Right hepatic artery branching off the superior mesenteric artery and its potential implications
Judy J. Moon, Coen A. Wijdicks, James M. Williams*
Department of Anatomy and Cell Biology, Rush Medical College, Rush University Medical Center, Chicago, Illinois, USA.
- *Corresponding Author:
- James M. Williams, PhD
Professor, Department of Anatomy and Cell Biology, Rush Medical College, 600 S. Paulina Street, Room 507 AAC, Chicago, IL 60612, USA.
Tel: +1 312 942-3598
E-mail: James_M_Williams@rush.edu
Date of Received: June 9th, 2009
Date of Accepted: September 11th, 2009
Published Online: December 10th, 2009
© IJAV. 2009; 2: 143–145.
[ft_below_content] =>Keywords
arterial variation, hepatic artery, liver, transplant, biliary tract
Introduction
The arterial supply to the liver varies substantially. In some series, up to 75% of patients
possessed a variant of the celiac trunk [1]. In most instances, such variants are inconsequential, since blood supply to the liver comes from the left gastric artery, aorta, and the superior mesenteric artery. However, under certain circumstances, a variant can pose significant risks, especially if undetected by the treating physician.
Case Report
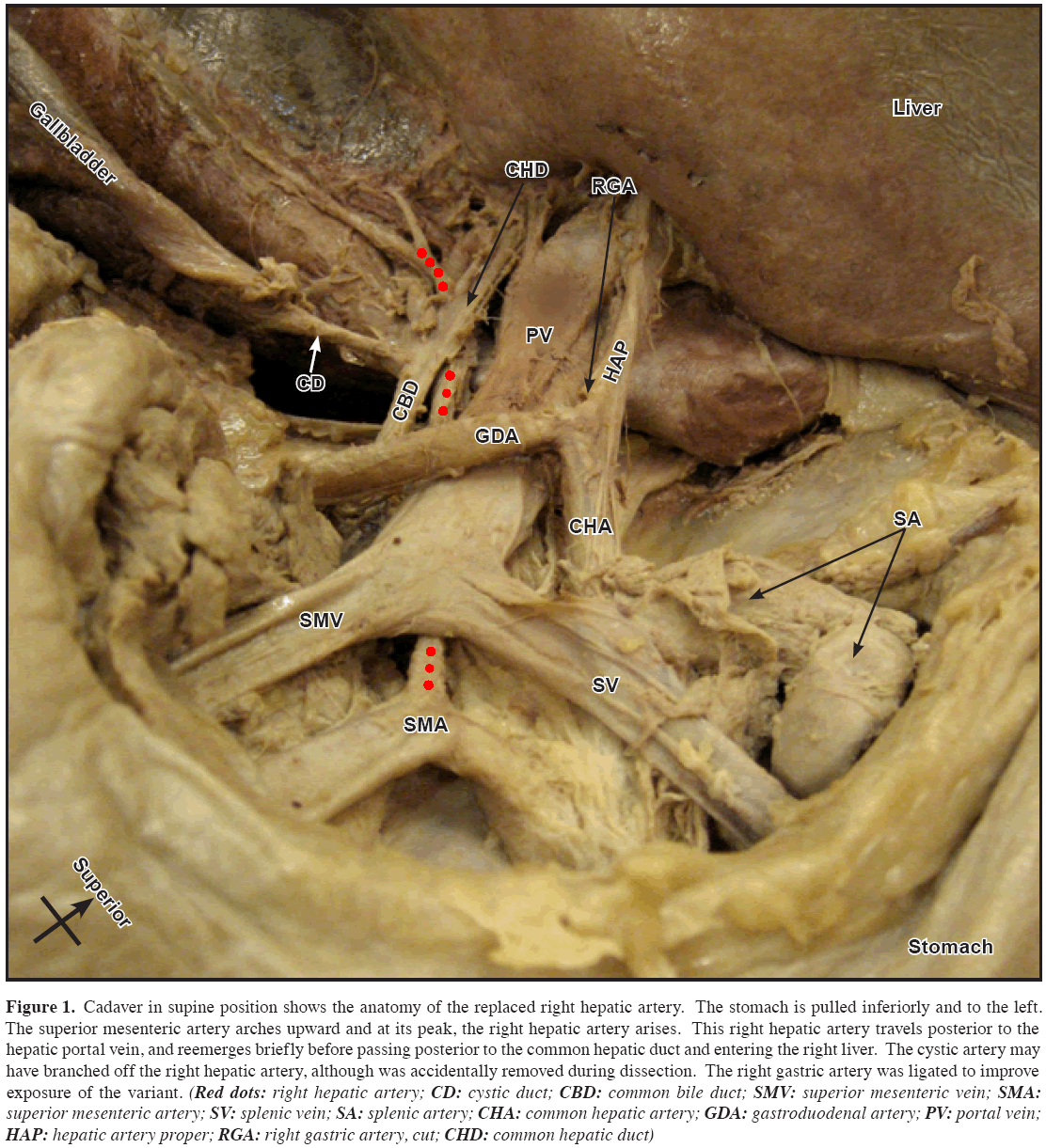
Routine dissection of a 64-year-old Caucasian male cadaver revealed a right hepatic artery (RHA) branching off of the superior mesenteric artery (SMA) and crossing posterior to the portal vein (Figures 1 and 2). This artery served as the sole arterial supply to the right lobe, and was identified as a replaced RHA. The length of the replaced RHA was 11 cm from the SMA to the liver. The hepatic artery proper branching from the common hepatic artery was 4 cm and normal in its trajectory. The aorta revealed no evidence of aneurysmal dilation. The liver was normal in appearance. A prior lethal myocardial infarction was noted in the history.
