSA. The recurrent laryngeal nerve does not pass around the ARSA in any of these three path scenarios [67]. If the regression occurs on the left arch, the result is an ALSA [11-14,21,27,37,57,64].
ARSA has been reported more frequently in females. The prevalence of ARSA rises exponentially to 26%-34% in individuals with Down syndrome (Trisomy 21) and other chromosomal defects, including microdeletion 22q11 (DiGeorge syndrome) and Trisomy 18 (Edward syndrome) [7,11,15-17,19,29,32,37,54,55,68-71]. These are genetic conditions likely to survive into postnatal, and increasingly adult life. Such conditions frequently demonstrate vascular variations, particularly those of the heart, great vessels, and the AA.
Accessory vertebral artery
Unilateral duplicate origin of the VA or accessory vertebral artery (AVA) is considered rare, with a reported incidence of 0.295%-0.72% [1,6,40,50-53]. In this individual, the left fifth intersegmental artery persisted. AVAs are equally frequent on both the right and left sides [72]. This developmental variation associates with a dual origin and variable level of fusion with the VA in the neck [1,51,73-76]. The AVA and VA can enter in the same foramen transversarium or in different foramina transversarium; usually the medial one enters at the higher level. Fusion of the AVA and VA occurs at the C5-C6 in 42.3% of cases, C4-C5 in 30.8%, C3-C4 in 15.4%, and C6-C7 in 11.5% [72,77]. We found reports of 58 cases involving this vascular variant, with the first reported case in 1844 [2,6,9,10,40-46,51,52,73-75,77-92].
AVAs are usually asymptomatic clinically [1,3,4,6,28,53,78,88,93,94]. This origin variation may alter cerebral hemodynamics, however, and lead to cerebral dysfunction. Such dysfunction may predispose a patient to cerebrovascular pathologies, such as aneurysm and dissection [2,3,6,9,75,81,85,95-100].
AVAs result from a developmental variant of the AA with the persistence of intersegmental arteries [5,101]. For accessory RVAs (ARVAs), originating from the RSA or BT, the right fourth or fifth or sixth (or less frequently the third) intersegmental artery persists. For the ALVA, the left fourth or fifth or sixth (less frequently the third) intersegmental artery persists [1,2,25,46,51,77]. Additionally, the level at which the accessory VA enters the foramen transversarium indicates which intersegmental artery or arteries persist [6,9,51,82,85].
Circle of willis variations
The vascular variations we observed within the circle of Willis and cerebellum in this case are well-documented in the literature. Hypoplasia is one of the most frequent vascular anomalies in the circle of Willis, ranging from a 23%-27% incidence rate [102-105]. Circle of Willis arteries less than 1 mm in external diameter are considered hypoplastic, with the exception of the communicating arteries. These are considered hypoplastic when less than 0.5 mm [102,104]. The posterior communicating artery variations are among the most common, with hypoplasia observed in 12% to 60% of carefully studied samples, followed by variations in the posterior cerebral artery in 10% [106,107].
Variations in subclavian branches
The branching variations of the RRSA and LSA we observed are found in the literature. In about 38% of cases, the transverse cervical artery arises from the thyrocervical trunk [37]. In Takafuji and Sato 1991 study of 72 Japanese adults, only 3.5% exhibited a thyrocervical trunk that arose from a common trunk with the internal mammary (thoracic) artery [108,109]. Its branches were the suprascapular artery and transverse cervical artery. In the same study, they found that the combination of ascending cervical and inferior thyroid arteries originating from a common trunk with the transverse cervical and suprascapular arteries originating from the thyrocervical trunk occurred in only 1.4% [108]. The inferior thyroid artery originated directly from the subclavian artery in 8% of cases in another study [37]. Variant origin of the RIMA occurred in 5% of cases examined with a frequency of 30% in LIMA in yet another study [110].
Another five-branched aortic arch description
Ma et al. described five vessels arising from the aortic arch. However, their unique case study reveals the following branches: RCCA, LCCA, left thyrocervical trunk (LTT), LSA, and RRSA [59]. Our findings differ in that we found our case study to exhibit: RCCA, LCCA, LSA, RRSA, and an ALVA.
