RENAL PATHOLOGY OF TROPICAL INFECTIOUS DISEASES IN THE PEDIATRIC POPULATION
2 Department of Pathology, University of Texas Medical Branch, Galveston, Texas, USA, Email: marjan.afrouzian@gmail.com
Received: 04-Jul-2024, Manuscript No. PULCNR-22-5123; Editor assigned: 06-Jul-2024, Pre QC No. PULCNR-22-5123(QC); Accepted Date: Jul 15, 2024; Reviewed: 20-Jul-2024 QC No. PULCNR-22-5123; Revised: 12-Jul-2024, Manuscript No. PULCNR-22-5123(R); Published: 30-Jul-2024
Citation: Afrouzian M, Reisler J, James T, et al. Renal pathology of tropical infectious diseases in the pediatric population. Clin Nephrol Res 2022;6(4):1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Infective agents affect the kidneys of children mainly through indirect mechanisms, i.e., via immunological reaction as part of an antigenic response. However, for children living in the tropics, there is also a direct mechanism of kidney injury which is less known by the medical community, simply because the direct mechanism is rarely seen in non-tropical countries. In some infectious diseases, both indirect and direct pathways are responsible in inducing two sets of morphologically separate renal lesions.
Six tropical infectious diseases affecting the kidney of children were reviewed in terms of their direct and/or indirect pathogenetic mechanism in inducing renal damage: Renal cryptococcosis to represent involvement of pure direct pathway; schistosomiasis and dengue fever as examples of dual direct and indirect pathways; and congenital syphilis, visceral leishmaniasis and chagas disease representing indirect pathway. Clinical manifestations, pathogenesis, renal pathology, and laboratory diagnostic methods for these tropical infectious diseases are reviewed and examples of each entity are illustrated.
Keywords
Immunological reaction; Antigenic response; Schistosomiasis; Chagas disease; Kidney injury
Introduction
The kidney involvement during an infectious process may follow one of the two pathways in children as in adults. The most common pathway involves a pathway in which the pathogen indirectly affects the kidney, by generating an immune response in the host. The kidney in this case is targeted following an antibody mediated or a cell mediated immunologic reaction which usually lags behind the initial infection by weeks. The second pathway, which is less seen by most nephrologists and nephropathologists, is the direct pathway. In these cases, the pathogen invades all compartments of the kidney including glomeruli, vessels, tubules and interstitial. Infectious diseases using the direct pathway to damage the kidney are less known because they occur in the tropics where they are endemic. Indeed, in immunocompromised children living in non-tropical areas, the direct pathway is responsible for most damage; however, in such cases, usually the lungs or the central nervous system are targeted by the pathogen, and renal involvement is not common. Transplantation and immunosuppression are other situations in which a limited number of pathogens such as polyoma virus and adenovirus may affect the kidney. Therefore, the authors aimed at raising awareness among pediatric nephrologists practicing in non-tropical areas about some of the uncommon pathogens involving the kidney [1-5]
The WHO’s list of 20 neglected tropical diseases in 2021 includes 11 parasitic diseases, of which four chagas disease, filariasis, leishmaniasis and schistosomiasis are often associated with kidney damage. Tropical diseases in general, and their effects on the kidney of children specifically, occur in regions with high levels of poverty, poor sanitation and exposure to the transmitting agents [6-10]. Additionally, the poor access to healthcare in tropical regions results in paucity of reporting of these diseases in the literature. On the other hand, tourism, increased migration, and business related travels have helped in propagation of this disease to high income and non-endemic countries. Therefore, to raise awareness among pediatric nephropathologists and nephrologists, the authors have provided below, a review of clinical and pathological manifestations of a number of tropical diseases involving the kidney. Additionally, illustrations obtained from one of the author’s nephropathology practice in the tropics (SDA) are included [11-15].
