Results of two times-cytoreduction for patients with pseudomyxoma peritonei
2 Department of Surgery, Tokyo University Hospital, Japan, Email: y.yonemura@coda.ocn.ne.jp
3 Department of Peritoneal Surface Oncology, Beijing Shijitan Hospital of Capital Medical University, Beijing, China, Email: y.yonemura@coda.ocn.ne.jp
Received: 06-Feb-2018 Accepted Date: Feb 10, 2018; Published: 05-Mar-2018
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The two times-cytoreductive surgery (CRS) seems to be effective treatment because it allows complete removal of widespread masses and a reduction of surgical complications for pseudomyxoma peritonei (PMP) with high PCI. The present study verified the indication and prognostic factors of two times-CRS for patients with PMP. Between 2006 and 2017, 85(22.8%) patients were programmed a two times-surgical approach to remove the residual tumors. At 2nd CRS, complete cytoreduction (CCR-0) was done in 35(41.2%) patients. CCR-0 resection was performed significantly higher in patients with PCI ≤ 23, small bowel PCI ≤ 8 and low grade mucinous neoplasm (LAMN). Causes of incomplete cytoreduction (CCR-1) at 2nd CRS were diffuse involvement of small bowel, deep pushing invasion, massive bleeding during CRS, elderly patients older than 75 years old, and severe adhesion. Postoperative major complications after 1st and 2nd CRS were experienced in 16(18.8%) and 33(38.9%) patients, respectively. Postoperative mortality after 2nd CRS was found in 5(5.9%) patients. The 5-year overall survival of CCR-0 and CCR-1 group were 66.6% and 7.2%, respectively. CCR score and postoperative major complications after 2nd CRS were independent prognostic factors affecting overall survival. Patients with LAMN, PCI ≤ 23, and SB-PCI ≤ 8 are recommended to undergo 2nd CRS. Training of careful and gentle operation techniques are essential for the prevention of postoperative complications and survival improvement.
Keywords
Cytoreductive surgery; pseudomyxoma peritonei; mucinous neoplasm
Introduction
Pseudomyxoma peritonei is a rare disease characterized by mucinous ascites and peritoneal implants, generally starting from appendiceal mucinous neoplasm [1]. Reported incidences were approximately one to 1.5 per million per year [2,3]. Due to rarity of the disease, widespread peritoneal metastasis at the time of diagnosis and resistance to systemic chemotherapy, patients had been treated with repeat incomplete cytoreduction until early 1990s. In 1996, Sugarbaker developed an innovative treatment for this neoplasm consisting of aggressive cytoreduction of metastases on visceral and peritoneal surface (peritonectomy) and hyperthermic intraperitoneal chemo-perfusion (HIPEC) [4]. The treatment is now designated a comprehensive treatment. After complete cytoreduction by peritonectomy, 5-year survival was reported about 80-90% [4,5], but that after incomplete cytoreduction was only 15% [4]. Accordingly, complete CRS is considered effective surgical procedure for cure of the disease. However, patients with wide spread neoplastic invasion into vital organs requires a particularly complex cytoreductive surgery (CRS) of multiple organs and peritoneal sectors, making the complete resection very dangerous during the same time. Two stage surgical approach was proposed for patients with massive amount of peritoneal implants, elderly patients older than 75 years old, or patients with comorbidity. The two times-CRS seems to be effective treatment because it allows complete removal of widespread masses and a reduction of surgical complications.
The present study represents the results of two times-CRS for pseudomyxoma peritonei patients.
Methods
Patients selection
Between June 2006 and April 2017, 1034 patients with paeudomyxoma peritonei received laparotomy to perform cytoreduction (CRS). Volume and extent of the tumor deposits were recorded as peritoneal cancer index (PCI) [6]. Sum of PCI scores on small bowel and its mesentery (sector 9 to 12) was designated as SB-PCI [7-9].
