Role of intracellular Ca2+ overload in inducing changes in cardiac gene expression
- *Corresponding Author:
- Dr Naranjan S Dhalla
Institute of Cardiovascular Sciences, St Boniface Hospital Research, 351 Tache Avenue, Winnipeg, Manitoba R2H 2A6
Telephone: 204-235-3417
Fax: 204-237-0347
E-mail: nsdhalla@sbrc.ca
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
[ft_below_content] =>Keywords
Cardiac gene expression; Ca2+-pump ATPase; Ca2+-release channel; Intracellular Ca2+ overload; Myosin heavy chains; Na+-K+-ATPase; Na+-Ca2+ exchanger
Normal expression of cardiac genes for Ca2+transport and contractile proteins is known to play a critical role in the maintenance of the function of subcellular organelles such as sarcolemma (SL), sarcoplasmic reticulum (SR) and myofibrils (MFs) in cardiomyocytes. Previous studies have revealed varying degrees of depression in gene expression for SL, SR and MF proteins, as well as defects in subcellular organelles during the development of heart failure in patients and experimental animal models [1-6]. Accordingly, it has been suggested that cardiac dysfunction in failing hearts is due to subcellular remodelling as a consequence of changes in cardiac gene expression (2,7-9). Although the occurrence of intracellular Ca2+ overload is believed to be intimately involved in the genesis of cardiac dysfunction in heart failure [10-15], its role in inducing defects in cardiac gene expression is not fully understood. It was, therefore, the purpose of the present study to investigate whether alterations in gene expression of SL, SR and MF proteins occur during the development of intracellular Ca2+ overload in the myocardium.
Isolated heart reperfused with high concentrations of Ca2+ following a brief period of perfusion with Ca2+-free medium has been demonstrated to be an excellent model (Ca2+paradox [CP]) for investigating the effects of intracellular Ca2+overload [16-26]. We have reported that the inability of CP hearts to recover contractile function was associated with a marked increase in intracellular Ca2+, as well as the development of cardiac contracture, ultrastructural damage and subcellular defects [11,13,15,16,21,23]. In the present study, we examined whether hearts perfused with Ca2+-free medium followed by reperfusion with medium containing different concentrations of Ca2+ exhibit alterations in gene expression for SL Na+-K+ ATPase and Na+-Ca2+ exchanger, SR Ca2+-pump ATPase, Ca2+-release channel and phospholamban (PLB), as well as MF α- and β-myosin heavy chain proteins. Because subcellular remodelling in the failing heart is dependent on the balance between changes in gene expression and activities of proteolytic enzymes, such as calpain [27-33], messenger RNA (mRNA) levels for both calpain-1 and calpain-2 were measured in CP hearts. It may be noted that subcellular remodelling in failing hearts has also been suggested to be the result of activation of different proteolytic enzymes [27,28].
Methods
Male Sprague Dawley rats, each weighing 250 g to 300 g, were used in the present study. All experiments were conducted according to the protocol approved by the Animal Care Committee of the University of Manitoba (Winnipeg, Manitoba) as per guidelines established by the Canadian Council on Animal Care. Isolated hearts were perfused according to the Langendorff technique with Krebs-Henselite medium (gassed with 95% O2 and 5% CO2) containing 1.25 mM Ca2+ at 37°C for 20 min. The left ventricular developed pressure and the left ventricular end diastolic pressure (LVEDP) were recorded using a microtip catheter (Millar Instruments Inc, USA) and AcqKnowledge software (Biopac Systems, USA). After a stabilizing period, the hearts were perfused with Ca2+-free medium for 5 min and then reperfused with medium containing different concentrations of Ca2+ for 30 min. Control hearts were perfused for 35 min with normal medium containing 1.25 mM Ca2+. Methods for the perfusion of heart, recording of contractile parameters and induction of CP were similar to those used previously [22-26].
At the end of perfusion-reperfusion protocol, total RNA was extracted from the control and experimental left ventricular tissue using the guanidinium thiocyanate method [34]. mRNA levels for different subunits (α1, α2 and β-isoforms) of SL Na+-K+ ATPase and Na+- Ca2+-exchanger, SR SERCA2a (Ca2+-pump ATPase), ryanodine receptor (Ca2+-release channel) and PLB, as well as MF α-myosin heavy chain and β-myosin heavy chain proteins were measured using previously described Northern blotting techniques [33,35,36]. In one set of experiments, gene expression for calpain-1 and calpain-2 proteins was determined using real-time polymerase chain reaction techniques described previously (37). Values are presented as mean ± SE and were statistically evaluated using one-way ANOVA; differences between the control and experimental groups were considered to be statistically significant at P<0.05.