Conclusion
Aortic arch and subclavian branching variations are common and significant in both their origin and course of arteries. Many are asymptomatic but can have serious implications for clinical diagnosis, angiographies, interventional and surgical procedures in the head and neck. With the increasing use of a transradial approach for coronary angiography, due to its lower risk of associated access site related complications, ARSAs will be encountered more frequently. Awareness of the presence of ARSA together with the presence of a right non-recurrent laryngeal nerve is clinically important when performing thyroid surgery. Awareness of an RRSA is significant, especially during right axillary, brachial, or radial angiographic approaches to the ascending thoracic aorta. The potential, but rare complication of a nearby arterioesophageal fistula carries a 50% risk of rupture and fatality. In this case, we present the presence of the ALVA rather than a LVA originating from the AA as a genuine potential variation in this region. Its presentation with a hypoplastic LVA could have been missed easily had we not engaged in a continued thorough anatomic cadaveric dissection of this individual. Anatomists, radiologists, and surgeons need to recognize this possibility and thus, the importance of thorough dissection and imaging of this region.
Acknowledgement
We would like to thank the family of our donor for their beneficent contribution. Without their generosity, this article would not have been possible. We would also like to thank Dr. Gary Wind for providing us an accurate anatomical rendering of the neurovasculature discussed in this article.
REFERENCES
- Kim MS. Duplicated vertebral artery: Literature review and clinical significance. J Korean Neurosurg Soc. 2018;61:28-34.
- Rameshbabu CS, Gupta OMP, Gupta KK, et al. Bilateral asymmetrical duplicated origin of vertebral arteries: Multidetector row CT angiographic study. Indian J Radiol Imaging. 2014;24:61-5.
- Satheesha BN, Sirasanagandla SR, Surekha DS, et al. Variant origin of the left vertebral artery from a vertebro-subclavian trunk associated with an unusual branch arising from the brachiocephalic trunk. J Surg Academia. 2014;4:73-5.
- Shi SK. Arterial vascular variation of the head and neck and its clinical significance. J Neurol Neurophysiol. 2017;8:442-3.
- Cheng M, Xie X, Wang C, et al. Two anatomic variations of the vertebral artery in four patients. Ann Vasc Surg. 2009;23:689.e1-5.
- Gupta OMP, Gupta KK, Qasim M, et al. Bilateral asymmetrical duplicated origin of vertebral arteries: Multidetector row CT angiographic study. Indian J Radiol Imaging. 2014;24:61-5.
- Kanaskar N, Vatslalswamy P, Sonje P, et al. Retroesophageal right subclavian artery. Adv Anat. 2014;2014:1-3.
- Lale P, Toprak U, Yagiz G, et al. Variations in the branching pattern of the aortic arch detected with computerized tomography angiography. Adv Radiol. 2014;2014:1-6.
- Jung S, Jung C, Jung Bae Y, et al. Duplicated Origin of the Left Vertebral Artery: A Case Report and Embryological Review. Neurointervention. 2016;11:50-4.
- Watanabe K, Saga T, Iwanaga J, et al. A rare case of dual origin of the left vertebral artery without convergence. Folia Morphol (Warsz). 2016;75:136-42.
- Chaoui R, Rake A, Heling KS. Aortic arch with four vessels: aberrant right subclavian artery. Ultrsound Obstet Gynecol. 2008;31:115-7.
- Atay Y, Engin C, Posacioglu H, et al. Surgical approaches to the aberrant right subclavian artery. Tex Heart Inst J. 2006;33:477-81.
- Darwazah AK, Eida M, Khalil RA, et al. Non-aneurysmal aberrant right subclavian artery causing dysphagia in a young girl: Challenges encountered using supraclavicular approach. J Cardiothorac Surg. 2015;10:92-5.
- Epstein DA, De Bord JR. Abnormalities associated with aberrant right subclavian arteries: A case report. Vasc Endovasc Surg. 2002;36:297-303.
- Fazan VPS, Ribeiro RA, Ribeiro JAS, et al. Right retroesophageal subclavian artery. Acta Cir Bras 2003;18:54-6.
- Kieffer E, Bahnini A, Koskas F. Aberrant subclavian artery: surgical treatment in thirty-three adult patients. J Vasc Surg 1994;19:100-11.