Literature Review
Direct pathway
Renal cryptococcosis: is an opportunistic pathogen that is a genus of fungi in the family Cryptococcaceae that includes both yeasts and filamentous species. This encapsulated fungus is often found in soil and pigeon droppings. Humans are infected through inhalation of spores which disseminate hematogenously. The two most commonly affected organs are lung and central nervous system. Pediatric cryptococcosis primarily appears in immunocompromised children, such as HIV positive or transplant patients. Pediatric cryptococcosis has been identified in the central nervous system, lung, and skin, but Renal cryptococcosis (RC) has rarely been reported. Ramdial, et al. have described two cases of HIV-positive children with RC and based on other observations, patients with RC present with weight loss, fatigue, and pallor with evidence of chronic renal failure on laboratory investigations [16-20].
The pathogenesis in cryptococcosis is complex and incompletely understood. Experimental and clinical studies have shown that most patients with cryptococcosis suffer from a low T-cell count (specifically CD4+ cells), reflecting the important role that CD4+ T-cells play against this infection. Although humoral factors play a role, their contribution to antifungal defense is controversial. The pathology of RC in adults is characterized by focal or diffuse cortical, medullary, or perinephric involvement. Likewise, pediatric RC is characterized by histopathological heterogeneity. The most dramatic and clinically fatal pattern is cryptococcal pyelonephritis, in which renal papillary necrosis, destructive tubulointerstitial necrotizing granulomatous inflammation, and glomerular involvement by large numbers of C. neoformans form a distinctive histological phenotype. RC presenting with acute renal failure may be due to granulomatous interstitial nephritis. It is important to note that immunocompetent children may present with RC, albeit rarely. A newly described form of RC occurs in HIV positive children and is related to a complication of the administration of antiretroviral therapy (ARV). Weeks after administering ARV, the patient paradoxically develops a new infection called HIV associated Immune Reconstitution Inflammatory Syndrome (IRIS). IRIS is due to dysfunctional restoration of the immune system, resulting in exaggerated pathologic inflammatory reactions. Although pediatric renal cryptococcal IRIS has been reported in one child, it remains a poorly documented entity. The biopsy of the reported case showed collapsing variant of Focal Segmental Glomerulosclerosis (FSGS), and fungal yeasts were found within 3 tubular lumina and focally within the interstitial by methylamine silver stain. Southgate mucicarmine and Masson Fontana stains confirmed the presence of C. neoformans. Literature review of RC in the pediatric population yield only three reports, mentioned above. It is not clear whether the paucity of information on RC in the pediatric population is due to its low incidence, misdiagnosis, and/or underreporting of the entity. Therefore, RC in the pediatric population is a potential focus for future research on cryptococcus’s, particularly the role it can play in IRIS [21-25].
Direct and indirect pathways
Chagas disease: Chagas disease is a zoonotic and parasitic disease caused by Trypanosoma cruzi, a common disease seen in Central and South America and the southern regions of North America. More than 8 million people are estimated to be affected with chagas disease, with more than 300,000 infected patients located in the United States. Rural areas and rustic conditions seem to be preferred by Trypanosoma. In a study performed in Bolivia, 3% of a population composed of children between 5 and 14 years old tested positive for chagas disease [26-31].
Congenital trypanosomiasis is a rare form of the disease reported in the literature, acquired via vertical transmission. The infection occurs through direct contact with feces or urine infected with hematophagous Triatominae insects. T. cruzi subsequently propagates through circulating blood, reaching muscles and nerves. One of the preferred location is the myocardium and the myenteric plexus of the gastrointestinal tract. The trypomastigotes enters the cells and are transformed to amastigotes, which replicate intracellularly and mature into trypomastigotes. Subsequently, the host cell membrane is ruptured, and the trypomastigotes reach the blood and propagate throughout the body to infect other organs. This complex life cycle, naturally, is represented by a multitude of clinical manifestations depending on the organs involved.
The heart is not only the most common organ involved, but also the source of most of the lesions occurring in the kidney when cardio renal syndrome occurs. The disease initially presents with mild constitutional symptoms but may lead to fatal arrhythmias.
Systemic manifestations such as hemolysis, hemorrhage and rhabdomyolysis have been reported. Progressive dilated cardiomyopathy, generalized lymphadenopathy, fever, and splenomegaly are rare manifestations of the disease.
The kidney is rarely involved but once affected, the main presentation is Acute Kidney Injury (AKI) due to decreased renal perfusion leading to proximal tubular injury, necrosis, and loss of concentrating ability.