Figure 1: Cadaver in supine position shows the anatomy of the replaced right hepatic artery. The stomach is pulled inferiorly and to the left. The superior mesenteric artery arches upward and at its peak, the right hepatic artery arises. This right hepatic artery travels posterior to the hepatic portal vein, and reemerges briefly before passing posterior to the common hepatic duct and entering the right liver. The cystic artery may have branched off the right hepatic artery, although was accidentally removed during dissection. The right gastric artery was ligated to improve exposure of the variant. (Red dots: right hepatic artery; CD: cystic duct; CBD: common bile duct; SMV: superior mesenteric vein; SMA: superior mesenteric artery; SV: splenic vein; SA: splenic artery; CHA: common hepatic artery; GDA: gastroduodenal artery; PV: portal vein; HAP: hepatic artery proper; RGA: right gastric artery, cut; CHD: common hepatic duct)
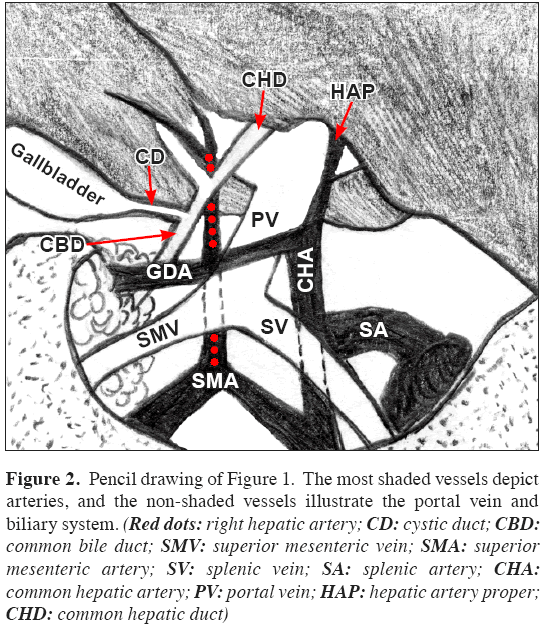
Figure 2: Pencil drawing of Figure 1. The most shaded vessels depict arteries, and the non-shaded vessels illustrate the portal vein and biliary system. (Red dots: right hepatic artery; CD: cystic duct; CBD: common bile duct; SMV: superior mesenteric vein; SMA: superior mesenteric artery; SV: splenic vein; SA: splenic artery; CHA: common hepatic artery; PV: portal vein; HAP: hepatic artery proper; CHD: common hepatic duct)
A replaced RHA has been documented in 5-25% of reported cases [1-7]. Previous literature has defined an RHA as replaced, if the artery supplies the right lobe in place of the typical celiac hepatic anatomy, but if the artery merely serves as an additional branch it would be referred to as aberrant [1].
