Refractory atrioventricular nodal re-entrant tachycardia: A rare manifestation of acute massive pulmonary embolism
- *Corresponding Author:
- Dr Jinu John
Department of Cardiology, Maimonides Medical Center, 4802 Tenth Avenue, Brooklyn, New York 11219, USA.
Telephone: 718-283-7489
Fax: 718-283-8546
E-mail: doctorjacob@gmail.com, jjohn3@maimonidesmed.org
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
AVNRT; Pulmonary embolism
Pulmonary embolism (PE) has always been a challenging diagnosis to establish due to its variable and nonspecific presentation. Despite advances in diagnostic and therapeutic modalities, PE accounts for 300,000 deaths annually in the United States, with the past 10 years witnessing a surprising uptrend in the incidence of PE hospitalization from 129 to 302 per 100,000 person-years (1,2). Hemodynamic status- based risk stratification of PE into massive, submassive and low-risk categories has helped to streamline therapeutic decision making (3). However, new-onset cardiac arrhythmia consequent to PE can also adversely influence hemodynamic characteristics, delaying timely recog- nition of PE and confounding the aforementioned risk stratification. Clinicians should remain vigilant to recognize PE in the backdrop of new-onset rhythm disturbances in appropriate settings.
Case Presentation
On the morning of admission, an 89-year-old Caucasian woman with a medical history of dementia, hypertension and chronic obstructive pul- monary disease was noted to experience sudden-onset dyspnea (oxygen desaturation to 80% on room air), hypotension and tachycardia at the nursing and rehabilitation centre; emergency medical services was called and she was intubated in the field. According to her history, she had not reported chest pain, diaphoresis, nausea or vomiting. She had been admit- ted to the rehabilitation centre one month previously, following conserva- tive management at another hospital for blunt sternal trauma secondary to a fall of unknown nature at her home. She had been mostly bedridden for the past month and was taking metoprolol 25 mg orally every 12 h, budesonide 0.5 mg nebulization every 12 h, famotidine 20 mg orally every 12 h and magestrol 400 mg orally once daily. She was brought to the emergency department (ED) having already been intubated by emergency medical services. On admission to the ED, her heart rate was regular but tachycardic (170 beats/min), with a respiratory rate of 34 breaths/min, an oxygen saturation of 96% on 100% FiO2 on a mech- anical ventilator, blood pressure (BP) of 73/60 mmHg and body temper- ature 98.8°C; she was drowsy and completely unaware of her surroundings. A respiratory system examination revealed bilateral equal air entry without any adventitious sounds, and cardiovascular examination was notable for tachycardia.
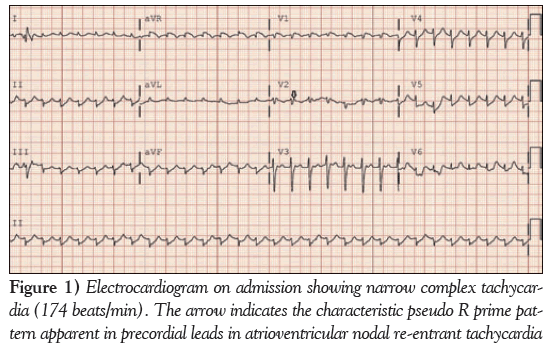
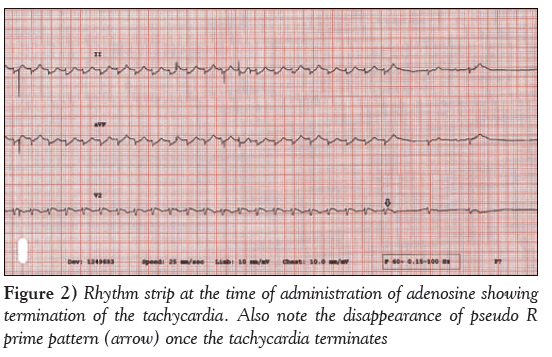
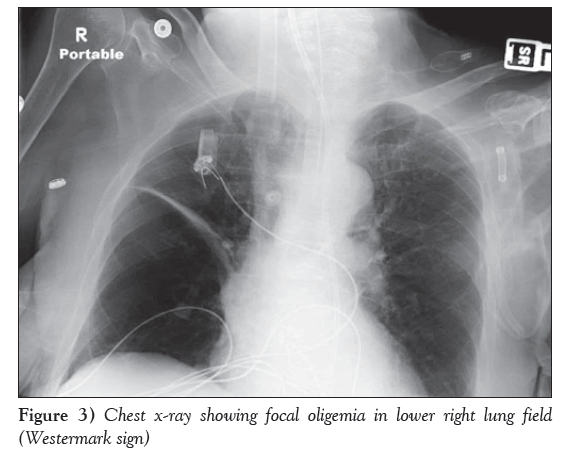