- Myers PO, Fasel JHD, Kalangos A, et al. Arteria lusoria: Developmental anatomy, clinical, radiological and surgical aspects. Ann Cardiol Angeiol. 2010;59:147-54.
- Abraham V, Mathew A, Cherian V, et al. Aberrant subclavian artery: Anatomical curiosity or clinical entity. Int J Surg. 2009;7:106-9.
- Polguj M, Chrzanowski L, Kasprzak JD, et al. The Aberrant right subclavian artery (Arteria Lusoria): The morphological and clinical aspects of one of the most important variations – a systematic study of 141 Reports. Sci World J. 2014;2014:1-6.
- Corral JS, Zuniga CG, Sanchez JB, et al. Treatment of aberrant right subclavian artery aneurysm with endovascular exclusion and adjunctive surgical bypass. J Vasc Interv Radiol. 2003;14:789-92.
- Freed K, Low VH. The aberrant subclavian artery. AJR Am J Roentgenol. 1997;168:481-4.
- Khatri R, Maud A, Rodriguez GJ. Aberrant right subclavian artery and common carotid trunk. J Vasc Interv Radiol. 2010;3:33-4.
- Erdem K, Ozden A, Erkuran MK, et al. Aberrant right subclavian artery (arteria lusoria): A case of symptomatic rupture. IJMSPH. 2014;3:99-101.
- Kopp R, Wizgall I, Kreuzer E, et al. Surgical and endovascular treatment of symptomatic aberrant right subclavian artery (arteria lusoria). Vascular. 2007;15:84-91.
- Loukas M, Louis Jr. RG, Gaspard J, et al. A retrotracheal right subclavian artery in association with a vertebral artery and thyroidea ima. Folia Morphol. 2006;65:236-41.
- Meena D. Aberrant right subclavian artery in association with common trunk of both carotid arteries: Diagnosis with CT. West Afr J Radiol. 2014;21:80-4.
- Naqvi SE, Beg MH, Thingam SK, et al. Aberrant right subclavian artery presenting as tracheoesophagial fistula in a 50-year-old lady: Case report of a rare presentation of a common arch anomaly. Ann Pediatr Card. 2017;10:190-3.
- Ocaya A. Retroesophageal right subclavian artery: A case report and review of the literature. Afri Health Sci. 2015;15:1034-7.
- Jain KK, Braze AJ, Shapiro MA, et al. Aberrant right subclavian artery-esophageal fistula and severe gastrointestinal bleeding after surgical correction of scimitar syndrome. Tex Heart Inst J. 2012;39:571-4.
- Rogers AD, Nel M, Eloff P, et al. Dysphagia Lusoria: A case of an aberrant right subclavian artery and a bicarotid trunk. international scholarly research network. 2011;2011:1-6.
- Rosa P, Gillespie DL, Goff JM, et al. Aberrant right subclavian artery syndrome: A case of chronic cough. J Vasc Surg. 2003;37:1318-21.
- Roszel AJ, Kiely ML. A Retroesophageal right subclavian artery with the right vertebral artery originating from the right common carotid artery. Clin Anat. 1991;4:373-9.
- Saito T, Tamatsukuri Y, Hitosugi T, et al. Three cases of retroesophageal right subclavian artery. J Nippon Med Sch 2005;72:375-82.
- Schneider J, Baier R, Dinges C, et al. Retroesophageal right subclavian artery (lusoria) as origin of traumatic aortic rupture. Eur J Cardiothorac Surg 2007;32:385-7.
- Stone WM, Brewster DC, Moncure AC, et al. Aberrant right subclavian artery: Varied presentations and management options. 1990;11:812-7.
- Tsai IC, Tzeng WS, Lee T, et al. Vertebral and carotid artery anomalies in patients with aberrant right subclavian arteries. Pediatr Radiol. 2007;37:1007-12.
- Tunali S. Subclavian Artery. In: Tubbs RS, Shoja MM, Loukas M, eds. Bergman’s Comprehensive Encyclopedia of Human Variation. 2016;575-82.
- Maiti TK, Konar SK, Bir S, et al. Anomalous origin of the right vertebral artery: Incidence and significance. World Neurosurg. 2016;89:601-10.