This variant can be attributed to the abnormal persistence or regression of an embryonic artery [8]. During embryonic development, the aorta gives off ventral segments, four of which become the celiac, splenic, common hepatic, and superior mesenteric arteries. A longitudinal ventral artery anastomoses these segments. The replaced right hepatic artery originates from the persistence of the longitudinal ventral arterial segment connected to the superior mesenteric artery.
This variant is of no clinical meaning unless the SMA becomes compromised. Occlusion of the SMA is a common clinical problem, and if the patient possesses a replaced RHA, not only the gut but also the liver will become necrotic. Approximately 4% of all arterial emboli lodge in the SMA and are characteristically cardiac in origin. Major risk factors include atrial fibrillation, mitral stenosis, left ventricular aneurysm with mural thrombus, and, as in this case, myocardial infarction. Patients with SMA embolism often present clinically with atrial fibrillation and persistent abdominal pain [9]. If the collateral circulation fails, SMA occlusion results in life threatening intestinal necrosis [9].
This anatomical variant must be identified prior to procedures such as laparoscopic cholecystectomy to prevent vascular or biliary damage, especially if the replaced RHA runs anterior to the common hepatic duct [10]. Angiography of the celiac and mesenteric arteries will define the vasculature pre-operatively [11]. Preoperative detection of an aberrant RHA in prospective transplant donors and recipients is essential for the proper management of living donor liver transplantation, as transplantation of the right lobe is heavily favored over the left, and the aberration affects the safety of both donor and recipient [12].
Studies suggest that a replaced RHA is a welcome discovery in right liver living donors [6,13]. A detrimental consequence of living donor transplantation is a shorter and thinner hepatic artery graft, since dissections of the right hepatic artery in living donors must be limited to the right side of the common bile duct to prevent its devascularization [2]. The smaller graft makes the artery more difficult to reconstruct, and hepatic artery thrombosis (HAT), a leading cause of postoperative graft loss, is more common where there exists diameter discrepancy between the recipient and graft arteries [2]. Moreover, emergency rearterialization to save a transplant in the event of HAT is often compromised by a thin, short graft. Replaced RHAs are longer and larger, decreasing the chance of HAT [5]. They also are easily isolated and can be anastomosed under loupe magnification [6,7].
The presence of a replaced RHA proximal to the bile ducts also may reduce the risk of post-operative ischemia in the biliary tract of the donor, an occurrence that is speculated to be caused by the loss of crucial feeding branches from the main or left hepatic artery during retrieval [14]. In contrast, caution must be applied where the recipient possesses a replaced RHA. Despite the development of branch patch reconstruction techniques, one study reported a higher incidence of postoperative HAT and stenosis in liver transplant recipients possessing a replaced RHA [15]. The suspected cause was reduced blood flow in the common hepatic artery, thinner due to lack of the right hepatic arterial branch [15].
Acknowledgments
This work was performed at the Rush University Medical College Gross Anatomy Laboratory. The authors would like to acknowledge Robert M. Leven, Ph.D. Department of Anatomy and Cell Biology, and Vassyl Lonchyna, MD, Department of Medicine, Division of Pulmonary and Critical Care, for their mentorship.
References
- Weiglein AH. Variations and topography of the arteries in the lesser omentum in humans. Clin Anat. 1996; 9: 143–150.
- Aramaki O, Sugawara Y, Kokudo N, Takayama T, Makuuchi M. Branch patch reconstruction in living donor liver transplantation: arterialization of grafts with replaced type arteries. Transplantation. 2006; 82: 1541–1543.
- Chaib E, Ribeiro MA Jr, Saad WA, Gama-Rodrigues J. The main hepatic anatomic variations for the purpose of split-liver transplantation. Transplant Proc. 2005; 37: 1063–1066.
- Kornasiewicz O, Krawczyk M, Paluszkiewicz R, Zieniewicz K, Hevelke P, Grzelak I, Pacho R, Rowinski O, Kalicinski P, Kaminski A, Pawlowska J. Anatomical alteration of the vascular tree observed during living related liver transplantation. Transplant Proc. 2003; 35: 2245–2247.
- Marcos A, Killackey M, Orloff MS, Mieles L, Bozorgzadeh A, Tan HP. Hepatic arterial reconstruction in 95 adult right lobe living donor liver transplants: evolution of anastomotic technique. Liver Transpl. 2003; 9: 570–574.
- Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, Kaihara S, Egawa H, Ozden I, Kobayashi N, Uemoto S. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002; 73: 1896–1903.
- Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, Schwartz ME, Miller CM, Sukru E. Anatomic variations in right liver living donors. J Am Coll Surg. 2004; 198: 577–582.
- Peschaud F, El-Hajjam M, Malafosse R, Goere D, Benoist S, Penna C, Nordlinger B. A common hepatic artery passing in front of the portal vein. Surg Radiol Anat. 2006; 28: 202–205.
- Barakate MS, Cappe I, Curtin A, Engel KD, Li-Kim-Moy J, Poon MS, Sandeman MD. Management of acute superior mesenteric artery occlusion. ANZ J Surg. 2002; 72: 25–29.
- Nicholson T, Travis S, Ettles D, Dyet J, Sedman P, Wedgewood K, Royston C. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999; 22: 20–24.
- Bradbury AW, Brittenden J, McBride K, Ruckley CV. Mesenteric ischaemia: a multidisciplinary approach. Br J Surg. 1995; 82: 1446–1459.
- Orguc S, Tercan M, Bozoklar A, Akyildiz M, Gurgan U, Celebi A, Nart D, Karasu Z, Icoz G, Zeytunlu M, Yuzer Y, Tokat Y, Kilic M. Variations of hepatic veins: helical computerized tomography experience in 100 consecutive living liver donors with emphasis on right lobe. Transplant Proc. 2004. 36: 2727–2732.
- Hwang S, Lee SG, Lee YJ, Park KM, Kim KH, Ahn CS, Sung KB, Moon DB, Ha TY, Kim KK, Kim YD. Donor selection for procurement of right posterior segment graft in living donor liver transplantation. Liver Transpl. 2004; 10: 1150–1155.
- Yeh BM, Coakley FV, Westphalen AC, Joe BN, Freise CE, Qayyum A, McTaggart RA, Roberts JP. Predicting biliary complications in right lobe liver transplant recipients according to distance between donor’s bile duct and corresponding hepatic artery. Radiology. 2007; 242: 144–151.
- Ishigami K, Zhang Y, Rayhill S, Katz D, Stolpen A. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol. 2004; 183: 1577–1584.
Judy J. Moon, Coen A. Wijdicks, James M. Williams*
Department of Anatomy and Cell Biology, Rush Medical College, Rush University Medical Center, Chicago, Illinois, USA.
- *Corresponding Author:
- James M. Williams, PhD
Professor, Department of Anatomy and Cell Biology, Rush Medical College, 600 S. Paulina Street, Room 507 AAC, Chicago, IL 60612, USA.
Tel: +1 312 942-3598
E-mail: James_M_Williams@rush.edu
Date of Received: June 9th, 2009
Date of Accepted: September 11th, 2009
Published Online: December 10th, 2009
© IJAV. 2009; 2: 143–145.
Abstract
Detection of a replaced right hepatic artery by surgeons and radiologists is crucial, not only for liver transplantation but prior to any abdominal surgery, for this unnoticed variant is vulnerable to inadvertent ligation. Despite potential risks, a replaced right hepatic artery in the right lobe of the donor liver will provide a larger graft, is more easily isolated, and can be anastomosed under loupe magnification. It may also reduce the risk of post-operative ischemia in the biliary tract of the recipient, since there is no threat of losing crucial feeding branches from the main or left hepatic artery during retrieval.
-Keywords
arterial variation, hepatic artery, liver, transplant, biliary tract
Introduction
The arterial supply to the liver varies substantially. In some series, up to 75% of patients
possessed a variant of the celiac trunk [1]. In most instances, such variants are inconsequential, since blood supply to the liver comes from the left gastric artery, aorta, and the superior mesenteric artery. However, under certain circumstances, a variant can pose significant risks, especially if undetected by the treating physician.
Case Report
Routine dissection of a 64-year-old Caucasian male cadaver revealed a right hepatic artery (RHA) branching off of the superior mesenteric artery (SMA) and crossing posterior to the portal vein (Figures 1 and 2). This artery served as the sole arterial supply to the right lobe, and was identified as a replaced RHA. The length of the replaced RHA was 11 cm from the SMA to the liver. The hepatic artery proper branching from the common hepatic artery was 4 cm and normal in its trajectory. The aorta revealed no evidence of aneurysmal dilation. The liver was normal in appearance. A prior lethal myocardial infarction was noted in the history.