In such cases, the proximal tubular epithelial cell damage is accompanied by interstitial infiltration of mononuclear inflammatory cells. Membranoproliferative Glomerulonephritis (MPGN) is the most common form of glomerular disease seen in cases of pediatric chagas disease.
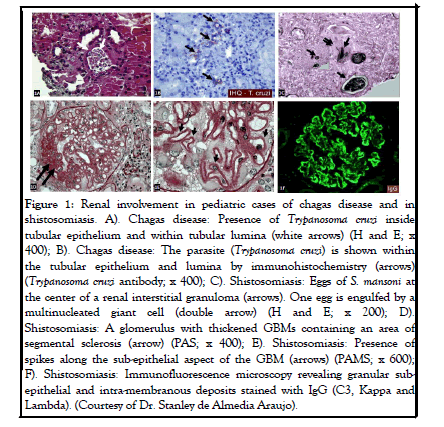
These patients may also present with AKI which is considered a poor prognostic factor. The underlying mechanisms of this parasitic disease associated kidney injury include direct parasite damage in the tubules as well as autoimmunity manifesting by immune complex deposition and inflammation. An example of tubular involvement in RC is illustrated in Figure 1.
Figure 1: Renal involvement in pediatric cases of chagas disease and in shistosomiasis. A). Chagas disease: Presence of Trypanosoma cruzi inside tubular epithelium and within tubular lumina (white arrows) (H and E; x 400); B). Chagas disease: The parasite (Trypanosoma cruzi) is shown within the tubular epithelium and lumina by immunohistochemistry (arrows) (Trypanosoma cruzi antibody; x 400); C). Shistosomiasis: Eggs of S. mansoni at the center of a renal interstitial granuloma (arrows). One egg is engulfed by a multinucleated giant cell (double arrow) (H and E; x 200); D). Shistosomiasis: A glomerulus with thickened GBMs containing an area of segmental sclerosis (arrow) (PAS; x 400); E). Shistosomiasis: Presence of spikes along the sub-epithelial aspect of the GBM (arrows) (PAMS; x 600); F). Shistosomiasis: Immunofluorescence microscopy revealing granular subepithelial and intra-membranous deposits stained with IgG (C3, Kappa and Lambda). (Courtesy of Dr. Stanley de Almedia Araujo).
Membranoproliferative glomerulonephritis in chagas disease is also likely associated with immune complex deposition. Glomerular deposits of IgM, predominantly in the meconium, occur in the early stages of infection and are associated with an inflammatory response and ischemia reperfusion injury, which lead to AKI.
When the disease is in its acute phase and the trypomastigotes are motile, identification can be made in fresh blood or buffy coat examined under the microscope. The parasites can be seen in peripheral blood smears if stained with Giemsa and can also be grown in blood cultures. A sensitive method for early detection is Polymerase Chain Reaction (PCR); however, during the chronic phase, IgG serologic testing with Enzyme Linked Immunosorbent Assay (ELISA) or with Immunofluorescent Antibody (IFA) is proven to be helpful diagnostic tests. Indeed, utilization of a single method may not lead to the diagnosis, as none of the above mentioned tests are specific or sensitive enough. Therefore, at least two different testing methods need to be used for diagnosis. Preferably, the two tests should include different antigens, such as whole parasite lysate and recombinant antigens. Early diagnosis of kidney involvement and adequate management is crucial to prevent progression of the kidney disease and to optimize patient recovery, and one should not overlook the use of nephrotoxic treatment of parasitic infections as an important cause of AKI in these patients.