- Kim YD, Yeo HT, Cho YD. Anomalous variations of the origin and course of vertebral arteries in patients with retroesophageal right subclavian artery. J Korean Neurosurg Soc. 2009;45:297-9.
- Mahmutyazicioglu K, Sarac K, Boluk A, et al. Duplicate origin of left vertebral artery with thrombosis at the origin: Color doppler sonography and CT angiography findings. J Clin Ultrasound 1998;26:323-5.
- Babin E, Haller M. Correlation between bony radiological signs and dolichoarterial loops of the cervical artery. Neuroradiol J. 1974;7:15.
- Harada J, Nishijima M, Yamatani K, et al. A case of the duplicate origin of right vertebral artery. No Shinkei Geka 1987;15:321-5.
- Harnier S, Harzheim A, Limmroth V, et al. Duplication of the common carotid artery and the ipsilateral vertebral artery with a fenestration of the contralateral common carotid artery. Neurol India 2008;56:491-3.
- Hashimoto H, Ohnishi H, Yuasa T, et al. Duplicate origin of the vertebral artery: Report of two cases. Neuroradiol J. 1987;29:301.
- Kiss J. Bifid origin of the right vertebral artery. A case report. Radiol. 1968;91:931.
- Suzuki S, Kuwabara Y, Hatano R, et al. Duplicate origin of left vertebral artery. Neuroradiol J. 1978;15:27-9.
- Ouchi H, Ohara I. Extracranial abnormalities of the vertebral artery detected by selective arteriography. J Cardiovasc Surg. 1968;9:250.
- Newton TH, Mani RL. The vertebral artery. In: Newton TH, Potts DG, eds. Radiology of the Skull and Brain. St. Louis: Mosby. 1974:1659-72.
- Kowada M, Yamaguchi K, Takahashi H. Fenestration of the vertebral artery with a review of 23 cases in Japan. Radiology. 1972;103:343-6.
- Bergman RA, Thompson SA, Afifi AK, et al. Compendium of human anatomic variation: Text, atlas and world literature. Baltimore: Urban and Schwarzenberg. 1988;pp:71-72.
- Baik J, Baek HJ, Shin HS, et al. Duplication of the right vertebral artery: MRA findings and review of the literature. Springer Plus. 2016;5:1123.
- Polguj M, Jęrdzejewski K, Topol M, et al. Duplication of the left vertebral artery in a patient with dissection of the right internal carotid artery and Ehlers-Danlos syndrome: Case report and review of the literature. Anat Sci Int. 2013;88:109-14.
- Yuan SM. Aberrant origin of vertebral artery and its clinical implications. Braz J Cardiovasc Surg. 2016;31:52-9.
- Allen D, Brews H, Vo M, et al. Arteria lusoria: An anomalous finding during right transradial coronary intervention. Case Reports in Cardiology 2016;2016:1-3.
- Jiang X-H, Zhu X-Y. Abnormal origin of the right subclavian artery: A case report. Chin Med J. 2017;130:1508-9.
- Mahmodlou R, Sepehrvand N, Hatami S. Aberrant right subclavian artery: A life-threatening anomaly that should be considered during Esophagectomy. J Surg Tech Case Rep. 2014;6:61-3.
- Adams HL, Kaplan HS. Angiographic interpretation in congenital heart disease. 2009;pp:54-57.
- Pifarre R, Jasuja M, Neville WE. Retroesophageal right subclavian artery in the adult. Dis Chest. 1969;56:538-9.
- Ma Z, Han J, Li H, et al. A unique variation with five branches of the aortic arch. Interact Cardiovasc Thorac Surg. 2018;26:165-6.
- Master AM, Lasser RP, Rosenfeld I, et al. Congenital anomalies of the aortic arch. In: The electrocardiogram and chest X-Ray in disease of the heart. Philadelphia, PA: Lea & Febiger, 1963:491-5.
- Hunauld PM. Examen de quelques parties d'un singe. Hist Acad Roy Sci. 1735;2:516-23.
- Bayford D. An account of a singular case of obstructed deglutition. Mem Med Soc Lond. 1794;2:271-86.
- Kommerell B. Verlagerung des osophagus durch eine abnorm verlaufende Arteria subclavia dextra (Arteria lusoria). Fortschr. Rontgenstr. 1936;54:590-5.