A 12-lead electrocardiogram obtained on admission (Figure 1) revealed typical AVNRT, with a heart rate of 174 beats/min and low voltage in the limb leads. Because she was hemodynamically unstable, synchronized cardioversion was attempted twice with 150 J followed by 200 J, which converted her to sinus rhythm only transiently, with improved BP. She again developed AVNRT, and 6 mg intravenous adenosine followed by 12 mg intravenously was attempted, which transiently normalized the arrhythmia; however, she again reverted into AVNRT (Figure 2). She received a bolus with 2 L normal saline in the first hour. After her BP improved, she received 10 mg of intravenous diltiazem and consequently main- tained sinus rhythm. Cardiac troponin and creatine kinase-MB were mildly elevated (0.43 ng/mL and 12.8 ng/mL, respectively) and her B-type natriuretic peptide level was 324 ng/L. A chest x-ray revealed unilateral pulmonary oligemia over the right lung field (Figure 3) and ABG showed a significantly elevated alveolar-arterial gradient. A bedside echocardiogram (Video 1) showed right ventricular dysfunction with free wall hypokinesis sparing the apex (McConnell’s sign) and normal left ventricular ejection fraction of 60% to 65%. At this point, with a high pretest probability for acute PE, she received an intravenous heparin bolus followed by intravenous heparin drip and underwent computed tomography pulmonary angiography, which revealed high-volume multilobar pulmonary emboli involving right upper, middle and lower lobe and left upper lobe and lingula (Video 2). She was transferred to the medical intensive care unit. Venous Doppler ultrasonography of her lower extremities showed acute partial thrombosis in the left common femoral vein. She was switched to rivaroxaban 15 mg through a feeding tube every 12 h beginning the day after her admission. During hospitaliza- tion, she developed left lower lobe consolidation and was treated with intravenous antibiotics. She was extubated on hospitalization day 6, maintained sinus rhythm throughout and remained stable during the remainder of her treatment.
Discussion
With 900,000 incident or recurrent annual cases and a high average fatality rate of 15%, PE remains the third most common cardiovascu- lar diagnosis in United States (4,5). Given the frequent disguised clinical presentation of PE, incorporation of basic diagnostic tools, such as electrocardiography, chest x-rays and two-dimensional echo- cardiography with clinical profile, is essential for the early identifi- cation of this ‘great masquerader’. Our patient had the Westermark sign on chest x-ray (focal pulmonary oligemia), AVNRT on electro- cardiography and McConnell’s sign on two-dimensional echocardi- ography with pertinent clinical background of being bedridden due to generalized debilitation
Preexisting cardiovascular status of the patient, in conjunction with different cardiovascular responses elicited by PE, results in the numerous electrocardiography patterns previously described in the literature. The markedly variable frequency of these electrocardio- graphic changes reported in various case series impairs sensitivity and specificity of electrocardiography as a diagnostic tool for PE (6,7). Nevertheless, new-onset atrial arrhythmias, new right bundle- branch block (complete or incomplete), Qr pattern in V1, S1Q3T3, negative T waves in V1 through V4, and ST-segment shift over V1 through V4 have been shown to correlate with poorer short-term prognosis in acute PE (8).