All the patients underwent CRS with intent to perform complete removal of visible peritoneal metastases using peritonectomy techniques [6]. After CRS, all sites and volumes of the residual disease were recorded in terms of the completeness of cytoreduction (CCR) score (9). A complete and incomplete cytoreduction were defined as CCR-0 and CCR-1. Residual PCI score and residual SB-PCI were designated as residual PCI and residual SB-PCI.
Among 1034 patients, 661(64%) patients were performed complete cytoreduction, but the other 373 patients inevitably underwent incomplete cytoreduction due to several reasons. Among 373 patients of CCR-1 group, 85(22.8%) patients were programmed a two times-surgical approach to remove the residual tumors. Among 85 patients, 40 patients underwent HIPEC at 1st CRS.
At the time of secondary CRS, midline incision was made from xyphoid process to pubic bone. Adhesiolysis was performed with an aqua dissection technique [6], and a thorough exploration of the entire abdomen was performed to calculate PCI. CRS was done again. Following CRS, 37(43.5%) patients were treated with HIPEC using MMC and CDDP.
The present study was performed to clarify the postoperative mortality and survival of the 85 patients who underwent two times-CRS.
Written informed consent was obtained from all patients. This study was carried out in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Kishiwada Tokushukai Hospital, with ethical approval number of H19.
Follow-up and timing of 2nd CRD
After discharge of 1st CRS, all patients had follow-up every 3 months, and magnetic resonance imaging (MRI) or contrast enhancement computed tomography (ceCT) were performed to examine the residual tumor. If the residual tumors were diagnosed to be able to remove by secondary CRS from the findings of MRI or ceCT, 2nd CRS was planned [4]. Thirty-five patients received 2nd CRS within 1 year after 1st CRS and, 2nd CRS was performed in 50 patients one year later after 1st CRS.
After discharge of 2nd CRS, follow-up was done every 3 months until 2 years and every 6 months after 2 years.
Data analysis
The association between variables were compared by X2 analysis or Fischer exact test for categorical variables and by t test for continuous variables. Overall survival was calculated from the date of therapeutic intervention (considered to be either the date of 1st CRS or the date of 2nd CRS) to the date of last follow-up or date of death from any cause. Survival analyses were calculated using Kaplan-Meier method. All data analyses were performed SPSS version 11.5(SPSS Inc., Chicago, IL). Significance was accepted at the P<0.05 level.
Results
Of 85 patients, 29 were men; the median age was 58 years (range 27~80 years). Also, 41 tumors were PMP with low grade histologic features and 2 showed acellular mucin (mucin without epithelial cells) [1]. These tumors were classified as low-grade mucinous neoplasm (LGMN). The other 40 tumors were PMP with high-grade histologic features and 2 were PMP with signet ring cells. These tumors were defined as high-grade mucinous neoplasm (HGMN) [1].
The mean PCI of the 1st CRS was 28.5 with a range of 8-39, and the mean residual PCI of 1st CRS was 15.0 with a range of 2-39 (Table 1). The mean SB-PCI at 1st CRS was 7.5 with range of 0-12, and the mean residual SB-PCI at 1st CRS was 6.0 (range; 0-12).
| Mean ± SE | Range | ||
|---|---|---|---|
| PCI | 0-39 | ||
| First operation | 28.5 ± 0.9 | Aug-38 | |
| residual PCI at first operation | 15.0 ± 1.5 | Feb-39 | |
| Second operation | 19.9 ± 1.1 | 0-39 | P<0.0001 |
| SB-PCI (small bowel PCI) | 0-12 | ||
| First operation | 7.5 ± 0.4 | 0-12 | |
| residual SB-PCI at first operation | 6.0 ± 0.5 | 0-12 | |
| Second operation | 6.9 ± 0.4 | 0-12 | P=0.036 |
| Bleeding volume (ml) | |||
| First operation | 2573 ± 237 | 200-11000 | |
| Second operation | 1956 ± 8.4 | 400-11000 | |
| Operation time (minutes) | |||
| First operation | 257 ± 9.5 | 60-437 | |
| Second operation | 244 ± 8.4 | 20-506 | |
| Postoperative stay (days) | |||
| First operation | 49.3 ± 42.5 | 9-240 | |
| Second operation | 68.5 ± 80.1 | 15-413 | P=0.019 |
Table 1: PCI, residual PCI and SB-PCI scores at 1st CRS, and 2nd CRS, and bleeding ime, operation time, and hospital stay at 1 st and 2nd CRS.