- Bull PG, Denck H. Aberrant right subclavian artery. Eur J Vasc Surg. 1994;8:757-60.
- Gray VJ. Diagnosing a freak of nature. Student Hospitalist. 2010;pp:1-2.
- Feugier P, Lemoine L, Gruner L, et al. Arterioesophageal fistula: A rare complication of retroesophageal subclavian arteries. Ann Vasc Surg. 2003;17:302-5.
- Green PH. An account of the varieties in the arterial system of the human body. Dublin, Ireland: James Marshall Leckie, 1830;pp:1-8.
- Chaoui R, Heling KS, Sarioglu N, et al. Aberrant right subclavian artery as a new cardiac sign in second- and third-trimester fetuses with Down syndrome. Am J Obstet Gynecol. 2005;192:257-63.
- Chaoui R, Thiel G, Heling KS. Prevalence of an aberrant right subclavian artery (ARSA) in fetuses with chromosomal aberrations. Ultrasound Obstet Gynecol 2006;28:414-5.
- Vibert-Guigue C, Fredouille C, Gricorescu R, et al. Données foetopathologiques sur une serie de foetus trisomique 21. Rev Pract Gynecol Obstet. 2006;103:35-40.
- Zalel Y, Achiron R, Yagel S, et al. Fetal aberrant right subclavian artery in normal and down syndrome fetuses. Ultrasound Obstet Gynecol. 2008;31:25-9.
- George B, Bruneau M. Vertebral artery. In: Tubbs RS, Shoja MM, Loukas M, eds. Bergman’s Comprehensive Encyclopedia of Human Variation. Hoboken, NJ: John Wiley & Sons, Inc. 2016:487-500.
- Ionete C, Omojola MF. MR angiographic demonstration of bilateral duplication of the extracranial vertebral artery: Unusual course and review of the literature. Am J Neuroradiol. 2006;27:1304-6.
- Goddard AJ, Annesley-Williams D, Guthrie JA, et al. Duplication of the vertebral artery: Report of two cases and review of the literature. Neuroradiol J. 2001;43:477-80.
- Melki E, Nasser G, Vandendries C, et al. Congenital vertebral duplication: A predisposing risk factor for dissection. J Neurol Sci 2012;314:161-2.
- Ozpinar A, Magill ST, Davies JM, et al. Vertebral Artery Fenestration. Cureus 2015;7:e245.
- Komiyama M, Nakajima H, Yamanaka K, et al. Dual origin of the vertebral artery-case report. Neurol Med Chir (Tokyo). 1999;39:932-7.
- Lemke AJ, Benndorf G, Liebig T, et al. Anomalous origin of the right vertebral artery: Review of the literature and case report of right vertebral artery origin distal to the left subclavian artery. Am J Neuroradiol. 1999;20:1318-21.
- Kuwujara H, Aoto K, Uno J. A case of duplicate origin of the left vertebral artery associated with moyamoya disease. J Clin Med. 1988;66:1174.
- Mashiyama S, Watanabe T. Duplicate origin of vertebral artery combined with brain tumor [in Japanese]. J Clin Radiol. 1989;34:727.
- Thomas AJ, Germanwala AV, Vora N, et al. Dual origin extracranial vertebral artery: Case report and embryology. J Neuroimaging. 2008;18:173-6.
- Meila D, Tysiac M, Petersen M, et al. Origin and course of the extracranial vertebral artery-CTA findings, association with vascular lesions and embryologic considerations. Clin Neuroradiol. 2012;22:327-33.
- Mordasisni P, Schmidt F, Schroth G, et al. Asymmetrical bilateral duplication of the extra cranial vertebral arteries: Report of a unique case. Eur J Radiol Extra. 2008;67:e91-4.
- Nishijima M, Harada J, Akai T, et al. Operative correction of a kinked duplicate origin of the vertebral artery in a patient with dizziness. Case report. Surg Neurol. 1989;32:356.
- Rieger P, Huber G. Fenestration and duplicate origin of the left vertebral artery in angiography. Report of three cases. Neuroradiol J. 1983;25:45.
- Shin SW, Park DW, Park CK, et al. Duplication of the left vertebral artery origin: A case report. J Korean Soc Radiol. 2013;68:1-4.