Hemodynamic response to PE is determined by the size of the embolus, the patient’s underlying cardiopulmonary status and com- pensatory neurohumoral adaptations (9). However, when PE mani- fests as new-onset cardiac arrhythmia, the rhythm disturbance is not only usually attributed as a primary culprit for the patient’s altered hemodynamics, but may also delay identifying PE as primary insult. PE presenting as typical slow-fast AVNRT is rarely described in lit- erature. In our patient, we believe that multiple atrial premature complexes due to the underlying PE persisted in triggering AVNRT refractory to standard treatment including adenosine and synchron- ized DC cardioversion. By definition, our patient experienced a massive PE; however, considering her advanced age, overall poor functional status, confounding effects of refractory AVNRT on hemodynamics and improvement in hemodynamic status, throm- bolysis or embolectomy were not considered and she was treated with heparin infusion and, subsequently, with rivaroxaban, with favourable outcomes.
We reported an often unrecognized causal relationship between PE and typical AVNRT refractory to standard treatment, timely identifi- cation of which can be lifesaving. Complex interplay of new-onset cardiac arrhythmia with underlying PE and its influence on the patient’s hemodynamics should be taken into consideration before selecting contemporary management strategy for PE based on defin- ition of massive versus submassive PE.
Disclosures: The authors have no financial disclosures or conflicts of intertest to declare
Acknowledgements
The authors thank Svetlana Khentov, a sonographer at Maimonides Medical Center.
References
- McCord J, Borzak S. Multifocal atrial tachycardia. Chest 1998;113:203-09.
- Minges KE, Bikdeli B, Wang Y, et al. National trends in pulmonary embolism hospitalization rates and outcomes for Medicare beneficiaries, 1999-2010. J Am Coll Cardiol 2013;61.
- Tapson VF. Medical progress: Acute pulmonary embolism. N Engl J Med 2008;358:1037-52.
- Goldhaber SZ Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER): Lancet 1999;353:1386-9
- Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2012 update: A report from the American Heart Association. Circulation 2012;125:e2-e220
- Rodger M, Makropoulos D, Turek M, et al. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol 2000;86:807-9.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991;100:598-603.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011;123:1788-830
- Piazza G, Goldhaber SZ. Acute pulmonary embolism, part I: Epidemiology and diagnosis. Circulation 2006;114:e28-e32.
- *Corresponding Author:
- Dr Jinu John
Department of Cardiology, Maimonides Medical Center, 4802 Tenth Avenue, Brooklyn, New York 11219, USA.
Telephone: 718-283-7489
Fax: 718-283-8546
E-mail: doctorjacob@gmail.com, jjohn3@maimonidesmed.org
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http:// creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
With a wide array of manifestations ranging from mild dyspnea to fatal cardiogenic shock, acute pulmonary embolism (PE) remains an enigmatic clinical entity. Vivid electrocardiography (ECG) patterns portrayed in different clinical instances of acute PE have always remained an interesting, yet debatable subject since the classic S1Q3T3 pattern on ECG secondary to PE was first described in 1935. Atrial fibrillation, atrial flutter and atrial tachycardia are commonly described supraventricular tachycardias in association with PE; however, there is scant description of PE in the literature presenting as atrioventricular nodal re-entrant tachycardia (AVNRT). The present report describes the clinical course of an elderly woman presenting with refractory typical AVNRT who was subsequently found to have bilateral multilobar acute PE. To the authors’ knowledge, the present report is the first to describe a case involving acute PE presenting as hemodynamically unstable AVNRT requiring cardioversion.