Causes of incomplete cytoreduction at 1st CRS were high PCI scores, diffuse involvement of small bowel and its mesentery, comorbidity, deep pushing invasion to vital organs, massive bleeding during 1st CRS, emergency CRS, elderly patients older than 75 years, and fertility sparing operation in, 38, 28, 3, 4, 3, 2, 2, and 1 patients, respectively.
PCI at 2nd CRS (mean; 19.9; range 2-39) was significantly higher than the residual PCI at 1st CRS (mean; 15.0, range; 2-39) (P<0.0001) (Table 1).
The SB-PCI at 2nd CRS (mean 6.9, range; 0-12) was significantly higher than that of residual SB-PCI at 1st CRS (mean; 6.0; range 2-12) (Table 1).
Operation methods of 2nd CRS were shown in Table 2. Resection of small bowel and its mesentery was performed in 38 patients. Rectal and colon resection were done in 23, and 13 patients, respectively. To remove perigastric tumors, total and distal gastrectomy was performed in 20 and 3 patients, respectively. Right and left peritonectomy of diaphragm were performed in 12 and 9 patients. To remove tumors around spleen and splenic hilum, splenectomy and distal pancreatectomy were performed in 12, and 3 patients, respectively.
| Resected organs and operation methods | No of patients |
|---|---|
| Small bowel and its mesentery | 38 |
| Rectum | 23 |
| Colon | 13 |
| Total gastrectomy | 20 |
| Distal gastrectomy | 3 |
| Cholecystectomy | 14 |
| Splenectomy | 12 |
| Right diaphragmatic peritonectomy | 12 |
| Left diaphragmatic peritonectomy | 9 |
| Pancreas tail resection | 3 |
| Hysterectomy | 6 |
| Oophorectomy | 5 |
| Resection of vagina | 2 |
| Cytoreduction alone | 14 |
| Probe laparotomy | 7 |
Table 2: Operation methods and resected organs at 2nd CRS.
Mean bleeding volume, operation time during 2nd CRS and hospital stay after 2nd CRS were 1956 ml (range; from 400-11000 ml), 244 min. (range; from 20-506 min.), and 68.5 days (range; from 15-413days) (Table 1).
Postoperative morbidity and mortality was shown in Table 3. According to Clavian-Dindo’s criteria, major complications of grade 3, 4, and 5 after 1st and 2nd CRS were experienced in 16 (18.8%) and 33 (38.9%) patients, respectively. Postoperative mortality after 2nd CRS was experienced in 5 (5.9%) patients, and the causes of mortality after 2nd CRS were bleeding, pneumonia, pancreas fistula, and sepsis in 2, 1, 1, and 1 patients, respectively.
| First operation | Second operation | |
|---|---|---|
| Grade 0 | 53 (62.4%) | 33 (38.8%) |
| Grade 1-2 | 16 (18.8%) | 19 (22.3%) |
| Grade 3 | 10 (11.8%) | 14 (16.5%) |
| Grade 4 | 6 (7.0%) | 14 (16.5%) |
| Grade 5 | 0 (0%) | 5 (5.9%) |
| P=0.006, X2=7.340 |
Table 3: Postoperative complications after 1st and 2nd CRS.
At 2nd CRS, CCR-0 resection was done in 35 (41.2%) of 85 patients.