Results
Perfusion of hearts with Ca2+-free medium for 5 min resulted in an immediate loss of their ability to generate left ventricular developed pressure; no recovery of this function was observed on reperfusing these hearts with medium containing different concentrations of Ca2+ for a period of 30 min. In contrast, the LVEDP was increased three- to fourfold in hearts perfused with Ca2+-free medium; this elevated LVEDP was markedly augmented (eight- to 10-fold) on reperfusion with medium containing 100 μM to 1.25 mM (CP) Ca2+ (Table 1). No significant increase in the LVEDP was apparent when the 5 min Ca2+- free perfused hearts were reperfused for 30 min with either Ca2+-free medium or medium containing 30 μM Ca2+ (Table 1). Such augmentation of the LVEDP on reperfusion with medium containing 100 μM to 1.25 mM Ca2+ has been demonstrated to be due to the development of intracellular Ca2+ overload in hearts perfused with Ca2+-free medium [16,19,21].
| Concentration of Ca2+ in reperfusion medium | Perfusion in Ca2+-free medium for 5 min | Reperfusion with different Ca2+ concentrations for 30 min |
|---|---|---|
| Increase in lVeDP, mmHg | ||
| 1.25 mM (CP) | 25.2±2.6 | 78.5±5.1* |
| 0 µM | 28.5±2.2 | 29.3±2.0 |
| 30 µM | 31.1±3.8 | 32.2±1.9 |
| 100 µM | 25.3±2.3 | 60.7±4.3* |
| 300 µM | 29.1±2.1 | 85.3±6.5* |
LVEDP in hearts before initiating Ca2+-free perfusion varied between 6 mmHg and 8 mmHg. Controls were perfused for 35 min without subjecting to Ca2+- free medium whereas Ca2+-paradox (CP) hearts were subjected to 5 min of Ca2+-free medium followed by 30 min of reperfusion with normal medium containing 1.25 mM Ca2+. Data presented as mean ± SE of 5 to 7 experiments. *Statistically significant (P<0.05)
Table 1 effect of 30 min reperfusion with medium containing different concentrations of Ca2+ on the left ventricular end diastolic pressure (lVeDP) in isolated rat hearts following perfusion with Ca2+-free medium for 5 min
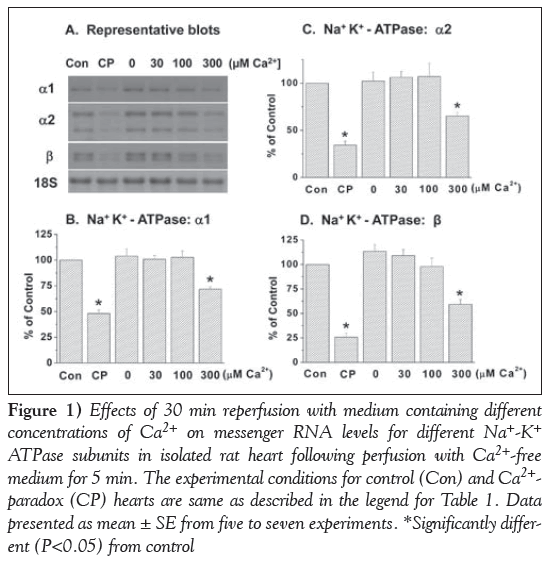
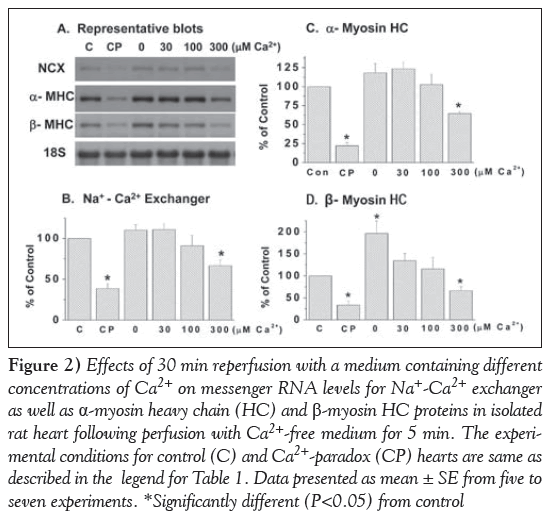
Reperfusion of the 5 min Ca2+-free perfused hearts with a medium containing 300 μM and 1.25 mM Ca2+ (CP) for 30 min produced varying degrees of depression in mRNA levels for different subunits (α1, α2 and β isoforms) of SL Na+-K+ ATPase (Figure 1). No changes in mRNA levels for different Na+-K+ ATPase isoforms were apparent when the Ca2+-free perfused hearts were reperfused with medium containing no Ca2+ or 30 μM and 100 μM Ca2+ (Figure 1). It is evident from Figure 2 that mRNA levels for SL Na+-Ca2+ exchanger were also depressed significantly on reperfusing the Ca2+-free perfused hearts with medium containing 300 μM and 1.25 mM Ca2+, unlike that in hearts reperfused with no Ca2+ or 30 μM and 100 μM Ca2+.
Figure 1: Effects of 30 min reperfusion with medium containing different concentrations of Ca2+ on messenger RNA levels for different Na+-K+ ATPase subunits in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+- paradox (CP) hearts are same as described in the legend for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
The data in Figure 2 show that reperfusing the 5 min Ca2+-free perfused hearts with medium containing 300 μM or 1.25 mM (CP) Ca2+ depressed mRNA levels for both α- and β-myosin heavy chain in MF proteins significantly. While mRNA levels for β-myosin heavy chain, unlike that for α-myosin heavy chain, was increased in hearts reperfused with Ca2+-free medium for 30 min, mRNA levels for both α- and β-myosin heavy chains were not altered significantly on reperfusion with medium containing 30 μM or 100 μM Ca2+ (Figure 2).