Figure 1: Cadaver in supine position shows the anatomy of the replaced right hepatic artery. The stomach is pulled inferiorly and to the left. The superior mesenteric artery arches upward and at its peak, the right hepatic artery arises. This right hepatic artery travels posterior to the hepatic portal vein, and reemerges briefly before passing posterior to the common hepatic duct and entering the right liver. The cystic artery may have branched off the right hepatic artery, although was accidentally removed during dissection. The right gastric artery was ligated to improve exposure of the variant. (Red dots: right hepatic artery; CD: cystic duct; CBD: common bile duct; SMV: superior mesenteric vein; SMA: superior mesenteric artery; SV: splenic vein; SA: splenic artery; CHA: common hepatic artery; GDA: gastroduodenal artery; PV: portal vein; HAP: hepatic artery proper; RGA: right gastric artery, cut; CHD: common hepatic duct)
Figure 2: Pencil drawing of Figure 1. The most shaded vessels depict arteries, and the non-shaded vessels illustrate the portal vein and biliary system. (Red dots: right hepatic artery; CD: cystic duct; CBD: common bile duct; SMV: superior mesenteric vein; SMA: superior mesenteric artery; SV: splenic vein; SA: splenic artery; CHA: common hepatic artery; PV: portal vein; HAP: hepatic artery proper; CHD: common hepatic duct)
A replaced RHA has been documented in 5-25% of reported cases [1-7]. Previous literature has defined an RHA as replaced, if the artery supplies the right lobe in place of the typical celiac hepatic anatomy, but if the artery merely serves as an additional branch it would be referred to as aberrant [1].
This variant can be attributed to the abnormal persistence or regression of an embryonic artery [8]. During embryonic development, the aorta gives off ventral segments, four of which become the celiac, splenic, common hepatic, and superior mesenteric arteries. A longitudinal ventral artery anastomoses these segments. The replaced right hepatic artery originates from the persistence of the longitudinal ventral arterial segment connected to the superior mesenteric artery.
This variant is of no clinical meaning unless the SMA becomes compromised. Occlusion of the SMA is a common clinical problem, and if the patient possesses a replaced RHA, not only the gut but also the liver will become necrotic. Approximately 4% of all arterial emboli lodge in the SMA and are characteristically cardiac in origin. Major risk factors include atrial fibrillation, mitral stenosis, left ventricular aneurysm with mural thrombus, and, as in this case, myocardial infarction. Patients with SMA embolism often present clinically with atrial fibrillation and persistent abdominal pain [9]. If the collateral circulation fails, SMA occlusion results in life threatening intestinal necrosis [9].
This anatomical variant must be identified prior to procedures such as laparoscopic cholecystectomy to prevent vascular or biliary damage, especially if the replaced RHA runs anterior to the common hepatic duct [10]. Angiography of the celiac and mesenteric arteries will define the vasculature pre-operatively [11]. Preoperative detection of an aberrant RHA in prospective transplant donors and recipients is essential for the proper management of living donor liver transplantation, as transplantation of the right lobe is heavily favored over the left, and the aberration affects the safety of both donor and recipient [12].
Studies suggest that a replaced RHA is a welcome discovery in right liver living donors [6,13]. A detrimental consequence of living donor transplantation is a shorter and thinner hepatic artery graft, since dissections of the right hepatic artery in living donors must be limited to the right side of the common bile duct to prevent its devascularization [2]. The smaller graft makes the artery more difficult to reconstruct, and hepatic artery thrombosis (HAT), a leading cause of postoperative graft loss, is more common where there exists diameter discrepancy between the recipient and graft arteries [2]. Moreover, emergency rearterialization to save a transplant in the event of HAT is often compromised by a thin, short graft. Replaced RHAs are longer and larger, decreasing the chance of HAT [5]. They also are easily isolated and can be anastomosed under loupe magnification [6,7].
The presence of a replaced RHA proximal to the bile ducts also may reduce the risk of post-operative ischemia in the biliary tract of the donor, an occurrence that is speculated to be caused by the loss of crucial feeding branches from the main or left hepatic artery during retrieval [14]. In contrast, caution must be applied where the recipient possesses a replaced RHA. Despite the development of branch patch reconstruction techniques, one study reported a higher incidence of postoperative HAT and stenosis in liver transplant recipients possessing a replaced RHA [15]. The suspected cause was reduced blood flow in the common hepatic artery, thinner due to lack of the right hepatic arterial branch [15].
