Rare incidence of spontaneous malignant fibrous histiocytoma in the rhesus monkey
2 National Laboratory Animal Facility-CSIR Institute (CSIR-CDRI), Uttar Pradesh, India
Himangsu Kousik Bora, National Laboratory Animal Facility-CSIR Institute (CSIR-CDRI), Uttar Pradesh, India, Tel: +91-9161410225, Email: drhkb008@cdri.res.in
Received: 28-Oct-2021 Accepted Date: Nov 11, 2021; Published: 18-Nov-2021
Citation: Mugale MN, Upadhyay DS, Yadav CS, et al. Rare incidence of spontaneous malignant fibrous histiocytoma in the rhesus monkey. J Histol Histopathol Res 2021;5(4):1-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The rodents are being widely used for cancer research studies, however, being phylogenetically distant; rodents do not closely mimic the disease progression as in humans. Therefore, Non-human Primates (NHPs) which are genetically closer to human are evidently better models. The outcome of preclinical tumor studies on NHPs has greater applicability in humans than those on rodents. The incidence of spontaneous neoplasia in NHPs is rare. We observed a case of spontaneously occurring tumor located at the lateral side of the neck adjacent to the shoulder joint in an adult rhesus monkey. The tumor arose as subcutaneous reddish-white mass, multicentric firm, and pedunculated smooth nodules with tall warty growths, resembling cauliflower in appearance. Microscopically, five different types of pleomorphic cells such as fibroblast-like cells, histiocyte-like cells, undifferentiated cells, xanthomatous cells, and multinucleated giant cells were observed. All cells were of storiform pattern, with irregular fascicles and variable cellularity, including atypical forms with numerous mitotic figures, which was suggestive of Malignant Fibrous Histiocytoma (MFH). The study of such spontaneous tumors may assist researchers in developing animal cancer models for human malignancies and, owing to their similarity with human cancers, help in prevention and treatment.
Keywords
Giant cells; Histiocytoma; Monkeys; Pleomorphic; Storiform
Introduction
Rodents, because of their small size, low cost, rapid growth and wider availability, are extensively used in cancer studies. However, the clinical findings in rodents, mostly, do not translate well and fail in clinical trials [1,2]. The Non-human Primates (NHPs) are genetically closely related to humans and have been used as animal models for many disease and disorders such as viral infection, aging, neuro-behavioral and preclinical regulatory safety studies, etc. [3]. The use of NHPs in cancer research is limited because the incidences of the spontaneous tumor are very low [4]. It is dependent on many factors including age, sex, and species. The incidence increases with the age in both humans and primates, and those over 60 and 20 years of age, respectively are particularly at a greater risk of developing tumors [5,6]. The occurrence of tumor incidence has been reported to be higher in African green monkey (8%) than Cynomolgus (1.5%) and rhesus monkeys (2.8%) [7]. Further, various methods including chemical, biological and physical have been used to induce tumor in primates other than rhesus monkeys. The malignant mesenchymal tumors, fibro-sarcomas, originate from fibrous connective tissue. These are rare and aggressive in nature and only a few reports on spontaneous fibromas or fibrosarcomas in rhesus monkey are available [8]. Malignant Fibrous Histiocytoma (MFH) is one of the most common adult soft tissue neoplasia of other species but very rare in primates. Pathogenesis of this kind of sarcoma is very aggressive and is most commonly located in the extremities and the retro-peritoneum that can get manifested to other sites as well [9].