Figure 2: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for Na+-Ca2+ exchanger as well as α-myosin heavy chain (HC) and β-myosin HC proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (C) and Ca2+-paradox (CP) hearts are same as described in the legend for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
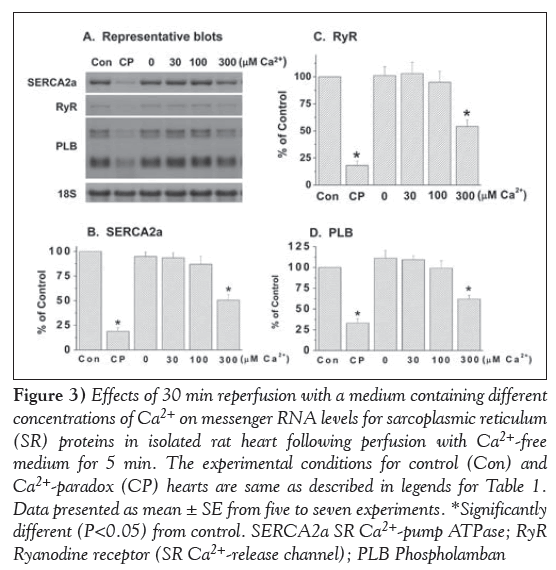
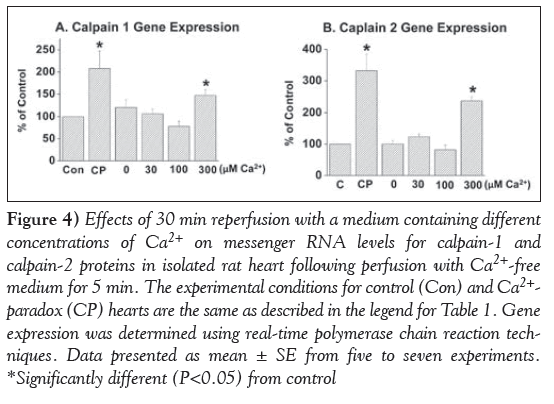
Marked depressions in mRNA levels for SR Ca2+-pump ATPase (SERCA2a), Ca2+-release channel (ryanodine receptor) and PLB proteins were observed in the 5 min Ca2+-free perfused hearts on reperfusion with medium containing 300 μM and 1.25 mM (CP) Ca2+ (Figure 3). In contrast, no significant alterations were observed in mRNA levels for the Ca2+-pump ATPase, Ca2+-release channel and PLB proteins on reperfusing the 5 min Ca2+-free perfused hearts with medium containing no Ca2+ or 30 μM and 100 μM Ca2+ (Figure 3). In another set of experiments, mRNA levels for calpain-1 and -2 proteins were determined in CP hearts. From the results in Figure 4, it can be observed that mRNA level for both calpain-1 and calpain-2 proteins were markedly elevated in the 5 min Ca2+-perfused hearts on reperfusion with medium containing 300 μM or 1.25 mM (CP) Ca2+ for 30 min. In contrast, no significant changes in mRNA levels for both calpain-1 and -2 proteins were observed on reperfusion with medium containing no Ca2+, or 30 μM and 100 μM Ca2+ (Figure 4).
Figure 3: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for sarcoplasmic reticulum (SR) proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+-paradox (CP) hearts are same as described in legends for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control. SERCA2a SR Ca2+-pump ATPase; RyR Ryanodine receptor (SR Ca2+-release channel); PLB Phospholamban
Figure 4: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for calpain-1 and calpain-2 proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+- paradox (CP) hearts are the same as described in the legend for Table 1. Gene expression was determined using real-time olymerase chain reaction techniques. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
Discussion
We have shown a marked increase in LVEDP and dramatic depressions in mRNA levels for SL Na+-K+ ATPase (different isoforms) and Na+- Ca2+ exchanger, MF α- and β-isoforms of myosin heavy chain, SR Ca2+-pump ATPase, Ca2+-release channel and PLB, as well as calpain-1 and -2 proteins in hearts on reperfusion with 1.25 mM Ca2+ for 30 min following perfusion with Ca2+-free medium for 5 min. Because the magnitude of changes in cardiac LVEDP and gene expression were found to be dependent on the concentration of Ca2+ in the reperfusion medium, it is likely that the observed alterations in cardiac gene expression in hearts subjected to CP are due to the development of intracellular Ca2+ overload. This view is supported by the fact that a marked cardiac contracture was demonstrated to be intimately associated with a marked increase in the intracellular Ca2+ content in the CP heart [16]. Varying degrees of alterations in functional and biochemical activities of SL, SR and MF activities have also been shown to occur in CP hearts as a consequence of intracellular Ca2+ overload [13-15,17,21-23]. Although elevated levels of intracellular Ca2+ have been observed to activate proteases, such as calpains, directly the observed increase in mRNA levels for both calpain-1 and -2 proteins may also contribute to increased calpain activity in hearts under different pathological conditions associated with the occurrence of intracellular Ca2+ overload [27-31]. Thus, it appears that the observed depressions in SL, SR and MF gene expression, as well as the increased gene expression for calpains due to intracellular Ca2+ overload, may account for remodelling of subcellular organelles observed in the hearts subjected to CP.