-Keywords
AVNRT; Pulmonary embolism
Pulmonary embolism (PE) has always been a challenging diagnosis to establish due to its variable and nonspecific presentation. Despite advances in diagnostic and therapeutic modalities, PE accounts for 300,000 deaths annually in the United States, with the past 10 years witnessing a surprising uptrend in the incidence of PE hospitalization from 129 to 302 per 100,000 person-years (1,2). Hemodynamic status- based risk stratification of PE into massive, submassive and low-risk categories has helped to streamline therapeutic decision making (3). However, new-onset cardiac arrhythmia consequent to PE can also adversely influence hemodynamic characteristics, delaying timely recog- nition of PE and confounding the aforementioned risk stratification. Clinicians should remain vigilant to recognize PE in the backdrop of new-onset rhythm disturbances in appropriate settings.
Case Presentation
On the morning of admission, an 89-year-old Caucasian woman with a medical history of dementia, hypertension and chronic obstructive pul- monary disease was noted to experience sudden-onset dyspnea (oxygen desaturation to 80% on room air), hypotension and tachycardia at the nursing and rehabilitation centre; emergency medical services was called and she was intubated in the field. According to her history, she had not reported chest pain, diaphoresis, nausea or vomiting. She had been admit- ted to the rehabilitation centre one month previously, following conserva- tive management at another hospital for blunt sternal trauma secondary to a fall of unknown nature at her home. She had been mostly bedridden for the past month and was taking metoprolol 25 mg orally every 12 h, budesonide 0.5 mg nebulization every 12 h, famotidine 20 mg orally every 12 h and magestrol 400 mg orally once daily. She was brought to the emergency department (ED) having already been intubated by emergency medical services. On admission to the ED, her heart rate was regular but tachycardic (170 beats/min), with a respiratory rate of 34 breaths/min, an oxygen saturation of 96% on 100% FiO2 on a mech- anical ventilator, blood pressure (BP) of 73/60 mmHg and body temper- ature 98.8°C; she was drowsy and completely unaware of her surroundings. A respiratory system examination revealed bilateral equal air entry without any adventitious sounds, and cardiovascular examination was notable for tachycardia.
A 12-lead electrocardiogram obtained on admission (Figure 1) revealed typical AVNRT, with a heart rate of 174 beats/min and low voltage in the limb leads. Because she was hemodynamically unstable, synchronized cardioversion was attempted twice with 150 J followed by 200 J, which converted her to sinus rhythm only transiently, with improved BP. She again developed AVNRT, and 6 mg intravenous adenosine followed by 12 mg intravenously was attempted, which transiently normalized the arrhythmia; however, she again reverted into AVNRT (Figure 2). She received a bolus with 2 L normal saline in the first hour. After her BP improved, she received 10 mg of intravenous diltiazem and consequently main- tained sinus rhythm. Cardiac troponin and creatine kinase-MB were mildly elevated (0.43 ng/mL and 12.8 ng/mL, respectively) and her B-type natriuretic peptide level was 324 ng/L. A chest x-ray revealed unilateral pulmonary oligemia over the right lung field (Figure 3) and ABG showed a significantly elevated alveolar-arterial gradient. A bedside echocardiogram (Video 1) showed right ventricular dysfunction with free wall hypokinesis sparing the apex (McConnell’s sign) and normal left ventricular ejection fraction of 60% to 65%. At this point, with a high pretest probability for acute PE, she received an intravenous heparin bolus followed by intravenous heparin drip and underwent computed tomography pulmonary angiography, which revealed high-volume multilobar pulmonary emboli involving right upper, middle and lower lobe and left upper lobe and lingula (Video 2). She was transferred to the medical intensive care unit. Venous Doppler ultrasonography of her lower extremities showed acute partial thrombosis in the left common femoral vein. She was switched to rivaroxaban 15 mg through a feeding tube every 12 h beginning the day after her admission. During hospitaliza- tion, she developed left lower lobe consolidation and was treated with intravenous antibiotics. She was extubated on hospitalization day 6, maintained sinus rhythm throughout and remained stable during the remainder of her treatment.