Schistosomiasis
Tropical regions and developing countries are the most common regions where schistosomiasis is endemic. Schistosomiasis, also known as bilharziasis, is a parasitic infection caused by trematodes of the genus Schistosoma. Human infection is caused by three variants of Schistosoma: S. mansoni, S. japonicum, and S. haematobium. The parasites are released by freshwater snails and transmitted to humans via contact with contaminated water. Circulating schistosomal antigens from the eggs and digestive tract of the parasites result in the formation of immune complexes. Deposition of these antigens and immune complexes in the glomeruli lead to glomerulonephritis. Clinical manifestations of schistosomiasis are diverse due to the following factors: 1) Involvement of multiple organs, 2) The complexity and multiple stages of the organism‘s life cycle, 3) Difference in host inflammatory reactions, and 4) The fact that different species of Schistosoma affect different organs. For example, S. haematobium, which often affects the bladder and ureter (urogenital schistosomiasis), causes inflammation by laying its eggs in the bladder and ureteral walls using the proteolytic effect of its enzymes. Common symptoms of pediatric urogenital schistosomiasis include hematuria, proteinuria, dysuria, and frequency.
The renal pathology of schistosomiasis in pediatric population has not been reported in a systemic fashion. Schistosomiasis usually affects the bladder and ureter; however, if the kidney is involved, the renal interstitial is usually suspected to be the location. Biopsy in these cases shows presence of an interstitial non necrotizing granulomatous reaction around Schistosoma’s eggs. Figure 1 illustrates a renal interstitial granulomatous reaction around the eggs of S. mansoni. About 6% of patients with chronic urogenital schistosomiasis may also demonstrate glomerular involvement. These patients are usually asymptomatic or present with persistent proteinuria or hematuria. The reported glomerular lesions are MPGN, membranous glomerulopathy, FSGS, and amyloidosis, probably due to chronic and untreated forms of the disease. These patients therefore will present with hematuria or proteinuria. Cases of schistosomiasis with interstitial granulomata containing S. mansoni eggs and concomitant membranous glomerulopathy have also been reported.
An example of glomerular involvement during schistosomiasis is described: A 17 years old male, born in southeastern Brazil, presented with ascites, hepatosplenomegaly, lower extremity edema, proteinuria and microscopic hematuria. A renal biopsy was performed revealing multiple interstitial granulomata containing S. mansoni eggs on light microscopy. The glomeruli showed segmental glomerulosclerosis associated with thickened Glomerular Basement Membrane (GBM) and silver stain revealed spikes along the sub epithelial aspect of the GBM. By immunofluorescence microscopy, granular deposits of IgG, C3, Kappa and Lambda were found along the GBM. Electron microscopy confirmed the presence of the electron dense deposits in sub epithelial and intramembranous locations.
Methods of detection include identification of Schistosoma eggs in the urine and stool, detection of Schistosoma antigens in the serum, cystoscopy and contrast studies, or ultrasonography of the urogenital system. Ultrasound is not only a diagnostic modality but also serves as a tool to follow up and evaluate post therapeutic progress. On ultrasound of children affected by S. haematobium, urinary bladder wall thickening and polyps, calcification, ureteric dilatation, and hydronephrosis (most likely due to fibrosis) may be observed. Although micro hematuria and proteinuria lack specificity, they are the best markers of morbidity.
Dengue fever: Dengue fever is another infectious process in which both indirect (most common) and direct pathways may be involved. Dengue fever is caused by the dengue virus, which belongs to the Flavivirus family. All four serotypes of the virus can cause the disease. The virus is transmitted by the bite of the female Aedes Aegyptus mosquito. The renal manifestations in patients with dengue fever vary from minor electrolyte disturbances to hematuria, proteinuria and AKI. AKI is most likely due to the indirect pathway and is often secondary to ischemic injury following hypotension or inflammatory cytokines, resulting in Acute Tubular Necrosis (ATN). Dengue Shock Syndrome (DSS) is the severe form of the disease characterized by the presence of shock along with other features of Dengue Hemorrhagic Fever (DHF), and is often associated with multi organ dysfunction, including AKI. AKI may also be due to additional factors, such as immune dysregulation, immune complex deposition, cytopathic effects, hemolysis or rhabdomyolysis, thrombotic microangiopathy, multiorgan dysfunction, and complications associated with intravenous fluid therapy. The WHO guidelines classify dengue fever into various grades, i.e. mild, moderate, and severe, based on clinical and laboratory manifestations, such as the absence or presence of warning signs and shock. The use of nephrotoxic drugs, hemolysis or rhabdomyolysis can also be additional factors causing AKI. Various studies have shown that the prevalence of AKI in dengue infection varies from 0.9 to 10% in children and 2.2 to 35.7% in adults.Dengue is known to induce interstitial injury and ATN. Abnormal urinary sediment, proteinuria, and microscopic hematuria may accompany AKI in children. MPGN and thrombotic microangiopathy are additional renal lesions reported in dengue infections, which may be due to related to an immune complex mediated injury in the glomerulus. Renal biopsy has shown presence of immune complexes; however, renal histopathology is rarely reported, since biopsy carries a risk of bleeding in these patients. In one study, the biopsy showed red blood cells in the tubular lumen, interstitial nephritis, and immune complex mediated glomerulonephritis with deposition of IgG, IgM and C3 along the peripheral capillaries. Transient IgA immune complex disease following infection with dengue has also been reported.