Although perfusing the hearts for 35 min with no Ca2+ was observed to significantly increase LVEDP, no depression in cardiac gene expression for different subcellular proteins was observed under this experimental condition. In fact, mRNA levels for β-myosin heavy chain were increased on perfusing the heart with no Ca2+ for 35 min. It appears that a critical level of LVEDP needs to be achieved for the occurrence of intracellular Ca2+ overload for inducing depression in cardiac gene expression in hearts subjected to CP. It should be emphasized that there are varying degrees of depression in SL, SR and MF gene expression, protein content and functional activities in hearts subjected to ischemia-reperfusion and these alterations have been attributed to the occurrence of intracellular Ca2+ overload [7-9,31-36,38]. Furthermore, intracellular Ca2+ overload has been suggested to play a critical role in the development of alterations in SL, SR and MF gene expression, protein content and functional activities in hearts failing due to myocardial infarction (1-6). Although the exact mechanisms by which intracellular Ca2+ overload induces changes in cardiac gene expression and subsequent subcellular remodelling are not clear at present, a marked increase in the production of different cytokines including tumour necrosis factor and the activation of nuclear factor-kB have been observed in the CP heart [24,39]. Furthermore, the role of various microRNAs and other transcription factors, which are known to regulate cardiac gene expression [40], needs to be investigated in hearts on inducing intracellular Ca2+ overload. Nonetheless, the experiments performed in the present study provide compelling evidence that intracellular Ca2+ overload is a potential mediator of inducing defects in gene expression and subsequent subcellular remodelling and cardiac dysfunction.
Acknowledgements
This study was supported by a grant from the Canadian Institutes of Health Research. The infrastructural support was provided by the St Boniface Hospital Research Foundation. Dr AT Ozcelikay was a visiting professor from the Ankara University (Ankara, Turkey). His present address is at Department of Pharmacology, Faculty of Pharmacy, Ankara University, Turkey.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Dhalla NS, Dent MR, Tappia PS, Sethi R, Barta J, Goyal RK. Subcellular remodeling as a viable target for the treatment of congestive heart failure. J Cardiovasc Pharmacol Therapeut 2006;11:31-45.
- Dhalla NS, Saini-Chohan HK, Rodriguez-Leyva D, Elimban V, Dent MR, Tappia PS. Subcellular remodeling may induce cardiac dysfunction in congestive heart failure. Cardiovasc Res 2009;81:429-38.
- Machackova J, Sanganalmath SK, Barta J, Dhalla KS, Dhalla NS. Amelioration of cardiac remodeling in congestive heart failure by β-adrenoceptor blockade is associated with depression in sympathetic activity. Cardiovasc Toxicol 2010;10:9-16.
- Shao Q, Ren B, Elimban V, Tappia PS, Takeda N, Dhalla NS. Modification of sarcolemmal Na+-K+-ATPase and Na+/Ca2+ exchanger expression in heart failure by blockade of reninangiotensin system. Am J Physiol Heart Circ Physiol 2005;288:H2637-46.
- Shao Q, Ren B, Saini HK, Netticadan T, Takeda N, Dhalla NS. Sarcoplasmic reticulum Ca2+-transport and gene expression in congestive heart failure are modified by imidapril treatment. Am J Physiol Heart Circ Physiol 2005;288:H1674-82.
- Wang J, Liu X, Ren B, Rupp H, Takeda N, Dhalla NS. Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. J Mol Cell Cardiol 2002;34:847-57.
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertension 2000;18:655-73.
- Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia reperfusion injury. Cardiovasc Res 2000;47:446-56.
- Dhalla NS, Saini HK, Tappia PS, Sethi R, Mengi SA, Gupta SK. Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 2007;8:238-50.
- Zimmerman AN, Daems W, Hulsmann WC, Sinjder J, Wisse E, Durrer D. Morphological changes of heart muscle caused by successive perfusion with calcium-free and calcium-containing solutions (calcium paradox). Cardiovasc Res 1967;1:201-9.
- Yates JC, Dhalla NS. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca++-free medium. J Mol Cell Cardiol 1975;7:91-103.
- Chapman RA, Suleiman MS, Rodrigo GC, Tunstall J. The calcium paradox: A role for [Na]i, a cellular or tissue basis, a property unique to the Langendorff perfused heart? A bundle of contradictions. J Mol Cell Cardiol 1991;23:773-7.
- Dhalla NS, Singh JN, McNamara DB, Bernatsky A, Singh A, Harrow JA. Energy production and utilization in contractile failure due to intracellular calcium overload. Adv Exp Med Biol 1983;161:305-16.
- Persad S, Vrbanova A, Meij JT, Panagia V, Dhalla NS. Possible role of phospholipase C in the induction of Ca2+-paradox in rat heart. Mol Cell Biochem 1993;121:181-90.
- Dhalla NS, Alto LE, Singal PK. Role of Na+-Ca2+ exchange in the development of cardiac abnormalities due to calcium paradox. Eur Heart J 1983;4(Suppl):51-6.
- Alto LE, Dhalla NS. Myocardial cation contents during induction of the calcium paradox. Am J Physiol 1979;237:H713-9.
- Persad S, Gupta KK, Dhalla NS. Status of Ca2+ channels in hearts perfused with Ca2+ free medium as well as upon reperfusion (Ca2+-paradox). J Mol Cell Cardiol 1995;27:513-22.