Regarding histologic subtype, CCR-0 resection was done significantly higher in patients with LGMN than those with HGMN (Table 4). With regards to PCI, CCR-0 resection was performed significantly higher in patients with PCI ≤ 23 or SP-PCI ≤ 8 than those with PCI ≥ 24 (P<0.0001) or SB-PCI ≥ 9 (P<0.001), respectively (Table 4). CCR-0 resection could not be performed in 16 patients with PCI ≥ 29. CCR-0 resection was performed only in 2 (4.8%) of 42 patients with SB-PCI ≥ 9. In contrast, 33 (76.7%) of 43 patients with SB-PCI ≤ 8 underwent CCR-0 resection. There was a significant difference in the incidence of CCR-0 resection between the two group (P<0.0001).
| CCR-0 | CCR-1 | ||
|---|---|---|---|
| Histologic subtype | |||
| Low grade mucinous neolasm (MN) | 25 (35.3%) | 18 | X2=8.97 |
| High grade MN or signet ring cell component | 10 (23.8%) | 32 | P=0.0025 |
| PCI cut-off = 3 vs=24 | |||
| =23 | 32 (57.1%) | 24 | X2=15.4 |
| =24 | 3 (10.3%) | 26 | P<0.0001 |
| SB-PCI (small bowel PCI) | |||
| =8 | 33 (76.7%) | 10 | X2=45.5 |
| =9 | 2 (4.8%) | 40 | P<0.0001 |
| Postoperative complication after 2nd CRS | |||
| Grade 0,1,2 | 25 (48.1%) | 27 | |
| Grade 3,4,5 | 10 (30%) | 23 | NS |
Table 4: CCR scores and PCI, SB-PCI score and postoperative complications after 2nd CRS.
Causes of incomplete CRS at 2nd CRS were diffuse involvement of small bowel, deep pushing invasion, massive bleeding during CRS, elderly patients older than 75 years old, and severe adhesion in 37, 5, 3, 2, and 2 patients, respectively.
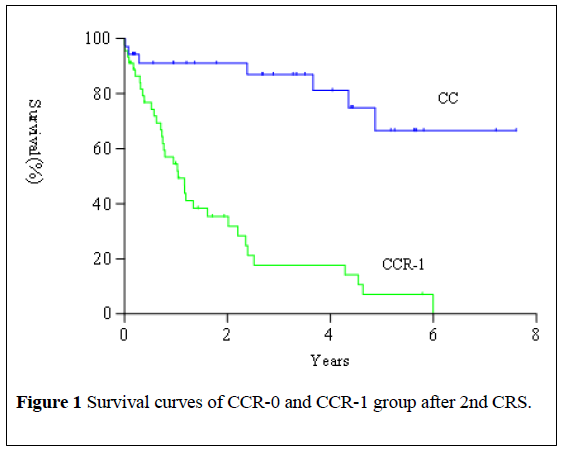
With median follow-up time of 42 months, the median overall survival was 28.7 months (range 6.0-91.2 months) from the date of 2nd CRS. Survival of patients who underwent CCR-0 resection at 2nd CRS was significantly higher than that of patients who received CCR-1 resection (Figure 1, X2=15.86, P<0.0001). The 5-year overall survival of CCR-0 and CCR-1 group were 66.6% and 7.2%, respectively. CCR score and complication after 2nd CRS were independent prognostic factors affecting overall survival (Table 5).
| Multivariate analysis | Univariate analysis | ||||
|---|---|---|---|---|---|
| p value | RR | CI | p value | X2 | |
| SB-PCI =8 vs. =9 | 0.8099 | 0.9185 | 0.4741-1.7794 | 0.0265 | 4.921 |
| Completeness of cytoreduction CCR-0 vs CCR-1 | <0.0001 | 7.4497 | 2.8488-19.482 | <0.0001 | 18.43 |
| PCI =23 vs. =24 | 0.2205 | 1.5608 | 0.7656-3.1819 | 0.0005 | 12.27 |
| Histologic subtype: low grade vs. high grade | 0.551 | 0.8078 | 0.4005-1.6294 | 0.0374 | 4.331 |
| Complication after 2nd CRS | |||||
| Grade 0,1,2 vs Grade 3,4,5 | <0.0001 | 4.4835 | 2.2173-9.0658 | <0.0001 | 22.87 |
Table 5: Prognostic factors at 2nd CRS for overall survival after 2nd CRS.