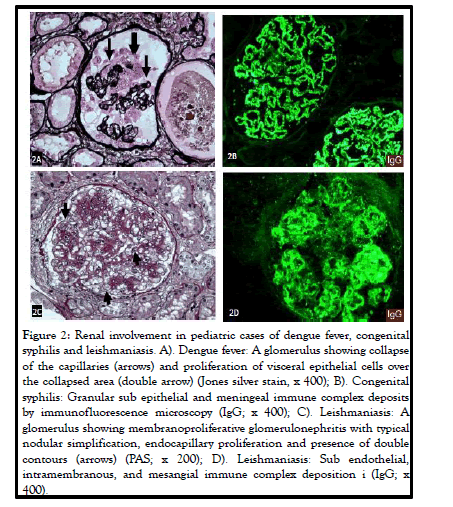
Illustrated in Figure 2, is an example of a rare association of dengue fever with collapsing glomerulopathy in a Brazilian child.
Figure 1: Renal involvement in pediatric cases of dengue fever, congenital syphilis and leishmaniasis. A). Dengue fever: A glomerulus showing collapse of the capillaries (arrows) and proliferation of visceral epithelial cells over the collapsed area (double arrow) (Jones silver stain, x 400); B). Congenital syphilis: Granular sub epithelial and meningeal immune complex deposits by immunofluorescence microscopy (IgG; x 400); C). Leishmaniasis: A glomerulus showing membranoproliferative glomerulonephritis with typical nodular simplification, endocapillary proliferation and presence of double contours (arrows) (PAS; x 200); D). Leishmaniasis: Sub endothelial, intramembranous, and mesangial immune complex deposition i (IgG; x 400).
In terms of laboratory testing, abnormal urinalysis has been seen in almost one third of patients, with proteinuria and microscopic hematuria being the most common findings. Nephrotic range proteinuria is uncommon, but has been reported, and may be explained by immune mediated mechanisms. Other laboratory findings include leukopenia with relative lymphocytosis, thrombocytopenia, increased hematocrit, and coagulopathy. Hyponatremia and hypokalemia are common electrolyte abnormalities seen in dengue infections, with the most severe hyponatremia being associated in patients with DSS. Diagnosis of dengue is made using antibody or antigen detection and requires either a positive NS1 antigen (non-structural protein) using ELISA or dengue IgM or IgG positivity associated with clinical manifestations of infection. The NS1 antigen test detects the presence of a viral nonstructural protein and can be positive at the time of onset of symptoms. Diagnosis of dengue requires either a positive NS1 antigen or dengue IgM positivity in a patient with a clinical presentation compatible to the infection. The management of dengue includes adequate and timely fluid therapy as per the WHO guidelines.
The mortality rate in dengue fever (DHF/DSS) ranges from 12 to 44%, and patients with renal failure have a higher risk of mortality. Mortality has been found to be around 60% in both adults and children with AKI.
Many studies have focused primarily on dengue infections in adult populations, however, more information is needed regarding outcomes of children with dengue, especially in areas where dengue is not endemic. In addition, there is a lack of studies that directly evaluate the various histopathology associated with dengue virus infection in pediatric populations.
Dengue has a wide range of complications, and so focusing solely on derangements in kidney function is difficult as individuals with severe dengue infections often have multiple and widespread multi organ involvement. In addition, the pathophysiology of kidney involvement in dengue infections is not well understood, since AKI is relatively uncommon.