- Gurinieri JJ. Decrease in the transmembrane sodium activity gradient in ferret papillary muscle as a prerequisite to the calcium paradox. J Clin Invest 1988;81:1938-44.
- Makino N, Panagia V, Gupta MP, Dhalla NS. Defects in sarcolemmal Ca2+ transport in hearts due to induction of calcium paradox. Circ Res 1988;63:313-21.
- Lamers JMJ, Ruigrok TJ. Diminished Na+/K+ and Ca2+ pump activities in the Ca2+ depleted heart: Possible role in the development of Ca2+ overload during Ca2+ paradox. Eur Heart J 1983;4 (Suppl H):73-9.
- Alto LE, Dhalla NS. Role of changes in microsomal calcium uptake in the effects of reperfusion of Ca2+-deprived rat hearts. Circ Res 1981;48:17-24.
- Alto LE, Elimban V, Dhalla NS. Alterations in sarcolemmal Ca2+/Mg2+ ecto-ATPase activity in hearts subjected to calcium paradox. Exp Clin Cardiol 1999;4:29-34.
- Alto LE, Elimban V, Lukas A, Dhalla NS. Modification of heart sarcolemmal Na+-K+ ATPase activity during development of the calcium paradox. Mol Cell Biochem 2000;207:87-94.
- Zhang M, Xu Y-J, Saini HK, Turan B, Liu PP, Dhalla NS. TNF-α as a potential mediator of cardiac dysfunction due to intracellular Ca2+-overload. Biochem Biophys Res Commun 2005;327:57-63.
- Kawabata K, Netticadan T, Osada M, Tamura K, Dhalla NS. Mechanisms of ischemic preconditioning effects on Ca2+-paradox induced changes in the heart. Am J Physiol Heart Circ Physiol 2000;278:H1008-15.
- Makazan Z, Saini-Chohan HK, Dhalla NS. Mitochondrial oxidative phosphorylation in hearts subjected to Ca2+-depletion and Ca2+-repletion. Can J Physiol Pharmacol 2009;87:789-97.
- Müller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 2012;17:395-409.
- Müller AL, Hryshko LV, Dhalla NS. Extracellular and intracellular proteases in cardiac dysfunction due to ischemia-reperfusion injury. Int J Cardiol 2013;164:39-47.
- Singh RB, Dhalla NS. Ischemia reperfusion-induced changes insarcolemmal Na+-K+ ATPase are due to the activation of calpain in the heart. Can J Physiol Pharmacol 2010;88:388-97.
- Singh RB, Hryshko L, Freed D, Dhalla NS. Activation of proteolytic enzymes and depression of the sarcolemmal Na+-K+ ATPase in ischemia-reperfused heart may be mediated through oxidative stress. Can J Physiol Pharmacol 2012;90:249-60.
- Müller AL, Freed D, Dhalla NS. Activation of proteases and changes in Na+-K+ ATPase subunits in hearts subjected to ischemia-reperfusion. J Appl Physiol 2013;114:351-60.
- Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemicreperfused heart. J Mol Cell Cardiol 2004;37:101-10.
- Maddika S, Elimban V, Chapman D, Dhalla NS. Role of oxidative stress in ischemia-reperfusion induced alterations in myofibrillar ATPase activities and gene expression in the heart. Can J Physiol Pharmacol 2009;87:120-9.
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156-9.
- Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemia-reperfused rat heart. Am J Physiol 1999;277:H584-94.
- Ostadal P, Elmoselhi AB, Zdobnicka I, Lukas A, Chapman D, Dhalla NS. Ischemia-reperfusion alters gene expression of Na+-K+ ATPase isoforms in rat heart. Biochem Biophys Res Commun 2003;306:457-62.
- Singal T, Dhalla NS, Tappia PS. Regulation of c-Fos and c-Jun gene expression by phospholipase C in adult cardiomyocytes. Mol Cell Biochem 2009;327:229-39.
- Saini HK, Dhalla NS. Defective calcium handling in cardiomyocytes isolated from hearts subjected to ischemiareperfusion. Am J Physiol Heart Circ Physiol 2005;288:H2260-70.
- Saini HK, Xu Y-J, Zhang M, Liu PP, Kirshenbaum LA, Dhalla NS. Role of tumor necrosis factor-alpha and other cytokines in ischemia-reperfusion induced injury in the heart. Exp Clin Cardiol 2005;10:213-22.
- Condorelli G, Latronico MVG, Cavarretta E. MicroRNAs in cardiovascular diseases. J Am Coll Cardiol 2014;63:2177-87.
- *Corresponding Author:
- Dr Naranjan S Dhalla
Institute of Cardiovascular Sciences, St Boniface Hospital Research, 351 Tache Avenue, Winnipeg, Manitoba R2H 2A6
Telephone: 204-235-3417
Fax: 204-237-0347
E-mail: nsdhalla@sbrc.ca
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact support@pulsus.com
Abstract
OBJECTIVES: Although intracellular Ca2+ overload is believed to cause cardiac abnormalities and subcellular remodelling, its role in inducing alterations in cardiac gene expression has not been investigated.