Discussion
Complete cytoreduction is an only treatment option to improve long term survival of patients with pseudomyxoma peritonei [5], because tumors are resistant to chemotherapy. In contrast, the rates of incomplete cytoreduction were reported to range from 18% to 27% [4-11]. Survival of patients after incomplete cytoreduction is very low with 5-year survival rate of 15% [5].
Main causes of CCR1 resection at 1st CRS were high PCI level, diffuse involvement of small bowel and its mesentery and deep pushing invasion to the vital organs. To perform complete cytoreduction for patients with these factors needs to resect multiple organs and wide peritoneal sectors, resulting in higher mortality and morbidity rates [12]. The postoperative morbidity and mortality were significantly associated with high PCI ≥ 20, blood loss ≥ 2.5 L, and operation time ≥ 5 hours [13]. From these results, two times-CRS was proposed for patients with high PCI [7-14]. However, the case numbers of the reports were small, and the effects of two times- CRS on survival remain unclear.
We performed 2 times-CRS for 85 patients among 373 patients who had received incomplete cytoreduction at the initial operation.
Main cause of incomplete CRS were high PCI of ≥ 24 and wide involvement of small bowel and its mesentery (SB-PCI of ≥ 9). Additionally, removal of tumors on the perihepatic region is the cause of intraoperative massive bleeding.
Bleeding over 1.5 times larger than the total circulation blood volume may cause coagulopathy [15]. In the situation, surgeons should inevitably interrupt to continue operation, and have to plan second time CRS. Present study demonstrated that the severe complications of Grade 3-4 were experienced in 16 (18.8%) of 85 patients after 1st CRS, and the incidence was significantly lower than those reported previously [12,13]. 1st CRS can be performed safely. When difficult CRS for patients with high PCI, deep pushing, or comorbidities is expected, two times-CRS are recommended.
After 1st CRS, timing and indication for 2nd CRS should be determined by periodical interviewing patients by physical and image diagnostic examination using ceCT or MRI 2 to 3 months interval.
PCIs at 2nd CRS was significantly higher than those of residual PCIs at 1st CRS. This means that the residual tumors grew after 1st CRS. However, there was no statistically different between PCIs at 2nd CRS of patients who underwent 2nd CRS 1 year earlier after 1st CRS. In contrast, PCIs of 2nd CRS of patients who underwent 2nd CRS 1 year later after 1st CRS were significantly higher than those at residual PCIs at 1st CRS. In addition, CCR-0 rate of patients who were performed 2nd CRS 1 year earlier after 1st CRS was significantly higher than those of patients who received 2nd CRS one year later after 1st CRS. These results indicate that 2nd CRS should be performed within 1 year after 1st CRS.
Five-year survival rate of patients who received CCR-0 resection at 2nd CRS was 66.5%. In contrast, that of patients who underwent CCR-1 resection was only 7.1%. Additionally, multivariate analysis showed that CCR-score at 2nd CRS revealed independent prognostic factor. CCR-0 resection was performed significantly higher in patients with LAMN, PCI ≤ 23, and SB-PCI ≤ 8 than in those with HGMN, PCI ≥ 24, and SB-PCI ≥ 9. Patients fulfill these criteria are recommended to undergo 2nd CRS.