Indirect pathway
Congenital syphilis: Congenital Syphilis (CS) is caused by trans placental transmission of the spirochete Treponema pallidum or exposure during the birthing process due to maternal syphilitic lesions. Untreated pregnant mothers have a greater than 70% chance of transmission to the fetus, and 40% of infected infants will die if infected at the time of birth, even though approximately two thirds of cases will be asymptomatic at birth. If left untreated, most infants develop symptoms within five weeks.
Congenital Nephrotic Syndrome (CNS) and acute Glomerulonephritis (GN) are the two main presentations of CS. Some infants may have features of both diseases. CNS is the result of either direct invasion of the kidney by spirochetes or due to an indirect mechanism linked to hypersensitivity reaction; therefore, both direct and indirect pathways are involved in CNS. The incidence of CNS in infected infants is not well known and detailed literature related to the renal lesions in syphilis has been sparse. Generalized edema, abdominal distension and gross hematuria presenting with brown urine are the first clinical manifestations. Initially the renal function may be normal but soon will become altered. In one report, a nine week old girl presented with a palmar erythematous rash, urticaria, and blisters preceding anasarca and periorbital edema. Renal biopsy of infants with CS who do not have CNS may reveal interstitial fibrosis containing large number of plasma cells and lymphocytes. Treponema can be demonstrated within the tubulo interstitial compartment. The perivascular arrangement of inflammatory cells is an important criterion in the histopathologic diagnosis of renal syphilis.
In CNS there is deposition of immune complexes in the sub epithelial region of the glomerulus leading to membranous glomerulopathy. Original studies of CNS secondary to syphilis reported diffuse involvement of all glomeruli showing endothelial and epithelial cell swelling and proliferation, glomerular and interstitial inflammation, glomerular basement membrane thickening, and presence of spikes on silver stain. By immunofluorescence microscopy, typical sub epithelial and mesangial granular deposits of IgG and C3 were present. An example is shown in Figure 2. Electron microscopic features of membranous glomerulopathy, including presence of sub epithelial and mesangial electron dense deposits, were reported later.
Once the diagnosis of CNS is made, serologic testing for syphilis is indicated; this is very important since CNS secondary to congenital syphilis is a curable disease. Tests for RPR, VDRL, and T. pallidum hemagglutination assay are utilized for diagnosis of CS. Additionally, X ray of long bones may reveal epiphysitis, periostitis, Wimberger’s sign. These findings are characteristic of CS.
Visceral leishmanias: Leishmania donovani is the causative agent of Leishmaniasis, which is a disease mainly seen in the Middle East, southern portions of Asia, Africa, and Latin America, with an estimated incidence of 0.7-1.2 million cases per year. Visceral leishmaniasis is a chronic inflammatory disease transmitted via the bite of sandflies infected with Leishmania spp. The promastigote form is inoculated into the human skin through the bite of the sandfly. Once within the dermis, the promastigote is phagocytosed by macrophages which provide an acidic milieu favorable for the transformation of the promastigote to the amastigote form. The proliferation of amastigotes within the macrophage leads to the demise of the macrophage and release of the amastigote which will be phagocytosed by other macrophages, continuing the process of infection.
The most common clinical manifestations of leishmaniasis in children include fever, splenomegaly, hepatomegaly, and anorexia. Visceral leishmaniasis may involve the kidney and present with AKI (as defined by the pediatric Risk, Injury, Failure, Loss, end stage kidney disease (pRIFLE) in up to 39.4% to 59.7% of affected children. Children with leishmaniasis associated AKI are more likely to show decreased albumin and PT (INR) as well as increased globulin levels as the main risk factors in the pediatric population have been shown to be secondary infection, hyperglobulinemia, and hypoalbuminemia. Creatinine clearance (eGFR) using the Schwartz formula to apply for the pRIFLE model allows for the determination of AKI and severity. A majority of these patients with AKI fall into the risk category followed by the injury category. Baseline clearance was 120 mL/min/1.73 m2.