- Sugita S, Abe T, Okura A, et al. MRI demonstration of double origin of the left vertebral artery: Case note. Neuroradiology 1995;37:295-6.
- Takasato Y, Hayashi H, Kobayashi T, et al. Duplicated origin of right vertebral artery with rudimentary and accessory left vertebral arteries. Neuroradiol J. 1992;34:287-9.
- Tobin WO, Killeen R, Kinsella JA, et al. Dual origin of the left vertebral artery: Extracranial MRA and CTA findings. J Neurol Sci. 2010;298:150-2.
- Nakamura M, Saga T, Tetsuka M, et al. A case of duplicated left vertebral artery and the research of the entrance of the vertebral artery in transverse foramen of cervical vertebra [Japanese]. Kurume igakukai zasshi. 2012;75:25-31.
- Eisenberg RA, Vines FS, Taylor SB. Bifid origin of left vertebral artery. Radiol. 1986;159:429.
- Onizuka K, Kurimoto M, Endo S, et al. A case of persistent first segmental artery associated with a duplicate origin of vertebral artery. J Clin Radiol. 1991;36:1707.
- Fazan VPS, Caetano AG, Filho OAR. Anomalous origin and cervical court of the vertebral artery in the presence of a retroesophageal right subclavian artery. Cli Anat. 2004;17:354-7.
- Alsaif HA, Ramadan WS. An anatomical study of the aortic arch variations. JKAU Med Sci. 2010;17:37-54.
- Bernardi L, Dettori P. Angiographic study of a rare anomalous origin of the vertebral artery. Neuroradiol J. 1975;9:43-7.
- Dare AO, Chaloupka JC, Putman CM, et al. Vertebrobasilar dissection in a duplicated cervical vertebral artery: A possible pathoetiologic association? A case report. Vasc Endovascular Surg. 1997;31:103-9.
- Satti SR, Cerniglia CA, Koenigsberg RA. Cervical vertebral artery variations: An anatomic study. Am J Neuroradiol. 2007;28:976-80.
- Kendi AT, Brace JR. Vertebral artery duplication and aneurysms: 64 slice multidetector CT findings. Br J Radiol. 2009;82:e216-8.
- Komiyama M, Morikawa T, Nakajiman H, et al. High incidence of arterial dissection associated with left vertebral artery of aortic origin. Neurol Med Chir (Tokyo). 2001;41:8-11.
- Kim DW. Concomitant dual origin and fenestration of the left vertebral artery resembling dissection. J Korean Neurosurg Soc. 2009;46:498-500.
- Polguj M, Podgorski M, Jędrzejewski K, et al. Fenestration and duplication of the vertebral artery: The anatomical and clinical points of view. Clin Anat. 2013;26:933-43.
- Iqbal S. A comprehensive study of the anatomical variations of the circle of Willis in adult human brains. J Clin Diagn Res. 2013;7:2423-7.
- Alpers BJ, Berry RG, Paddison RM. Anatomical studies of the circle of Willis in normal brain. Arch Neurol Psychiat. 1959;81:409-418.
- Kamath S. Observations on the length and diameter of the vessels forming the circle of Willis. J Anat. 1981;133:419-23.
- Fetterman GH, Moran TJ. Anomalies of the circle of Willis in relation to cerebral softening. Arch Pathol. 1941;32:251-7.
- Nordon DG, Rodrigues Junior OF. Variations in the brain circulation-the circle of Willis. J Morphol Sci. 2012;29:243-7.
- Foreman PM, Griessenauer CJ, Deveikis JP, et al. Internal carotid artery and the anterior cerebral circulation. In: Tubbs RS, Shoja MM, Loukas M, eds. Bergman’s Comprehensive Encyclopedia of Human Variation. Hoboken, NJ: John Wiley & Sons, Inc, 2016:449-60.
- Takafuji T, Sato Y. Study on the subclavian artery and its branches in japanese adults. Okajimos Folia Anat Jpn. 1991;68:171-86.
- Yucel AH, Kizilkanat E, Ozdemir CO. The variations of the subclavian artery and its branches. Okajimos Folia Anat Jpn. 1999;76:255-62.
- Panakkal BJ, Rajesh GN, Parakkal HB, et al. Bilateral variant origin of subclavian artery branches. BJR Case Rep. 2016;2.