Yan H et al reported that patients with HGMN had low chance to receive CCR-0 resection at 2nd CRS due to higher growth of residual tumors after 1st CRS [15]. De Simone described that systemic chemotherapy may have a role in suppress tumor growth after 1st CRS, and that systemic chemotherapy may increase the incidence of CCR0 resection [8]. In the present study, however, HIPEC at 1st CRS and systemic chemotherapy after 1st CRS did not decrease PCI. Accordingly, surgical complete cytoreduction is the most important option for the improvement of longterm survival.
Postoperative Grade 3 or Grade 4 morbidity and mortality were independent prognostic factor for poor prognosis. Mizumoto A et al. also reported a significant correlation between postoperative severe complications and prognosis [13]. Training of careful and gentle operation techniques is essential for the prevention of postoperative complications and survival improvement.
Conclusion
Patients with PCI ≥ 24, SB-PCI ≥ 9 and deep pushing invasion into the vital organs are not recommended for 2nd CRS, because of high incidence of postoperative morbidity and mortality. In contrast, 2nd CRS is recommended for patients with PCI ≤ 23, SB-PCI ≤ 8, and mild pushing invasion.
REFERENCES
- Carr NJ, Cecil TD, Mohamed F, et al. A consensus for classification and pathologic reporting of pseudomixomaperitonei and associated appendiceal neoplasm. The results of the Peritoneal Surface Oncology Group International (PSOGI) modified Delphi Process. Am J SurgPathol2016;40:14-26,
- Moran BJ, Cecil T. The etiology, clinical presentation, and management of pseudomyxomaperitonei. SurgOncolClin N Am 2003;12:585-603
- Kitai T, Hirai T, Fujita T, et al. Survay on the incidence and management of pseudomyxomaperitonei in Japan. Gan to KagakyRyoho 2013;40:1043-1048.
- Sugarbaker PH, Ronnett BM, Archer A, et al. Pseudomyxomaperitonei syndrome. Arch Surg 1996;30:233-280.
- Sugarbaker PH. Epithelial appendiceal neoplasm. The Cancer J2009;15:225-235.
- González MS, Kusamura S, Baratti D, et al. Postoperative residual disease evaluation in the locoregional treatment of peritoneal surface malignancy. J SurgOncol2008;98:237-241.
- Santoro B, Griffen WO, Wangensteen OH. The second-look procedure in the management of ovarian malignancies and pseudomyxomaperitonei. Surgery1961;50:354-358.
- De Siemone M, Scuderi S, Vaira M, et al. Treatment of pseudomyxomaperitonei with two times-cytoreduction and hyperthermicantiblastic peritoneal perfusion (HAPP). J ExpClinCncer Res2003;22:22-24.
- Yonemura Y, Elnemr A, Endou Y, et al. Surgical Results of Patients with Peritoneal Carcinomatosis Treated with Cytoreductive Surgery Using a New Technique Named Aqua Dissection. Gastroenterology Research and Practice 2012.
- DindoD, Demartines N, Clavian PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-213.
- Austin F, Mavanur A, Sathaiah M, et al. Aggressive management of peritoneal carcinomatosis from mucinous appendiceal neoplasms. Ann SurgOncol 2012;19-26
- Valle SJ, Alzahrani N, Alzaharani S, et al. Enterocutaneus fistula in patients with peritoneal malignancy following cyoreductive surgery and hyperthermicintraperitoneal chemotherapy: Incidence, management and outcomes. SurgOncol 2016;25;315-320.
- Mizumoto A, Canbay E, Hirano M, et al. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermicintraperitoneal chemotherapy at a sigle institution in Japan. Gastroente Res Prac 2012.
- Santoro BT, Griffen WO, Wangensteen OH. The second-look procedure in the management of ovarian malignancies and peudomyxomaperitonei. Surgery 1961;24:354-358.
- Satani M, Oomae N, Tomita M, et al. Blood transfusion therapy for massive hemorrhage associated with scheduled peritonectomy for pseudomyxomaperitonei and peritoneal dissemination. Masui. 2009;58:432-437.