Discussion
Literature review shows that up to 44.8% of pediatric patients presenting with AKI due to leishmaniasis achieve complete recovery of the kidney function before discharge from the hospital. The light chains derived from hyperglobulinemia may play a role in the renal pathology, as light chains have toxic effects on renal tubular cells. Hypoalbuminemia may be a risk factor for AKI as albumin plays a crucial role in scavenging reactive oxygen species and promoting DNA synthesis in renal tubular cells. According to da Silva’s review, the most common renal pathologies in leishmaniasis are mesangial proliferative glomerulonephritis, MPGN, and collapsing FSGS. An example of MPGN in a child with leishmaniasis is shown in Figure 2. The interstitial inflammation is usually composed of mononuclear cells including macrophages, lymphocytes, and plasma cells. The most common kidney lesions seen in experimental models are also tubular and interstitial. This review presents two cases: the first case is a 16 years old patient who had collapsing segmental, and the second case is a 20 years old who had necrotizing segmental and focal glomerular sclerosis. This article also put an emphasis on the role of IgG overload and the damage it might cause to the tubular cells affecting acidification and concentrating functions of the tubules, as mentioned above.
Conclusion
Tropical infections involving the kidney of children are rarely observed in non-tropical areas of the world and their pathology in the kidney is not reported most of the time. To familiarize the pediatric nephropathologists and nephrologists, the authors presented some of the rarest infections that could involve the kidney of a child in the tropics, describing their clinical and pathological manifestations which most of the time, result in unexpected and exotic morphological features.
Acknowledgement
The authors would like to thank Dr. Stanley de Almedia Araujo, nephropathologist at the Instituto de Nefropatologia LTDA e Hospital das Clínicas da UFMG, Universidade Federal de Ouro Preto, Brazil, for sharing the pathology images from his personal collection.
References
- Ramdial PK, Sing Y, Deonarain J, et al. Pediatric renal cryptococcosis: novel manifestations in the acquired immunodeficiency syndrome era. Int J Surg Pathol. 2011;19:386-92.
[Crossreff] [Googlescholar] [Indexed]
- Suárez-Rivera M, Abadeer RA, Kott MM, et al. Cryptococcosisassociated with crescentic glomerulonephritis. Pediat Neph. 2008; 23(5):827-30.
[Crossreff] [Googlescholar] [Indexed]
- Chung S, Park CW, Chung HW, et al. Acute renal failure presenting as a granulomatous interstitial nephritis due to cryptococcal infection. Kidney Int. 2009;76(4):453-8.
[Crossreff] [Googlescholar] [Indexed]
- Robello C, Maldonado DP, Hevia A, et al. The fecal, oral, and skin microbiota of children with Chagas disease treated with benznidazole. PLoS ONE. 2019;14(2):0212593.
- Goma MS, Nalubamba M, Kowa S, et al. Congenital trypanosomiasis. J Trop Pediatr. 2004;50(6):377-378
[Crossreff] [Googlescholar] [Indexed]
- Nagajyothi F, Machado FS, Burleigh BA, et al. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Biolo. 2012;14(5):634-43.
[Crossreff] [Googlescholar] [Indexed]
- Junior GS, Antunes VVH. Chagas disease associated kidney injury a review. Nefrología Latinoamericana. 2017;14(1): 22–6
- Lemos JR, Rodrigues WF, Miguel CB, et al. Influence of parasite load on renal function in mice acutely infected with Trypanosoma cruzi. PLoS One. 2013;8(8):71772.
[Crossreff] [Googlescholar] [Indexed]
- Bern C. Chagas’ disease. New England J Med. 2015;373(5):456-66
- Daher ED, da Silva Junior GB, et al. Kidney complications of parasitic diseases. Nat Rev Nephrol. 2022;(6):396-406.
- King CH. Bustinduy AL. Nelson Textbook of Pediatrics. 21st Edition, Elsevier. 2020; 326:1890-2
- Kamath N, Iyengar A. Infections and the kidney: a tale from the tropics. Pediatr Nephrol. 2018;33(8):1317-26.
- Kayange NM, Smart LR, Tallman JE, et al. Kidney disease among children in sub-Saharan Africa: systematic review. Pediatr Res. 2015; 77(2):272-81.