METHODS: Intracellular Ca2+ overload was induced in isolated rat hearts on perfusion with Ca2+-free medium for 5 min followed by reperfusion with medium containing different concentrations of Ca2+ for 30 min (Ca2+paradox). Changes in messenger RNA levels for various subcellular proteins were monitored either by Northern blotting or real-time polymerase chain reaction techniques.
RESULTS: Marked depressions in gene expression for sarcolemma Na+- K+-ATPase and Na+-Ca2+ exchanger, sarcoplasmic reticulum Ca2+-pump ATPase, Ca2+ release channel and phospholamban, as well as myofibrillar α- and β-myosin heavy chain proteins were observed in hearts reperfused with 1.25 mM Ca2+ following perfusion with Ca2+-free medium. In contrast, messenger RNA levels for calpain-1 and -2 proteins were elevated in hearts subjected to Ca2+paradox. These changes were dependent on the concentration of Ca2+ in the reperfusion medium.
CONCLUSIONS: The results suggest that intracellular Ca2+ overload is an important factor in the induction of defects in gene expression, subcellular remodelling and cardiac dysfunction in heart disease.
-Keywords
Cardiac gene expression; Ca2+-pump ATPase; Ca2+-release channel; Intracellular Ca2+ overload; Myosin heavy chains; Na+-K+-ATPase; Na+-Ca2+ exchanger
Normal expression of cardiac genes for Ca2+transport and contractile proteins is known to play a critical role in the maintenance of the function of subcellular organelles such as sarcolemma (SL), sarcoplasmic reticulum (SR) and myofibrils (MFs) in cardiomyocytes. Previous studies have revealed varying degrees of depression in gene expression for SL, SR and MF proteins, as well as defects in subcellular organelles during the development of heart failure in patients and experimental animal models [1-6]. Accordingly, it has been suggested that cardiac dysfunction in failing hearts is due to subcellular remodelling as a consequence of changes in cardiac gene expression (2,7-9). Although the occurrence of intracellular Ca2+ overload is believed to be intimately involved in the genesis of cardiac dysfunction in heart failure [10-15], its role in inducing defects in cardiac gene expression is not fully understood. It was, therefore, the purpose of the present study to investigate whether alterations in gene expression of SL, SR and MF proteins occur during the development of intracellular Ca2+ overload in the myocardium.
Isolated heart reperfused with high concentrations of Ca2+ following a brief period of perfusion with Ca2+-free medium has been demonstrated to be an excellent model (Ca2+paradox [CP]) for investigating the effects of intracellular Ca2+overload [16-26]. We have reported that the inability of CP hearts to recover contractile function was associated with a marked increase in intracellular Ca2+, as well as the development of cardiac contracture, ultrastructural damage and subcellular defects [11,13,15,16,21,23]. In the present study, we examined whether hearts perfused with Ca2+-free medium followed by reperfusion with medium containing different concentrations of Ca2+ exhibit alterations in gene expression for SL Na+-K+ ATPase and Na+-Ca2+ exchanger, SR Ca2+-pump ATPase, Ca2+-release channel and phospholamban (PLB), as well as MF α- and β-myosin heavy chain proteins. Because subcellular remodelling in the failing heart is dependent on the balance between changes in gene expression and activities of proteolytic enzymes, such as calpain [27-33], messenger RNA (mRNA) levels for both calpain-1 and calpain-2 were measured in CP hearts. It may be noted that subcellular remodelling in failing hearts has also been suggested to be the result of activation of different proteolytic enzymes [27,28].
Methods
Male Sprague Dawley rats, each weighing 250 g to 300 g, were used in the present study. All experiments were conducted according to the protocol approved by the Animal Care Committee of the University of Manitoba (Winnipeg, Manitoba) as per guidelines established by the Canadian Council on Animal Care. Isolated hearts were perfused according to the Langendorff technique with Krebs-Henselite medium (gassed with 95% O2 and 5% CO2) containing 1.25 mM Ca2+ at 37°C for 20 min. The left ventricular developed pressure and the left ventricular end diastolic pressure (LVEDP) were recorded using a microtip catheter (Millar Instruments Inc, USA) and AcqKnowledge software (Biopac Systems, USA). After a stabilizing period, the hearts were perfused with Ca2+-free medium for 5 min and then reperfused with medium containing different concentrations of Ca2+ for 30 min. Control hearts were perfused for 35 min with normal medium containing 1.25 mM Ca2+. Methods for the perfusion of heart, recording of contractile parameters and induction of CP were similar to those used previously [22-26].
At the end of perfusion-reperfusion protocol, total RNA was extracted from the control and experimental left ventricular tissue using the guanidinium thiocyanate method [34]. mRNA levels for different subunits (α1, α2 and β-isoforms) of SL Na+-K+ ATPase and Na+- Ca2+-exchanger, SR SERCA2a (Ca2+-pump ATPase), ryanodine receptor (Ca2+-release channel) and PLB, as well as MF α-myosin heavy chain and β-myosin heavy chain proteins were measured using previously described Northern blotting techniques [33,35,36]. In one set of experiments, gene expression for calpain-1 and calpain-2 proteins was determined using real-time polymerase chain reaction techniques described previously (37). Values are presented as mean ± SE and were statistically evaluated using one-way ANOVA; differences between the control and experimental groups were considered to be statistically significant at P<0.05.