Figure 2: Rhythm strip at the time of administration of adenosine showing termination of the tachycardia. Also note the disappearance of pseudo R prime pattern (arrow) once the tachycardia terminates
Discussion
With 900,000 incident or recurrent annual cases and a high average fatality rate of 15%, PE remains the third most common cardiovascu- lar diagnosis in United States (4,5). Given the frequent disguised clinical presentation of PE, incorporation of basic diagnostic tools, such as electrocardiography, chest x-rays and two-dimensional echo- cardiography with clinical profile, is essential for the early identifi- cation of this ‘great masquerader’. Our patient had the Westermark sign on chest x-ray (focal pulmonary oligemia), AVNRT on electro- cardiography and McConnell’s sign on two-dimensional echocardi- ography with pertinent clinical background of being bedridden due to generalized debilitation
Preexisting cardiovascular status of the patient, in conjunction with different cardiovascular responses elicited by PE, results in the numerous electrocardiography patterns previously described in the literature. The markedly variable frequency of these electrocardio- graphic changes reported in various case series impairs sensitivity and specificity of electrocardiography as a diagnostic tool for PE (6,7). Nevertheless, new-onset atrial arrhythmias, new right bundle- branch block (complete or incomplete), Qr pattern in V1, S1Q3T3, negative T waves in V1 through V4, and ST-segment shift over V1 through V4 have been shown to correlate with poorer short-term prognosis in acute PE (8).
Hemodynamic response to PE is determined by the size of the embolus, the patient’s underlying cardiopulmonary status and com- pensatory neurohumoral adaptations (9). However, when PE mani- fests as new-onset cardiac arrhythmia, the rhythm disturbance is not only usually attributed as a primary culprit for the patient’s altered hemodynamics, but may also delay identifying PE as primary insult. PE presenting as typical slow-fast AVNRT is rarely described in lit- erature. In our patient, we believe that multiple atrial premature complexes due to the underlying PE persisted in triggering AVNRT refractory to standard treatment including adenosine and synchron- ized DC cardioversion. By definition, our patient experienced a massive PE; however, considering her advanced age, overall poor functional status, confounding effects of refractory AVNRT on hemodynamics and improvement in hemodynamic status, throm- bolysis or embolectomy were not considered and she was treated with heparin infusion and, subsequently, with rivaroxaban, with favourable outcomes.
We reported an often unrecognized causal relationship between PE and typical AVNRT refractory to standard treatment, timely identifi- cation of which can be lifesaving. Complex interplay of new-onset cardiac arrhythmia with underlying PE and its influence on the patient’s hemodynamics should be taken into consideration before selecting contemporary management strategy for PE based on defin- ition of massive versus submassive PE.
Disclosures: The authors have no financial disclosures or conflicts of intertest to declare
Acknowledgements
The authors thank Svetlana Khentov, a sonographer at Maimonides Medical Center.
References
- McCord J, Borzak S. Multifocal atrial tachycardia. Chest 1998;113:203-09.
- Minges KE, Bikdeli B, Wang Y, et al. National trends in pulmonary embolism hospitalization rates and outcomes for Medicare beneficiaries, 1999-2010. J Am Coll Cardiol 2013;61.
- Tapson VF. Medical progress: Acute pulmonary embolism. N Engl J Med 2008;358:1037-52.
- Goldhaber SZ Visani L, De Rosa M. Acute pulmonary embolism: Clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER): Lancet 1999;353:1386-9
- Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics – 2012 update: A report from the American Heart Association. Circulation 2012;125:e2-e220
- Rodger M, Makropoulos D, Turek M, et al. Diagnostic value of the electrocardiogram in suspected pulmonary embolism. Am J Cardiol 2000;86:807-9.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991;100:598-603.
- Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: A scientific statement from the American Heart Association. Circulation 2011;123:1788-830
- Piazza G, Goldhaber SZ. Acute pulmonary embolism, part I: Epidemiology and diagnosis. Circulation 2006;114:e28-e32.