[Crossreff] [Googlescholar] [Indexed]
- Al-Mendalawi MD. Ultrasound findings in urinary schistosomiasis infection in school children in Gezira State, Central Sudan. Saudi J Kidney Dis Transpl. 2013;24(6):1252-53.
[Crossreff] [Googlescholar] [Indexed]
- Bocanegra C, Pintar Z, Mendioroz J, et al. Ultrasound Evolution of Pediatric Urinary Schistosomiasis after Treatment with Praziquantel in a Highly Endemic Area. Am J Trop Med Hyg. 2018; 99(4):1011-17.
[Crossreff] [Googlescholar] [Indexed]
- Vachvanichsanong P, Thisyakorn U, Thisyakorn C. Dengue hemorrhagic fever and the kidney. Arch Virol. 2016;161:771–7
[Crossreff] [Googlescholar] [Indexed]
- Lima EQ, Nogueira ML. Viral hemorrhagic fever induced acute kidney injury. Semin Nephrol. 2008;28:409–15
[Crossreff] [Googlescholar] [Indexed]
- Hebbal P, Darwich Y, Fong J, et al. Nephrotic range proteinuria in an eight year old traveler with severe dengue: Case report and review of the literature. Travel Med Infect Dis. 2016;14(1):45-8.
[Crossreff] [Googlescholar] [Indexed]
- Ismail J, Sankar J. Acute Kidney Injury in Dengue Not Unprecedented. Indian J Pediatr. 2020;87(12):993-4.
[Crossreff] [Googlescholar] [Indexed]
- Gurugama P, Jayarajah U, Wanigasuriya K, et al. Renal manifestations of dengue virus infections. J Clin Virol. 2018; 101:1-6.
- Rajan M, Geminiganesan S, Sankaranarayanan S, et al. Renal manifestations in children with dengue fever hospitalized in pediatric intensive care unit. Indian J Pediatr. 2020;87(12):1014-17.
[Crossreff] [Googlescholar] [Indexed]
- World Health Organization. Prevention and control of dengue and dengue haemorrhagic fever. WHO Regional Office for South-East Asia, 1999.
- Kim YH, Song JH, Kim CJ, et al. Congenital syphilis presenting with only nephrotic syndrome: reemergence of a forgotten disease. J Korean Med Sci. 2016;32;1374-76.
- Hill LL, Singer DB, Falletta J, et al. The nephrotic syndrome in congenital syphilis: an immunopathy. Pediatrics. 1972;49(2):260-266.
[Crossreff] [Googlescholar] [Indexed]
- Rich A R. The pathology of nineteen cases of a peculiar and special form of nephritis associated with acquired syphilis. Bull. John Hopkins Hosp. 1932;50:357.
- Yampolsky J, Mullins DF. Acute glomerular nephritis in an infant with congenital syphilis. American. J Arch Dis Child. 1945;69(3):163-72.
- Braunstein GD, Lewis EJ, Galvanek EG, et al. The nephrotic syndrome associated with secondary syphilis. An immune deposit disease. Amer J Med. 1970; 48:643.
[Crossreff] [Googlescholar] [Indexed]
- Kumar V, Abbas AK, Aster JC. Leishmaniasis. In: Robbins and Cotran Pathologic Basis of Disease. Elsevier. 2020;8:391-2
- Petrela R, Kuneshka L, Foto E, et al. Pediatric visceral leishmaniasis in Albania: a retrospective analysis of 1,210 consecutive hospitalized patients (1995–2009). PLoS Neglect Trop Dis. 2010;4(9):814.
[Crossreff] [Googlescholar] [Indexed]
- Libório AB, Rocha NA, Oliveira MJ, et al. Acute kidney injury in children with visceral leishmaniasis. J Pediatr Infect Dis. 2012;31(5):451-4.
[Crossreff] [Googlescholar] [Indexed]
- Wiedermann CJ, Wiedermann W, Joannidis M, et al. Causal relationship between hypoalbuminemia and acute kidney injury. World Nephrol. 2017;6(4):176.
[Crossreff] [Googlescholar] [Indexed]