Results
Perfusion of hearts with Ca2+-free medium for 5 min resulted in an immediate loss of their ability to generate left ventricular developed pressure; no recovery of this function was observed on reperfusing these hearts with medium containing different concentrations of Ca2+ for a period of 30 min. In contrast, the LVEDP was increased three- to fourfold in hearts perfused with Ca2+-free medium; this elevated LVEDP was markedly augmented (eight- to 10-fold) on reperfusion with medium containing 100 μM to 1.25 mM (CP) Ca2+ (Table 1). No significant increase in the LVEDP was apparent when the 5 min Ca2+- free perfused hearts were reperfused for 30 min with either Ca2+-free medium or medium containing 30 μM Ca2+ (Table 1). Such augmentation of the LVEDP on reperfusion with medium containing 100 μM to 1.25 mM Ca2+ has been demonstrated to be due to the development of intracellular Ca2+ overload in hearts perfused with Ca2+-free medium [16,19,21].
| Concentration of Ca2+ in reperfusion medium | Perfusion in Ca2+-free medium for 5 min | Reperfusion with different Ca2+ concentrations for 30 min |
|---|---|---|
| Increase in lVeDP, mmHg | ||
| 1.25 mM (CP) | 25.2±2.6 | 78.5±5.1* |
| 0 µM | 28.5±2.2 | 29.3±2.0 |
| 30 µM | 31.1±3.8 | 32.2±1.9 |
| 100 µM | 25.3±2.3 | 60.7±4.3* |
| 300 µM | 29.1±2.1 | 85.3±6.5* |
LVEDP in hearts before initiating Ca2+-free perfusion varied between 6 mmHg and 8 mmHg. Controls were perfused for 35 min without subjecting to Ca2+- free medium whereas Ca2+-paradox (CP) hearts were subjected to 5 min of Ca2+-free medium followed by 30 min of reperfusion with normal medium containing 1.25 mM Ca2+. Data presented as mean ± SE of 5 to 7 experiments. *Statistically significant (P<0.05)
Table 1 effect of 30 min reperfusion with medium containing different concentrations of Ca2+ on the left ventricular end diastolic pressure (lVeDP) in isolated rat hearts following perfusion with Ca2+-free medium for 5 min
Reperfusion of the 5 min Ca2+-free perfused hearts with a medium containing 300 μM and 1.25 mM Ca2+ (CP) for 30 min produced varying degrees of depression in mRNA levels for different subunits (α1, α2 and β isoforms) of SL Na+-K+ ATPase (Figure 1). No changes in mRNA levels for different Na+-K+ ATPase isoforms were apparent when the Ca2+-free perfused hearts were reperfused with medium containing no Ca2+ or 30 μM and 100 μM Ca2+ (Figure 1). It is evident from Figure 2 that mRNA levels for SL Na+-Ca2+ exchanger were also depressed significantly on reperfusing the Ca2+-free perfused hearts with medium containing 300 μM and 1.25 mM Ca2+, unlike that in hearts reperfused with no Ca2+ or 30 μM and 100 μM Ca2+.
Figure 1: Effects of 30 min reperfusion with medium containing different concentrations of Ca2+ on messenger RNA levels for different Na+-K+ ATPase subunits in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+- paradox (CP) hearts are same as described in the legend for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
The data in Figure 2 show that reperfusing the 5 min Ca2+-free perfused hearts with medium containing 300 μM or 1.25 mM (CP) Ca2+ depressed mRNA levels for both α- and β-myosin heavy chain in MF proteins significantly. While mRNA levels for β-myosin heavy chain, unlike that for α-myosin heavy chain, was increased in hearts reperfused with Ca2+-free medium for 30 min, mRNA levels for both α- and β-myosin heavy chains were not altered significantly on reperfusion with medium containing 30 μM or 100 μM Ca2+ (Figure 2).
Figure 2: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for Na+-Ca2+ exchanger as well as α-myosin heavy chain (HC) and β-myosin HC proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (C) and Ca2+-paradox (CP) hearts are same as described in the legend for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
Marked depressions in mRNA levels for SR Ca2+-pump ATPase (SERCA2a), Ca2+-release channel (ryanodine receptor) and PLB proteins were observed in the 5 min Ca2+-free perfused hearts on reperfusion with medium containing 300 μM and 1.25 mM (CP) Ca2+ (Figure 3). In contrast, no significant alterations were observed in mRNA levels for the Ca2+-pump ATPase, Ca2+-release channel and PLB proteins on reperfusing the 5 min Ca2+-free perfused hearts with medium containing no Ca2+ or 30 μM and 100 μM Ca2+ (Figure 3). In another set of experiments, mRNA levels for calpain-1 and -2 proteins were determined in CP hearts. From the results in Figure 4, it can be observed that mRNA level for both calpain-1 and calpain-2 proteins were markedly elevated in the 5 min Ca2+-perfused hearts on reperfusion with medium containing 300 μM or 1.25 mM (CP) Ca2+ for 30 min. In contrast, no significant changes in mRNA levels for both calpain-1 and -2 proteins were observed on reperfusion with medium containing no Ca2+, or 30 μM and 100 μM Ca2+ (Figure 4).