Case Representation
Total 30 (15 Male+15 Female) wild-caught adult Rhesus monkeys (Macacamulatta) weighing about 4-6 kilogram were supplied by CPCSEA (Committee for the Prevention, Control, and Supervision of Experiments in Animals) approved supplier M/S Indian Animals Suppliers, Lucknow (INDIA) and housed at primate unit, National laboratory Animal Facility, CSIR-CDRI, Lucknow. Upon receipt, the animals were clinically examined and housed individually at the quarantine unit at least for 45 days. Individual animals were marked with unique identification numbers written on their chests by tattooing with the help of indelible tattooing ink. Clean potable drinking water, standard balanced laboratory pelleted diet along with seasonal fruits and vegetables were provided ad libitum to the animals. During the quarantine period, the animals were subjected to multi-step screening programs like routine clinical observation, tuberculin testing, chest X-ray, and other regular veterinary preventive measures. Clinical signs such as general appearance, activity, behavior, movement, gait, posture and response to handling, eyes, lacrimation, fur-coat, mucous membranes, body orifices, consistency of excreta, unusual respiratory pattern, as well as the presence of clonic or tonic movements, stereotypes (e.g. excessive grooming, repetitive circling) or any strange behavior if any were duly recorded. The intradermal tuberculin test was performed by injecting 0.1 ml of PPD (1,000 to 1,500 TU, fresh solution) in the palpebral skin followed by a period of observation of 24-72 hours. Initial hematology, clinical biochemistry, urine analysis, ECG, and Ophthalmic examination were carried out. Towards the end of the quarantine period, one monkey was found to be anorectic, weak, and dehydrated. The monkey was immediately isolated and shifted to the isolation unit. On clinical examination, one enlarged palpable nodular growth was observed in the left lateral side of the neck above the shoulder joint. Therapeutic interventions like intravenous fluid therapy, vitamin supplements, and broad-spectrum antibiotic along with NSAID were provided but there were no positive responses and animals died one-month post-treatment. Necropsy was performed immediately; the excised tumor tissues along with other major visceral organs and tissues were collected and preserved in 10% neutral buffer formalin solution. After proper fixation, representative tissue samples were processed; paraffin sections of 4 to 5 μ were routinely prepared and stained with hematoxylin and eosin. Histopathological alteration of tumorous nodules was observed under a light microscope (Olympus BX61). Masson’s trichrome special staining was performed for collagen fibers using a commercial staining kit (Polysciences, Inc., Washington). Immuno-histochemistry examination for cytokeratin and Ki67 biomarker was carried out as per routine procedure following the protocols provided by the manufacturer (Thermo fisher scientific USA).
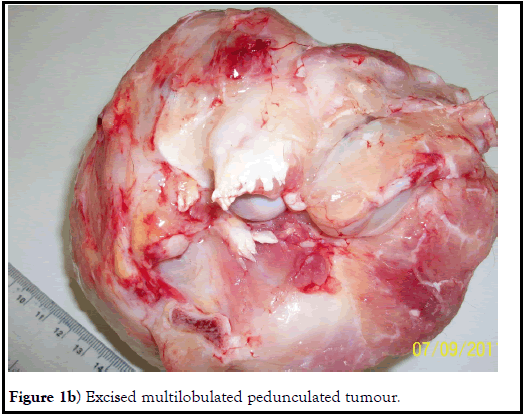
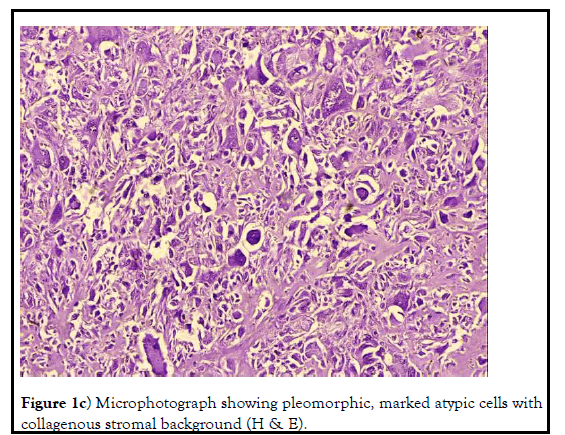
Externally, a tumor like nodular growth was noticed at the left lateral side of the neck region adjutant to the shoulder joint. Needle aspiration was performed to differentiate from any kind of abscess but no fluid or pus could be aspirated out. At necropsy, grossly the tumor ascended as a subcutaneous reddish-white mass, multicentric firm, and pedunculated smooth nodules with tall warty growths, resemblance to large cauliflower and of multiple confluence appearance. The weight of the excised tumor was about 882g. Morphologically, the tumor surface was observed multilobular with a dense fibrous capsule. Histopathological examination of the tumor sections revealed marked cellular proliferation arranged in a storiform pattern (cells emanate from a central focus), with irregular fascicles and variable cellularity. The majorities of the cells were pleomorphic in nature and bizarre neoplastic cells containing foamy cytoplasm and marked atypia embedded in the inflamed collagenous stromal matrix. These findings typically suggested the end stage of any sarcomas having a common morphological cellular pattern of pleomorphism and storiform cellular growth. Few polygonal cells resembling histiocytes, and malignant giant cells that can be classified as MFH-giant cells were observed. Atypical cells with anisocytosis, anisokaryosis, and numerous mitotic figures were suggestive of (MFH) or Malignant Fibrous Histiocytoma. Five different types of cells viz. fibroblastlike cells, histiocyte-like cells, undifferentiated cells, xanthomatous cells, and multinucleated giant cells were observed which was inconsistent with earlier reports on MFH. Mild bluish-green coloration indicating collagen fiber formation was observed in Masson’s trichrome special staining. Immunohistochemistry of tumor sections showed expression of vimentin and cytokeratin antibody but which was minimal. There was no metastasis observed in any other visceral organs (Figures 1a-1d).