Acknowledgments
This work was performed at the Rush University Medical College Gross Anatomy Laboratory. The authors would like to acknowledge Robert M. Leven, Ph.D. Department of Anatomy and Cell Biology, and Vassyl Lonchyna, MD, Department of Medicine, Division of Pulmonary and Critical Care, for their mentorship.
References
- Weiglein AH. Variations and topography of the arteries in the lesser omentum in humans. Clin Anat. 1996; 9: 143–150.
- Aramaki O, Sugawara Y, Kokudo N, Takayama T, Makuuchi M. Branch patch reconstruction in living donor liver transplantation: arterialization of grafts with replaced type arteries. Transplantation. 2006; 82: 1541–1543.
- Chaib E, Ribeiro MA Jr, Saad WA, Gama-Rodrigues J. The main hepatic anatomic variations for the purpose of split-liver transplantation. Transplant Proc. 2005; 37: 1063–1066.
- Kornasiewicz O, Krawczyk M, Paluszkiewicz R, Zieniewicz K, Hevelke P, Grzelak I, Pacho R, Rowinski O, Kalicinski P, Kaminski A, Pawlowska J. Anatomical alteration of the vascular tree observed during living related liver transplantation. Transplant Proc. 2003; 35: 2245–2247.
- Marcos A, Killackey M, Orloff MS, Mieles L, Bozorgzadeh A, Tan HP. Hepatic arterial reconstruction in 95 adult right lobe living donor liver transplants: evolution of anastomotic technique. Liver Transpl. 2003; 9: 570–574.
- Nakamura T, Tanaka K, Kiuchi T, Kasahara M, Oike F, Ueda M, Kaihara S, Egawa H, Ozden I, Kobayashi N, Uemoto S. Anatomical variations and surgical strategies in right lobe living donor liver transplantation: lessons from 120 cases. Transplantation. 2002; 73: 1896–1903.
- Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, Schwartz ME, Miller CM, Sukru E. Anatomic variations in right liver living donors. J Am Coll Surg. 2004; 198: 577–582.
- Peschaud F, El-Hajjam M, Malafosse R, Goere D, Benoist S, Penna C, Nordlinger B. A common hepatic artery passing in front of the portal vein. Surg Radiol Anat. 2006; 28: 202–205.
- Barakate MS, Cappe I, Curtin A, Engel KD, Li-Kim-Moy J, Poon MS, Sandeman MD. Management of acute superior mesenteric artery occlusion. ANZ J Surg. 2002; 72: 25–29.
- Nicholson T, Travis S, Ettles D, Dyet J, Sedman P, Wedgewood K, Royston C. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999; 22: 20–24.
- Bradbury AW, Brittenden J, McBride K, Ruckley CV. Mesenteric ischaemia: a multidisciplinary approach. Br J Surg. 1995; 82: 1446–1459.
- Orguc S, Tercan M, Bozoklar A, Akyildiz M, Gurgan U, Celebi A, Nart D, Karasu Z, Icoz G, Zeytunlu M, Yuzer Y, Tokat Y, Kilic M. Variations of hepatic veins: helical computerized tomography experience in 100 consecutive living liver donors with emphasis on right lobe. Transplant Proc. 2004. 36: 2727–2732.
- Hwang S, Lee SG, Lee YJ, Park KM, Kim KH, Ahn CS, Sung KB, Moon DB, Ha TY, Kim KK, Kim YD. Donor selection for procurement of right posterior segment graft in living donor liver transplantation. Liver Transpl. 2004; 10: 1150–1155.
- Yeh BM, Coakley FV, Westphalen AC, Joe BN, Freise CE, Qayyum A, McTaggart RA, Roberts JP. Predicting biliary complications in right lobe liver transplant recipients according to distance between donor’s bile duct and corresponding hepatic artery. Radiology. 2007; 242: 144–151.
- Ishigami K, Zhang Y, Rayhill S, Katz D, Stolpen A. Does variant hepatic artery anatomy in a liver transplant recipient increase the risk of hepatic artery complications after transplantation? AJR Am J Roentgenol. 2004; 183: 1577–1584.