Figure 1c): Microphotograph showing pleomorphic, marked atypic cells with collagenous stromal background (H & E).
Results and Discussion
A malignant fibrous histiocytoma is defined as a pleomorphic sarcoma of a varied pattern consisting of a mixture of histiocytic and fibroblastic elements [10]. Moreover, several mesenchymal origin malignant tumors contain fibroblastic elements, misleading to an over-diagnosis of fibrosarcoma. Furthermore, MFH is recognized recently as a diagnostic entity and histologically characterized by the mixture of spindle cells arranged in a storiform pattern, polygonal cells resembling histiocytes, and malignant giant cells [11]. MFH often recurs even after surgical removal of the tumor. It may get metastasized if the cells are large, have bizarre shapes, and are extremely invasive in nature although we did not observe metastasis in any other organs and tissues. In this case, atypical cells with anisocytosis and anisokaryosis were noticeable with infrequent abundant mitotic figures suggestive of fibrosarcoma [12]. There are numerous reports regarding incidences of fibrosarcomas of the head and neck region, including the nasal cavity, paranasal sinuses, and nasopharynx in other laboratory animals [13,14]. Based on the degrees of differentiations, these sarcomas may be graded as low grade, intermediate malignancy, and high malignancy (anaplastic).
Conclusion
Histological observation of proliferating cells revealed five different types of viz. fibroblast-like cells, histiocyte-like cells, undifferentiated cells, xanthomatous cells, and multinucleated giant cells were observed which was in agreement with earlier reports on MFH. The existence of pleomorphic tumor cells resembling histiocytes along with malignant giant cells differentiates MFH from other sarcomas such as osteosarcoma, fibrosarcoma, leomyosarcoma, rhabdomyosarcoma, and osteosarcoma. Tumors of these kinds are most commonly observed in the deep soft tissue of the lower extremities and followed by the upper trunk area.
Acknowledgment
The authors gratefully acknowledge Director, CSIR- Central Drug Research Institute for providing support and facility to carry out the work.
Animal Ethical Approval
The monkeys procured after obtaining ethical approval from Institutional Animal Ethics Committee (IAEC) and subsequent approval from Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India vide approval reference number CPCSEA Approval no: F.No 25/8/2016 CPCSEA (Part-I) dtd 25.05.2016.
Competing Interest
None
Funding
Part of the funding required for this study was provided by CSIR-CDRI, Lucknow (India).
REFERENCES
- Vandamme TF. Rodent models for human diseases. Eur J Pharmacol. 2015;759:84-89.
- Xia HJ, Chen CS. Progress of non-human primate animal models of cancers. Dongwuxue Yanjiu. 2011;32(1):70-80.
- Estes JD, Wong SW, Brenchley JM. Nonhuman primate models of human viral infections. Nat Rev Immunol. 2018;18(6):390-404.
- Simmons HA, Mattison JA. The incidence of spontaneous neoplasia in two populations of captive rhesus macaques (Macacamulatta). Antioxid Redox Signal. 2011;14(2):221-227.
- Kent SP. Spontaneous and induced malignant neoplasms in monkeys. Ann N Y Acad Sci. 1960;85(3):819-827 .
- Lombard LS, Witte EJ. Frequency and types of tumors in mammals and birds of the Philadelphia Zoological Garden. Cancer Res. 1959;19(2):127-141.
- Thorgeirsson UP, Dalgard DW, Reeves J, et al. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul Toxicol Pharmacol. 1994;19(2):130-151.
- Todd GC, Griffing WJ, Koenig GR. Fibrosarcoma with metastasis in a rhesus monkey. Vet Pathol.1973;10(4):342-346 .
- O’brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17(1):1445-1455.
- Skavlen PA, Speers WC, Peterson RR, et al. Malignant fibrous histiocytoma in a bonnet macaque (Macacaradiata). Lab Anim Sci. 1988;38(3):310-311.
- Furukawa SL, Wingenfeld I, Sakaguchi T, et al. Malignant Fibrous Histiocytoma in the heart; An Autopsy Case. Int J Cardiol. 2012;10(3):1-3.
- Petterino C, Modesto P, Strata D, et al. A case of interscapular fibrosarcoma in a dwarf rabbit (Oryctolagus cuniculus). J Vet Diagn Invest. 2009;21(6):900-905.
- Mugale M, Singh V. Rare Spontaneous Proliferating Tumour in Rat: Fibrosarcoma. Scand J Lab Anim Sci. 2012;39(1):61-64.
- Renlund RC, Pritzker KP. Malignant fibrous histiocytoma involving the digit in a cat. Vet Pathol. 1984;21(4):442-444 .