Figure 3: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for sarcoplasmic reticulum (SR) proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+-paradox (CP) hearts are same as described in legends for Table 1. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control. SERCA2a SR Ca2+-pump ATPase; RyR Ryanodine receptor (SR Ca2+-release channel); PLB Phospholamban
Figure 4: Effects of 30 min reperfusion with a medium containing different concentrations of Ca2+ on messenger RNA levels for calpain-1 and calpain-2 proteins in isolated rat heart following perfusion with Ca2+-free medium for 5 min. The experimental conditions for control (Con) and Ca2+- paradox (CP) hearts are the same as described in the legend for Table 1. Gene expression was determined using real-time olymerase chain reaction techniques. Data presented as mean ± SE from five to seven experiments. *Significantly different (P<0.05) from control
Discussion
We have shown a marked increase in LVEDP and dramatic depressions in mRNA levels for SL Na+-K+ ATPase (different isoforms) and Na+- Ca2+ exchanger, MF α- and β-isoforms of myosin heavy chain, SR Ca2+-pump ATPase, Ca2+-release channel and PLB, as well as calpain-1 and -2 proteins in hearts on reperfusion with 1.25 mM Ca2+ for 30 min following perfusion with Ca2+-free medium for 5 min. Because the magnitude of changes in cardiac LVEDP and gene expression were found to be dependent on the concentration of Ca2+ in the reperfusion medium, it is likely that the observed alterations in cardiac gene expression in hearts subjected to CP are due to the development of intracellular Ca2+ overload. This view is supported by the fact that a marked cardiac contracture was demonstrated to be intimately associated with a marked increase in the intracellular Ca2+ content in the CP heart [16]. Varying degrees of alterations in functional and biochemical activities of SL, SR and MF activities have also been shown to occur in CP hearts as a consequence of intracellular Ca2+ overload [13-15,17,21-23]. Although elevated levels of intracellular Ca2+ have been observed to activate proteases, such as calpains, directly the observed increase in mRNA levels for both calpain-1 and -2 proteins may also contribute to increased calpain activity in hearts under different pathological conditions associated with the occurrence of intracellular Ca2+ overload [27-31]. Thus, it appears that the observed depressions in SL, SR and MF gene expression, as well as the increased gene expression for calpains due to intracellular Ca2+ overload, may account for remodelling of subcellular organelles observed in the hearts subjected to CP.
Although perfusing the hearts for 35 min with no Ca2+ was observed to significantly increase LVEDP, no depression in cardiac gene expression for different subcellular proteins was observed under this experimental condition. In fact, mRNA levels for β-myosin heavy chain were increased on perfusing the heart with no Ca2+ for 35 min. It appears that a critical level of LVEDP needs to be achieved for the occurrence of intracellular Ca2+ overload for inducing depression in cardiac gene expression in hearts subjected to CP. It should be emphasized that there are varying degrees of depression in SL, SR and MF gene expression, protein content and functional activities in hearts subjected to ischemia-reperfusion and these alterations have been attributed to the occurrence of intracellular Ca2+ overload [7-9,31-36,38]. Furthermore, intracellular Ca2+ overload has been suggested to play a critical role in the development of alterations in SL, SR and MF gene expression, protein content and functional activities in hearts failing due to myocardial infarction (1-6). Although the exact mechanisms by which intracellular Ca2+ overload induces changes in cardiac gene expression and subsequent subcellular remodelling are not clear at present, a marked increase in the production of different cytokines including tumour necrosis factor and the activation of nuclear factor-kB have been observed in the CP heart [24,39]. Furthermore, the role of various microRNAs and other transcription factors, which are known to regulate cardiac gene expression [40], needs to be investigated in hearts on inducing intracellular Ca2+ overload. Nonetheless, the experiments performed in the present study provide compelling evidence that intracellular Ca2+ overload is a potential mediator of inducing defects in gene expression and subsequent subcellular remodelling and cardiac dysfunction.
Acknowledgements
This study was supported by a grant from the Canadian Institutes of Health Research. The infrastructural support was provided by the St Boniface Hospital Research Foundation. Dr AT Ozcelikay was a visiting professor from the Ankara University (Ankara, Turkey). His present address is at Department of Pharmacology, Faculty of Pharmacy, Ankara University, Turkey.
Disclosures
The authors have no financial disclosures or conflicts of interest to declare.
References
- Dhalla NS, Dent MR, Tappia PS, Sethi R, Barta J, Goyal RK. Subcellular remodeling as a viable target for the treatment of congestive heart failure. J Cardiovasc Pharmacol Therapeut 2006;11:31-45.
- Dhalla NS, Saini-Chohan HK, Rodriguez-Leyva D, Elimban V, Dent MR, Tappia PS. Subcellular remodeling may induce cardiac dysfunction in congestive heart failure. Cardiovasc Res 2009;81:429-38.
- Machackova J, Sanganalmath SK, Barta J, Dhalla KS, Dhalla NS. Amelioration of cardiac remodeling in congestive heart failure by β-adrenoceptor blockade is associated with depression in sympathetic activity. Cardiovasc Toxicol 2010;10:9-16.
- Shao Q, Ren B, Elimban V, Tappia PS, Takeda N, Dhalla NS. Modification of sarcolemmal Na+-K+-ATPase and Na+/Ca2+ exchanger expression in heart failure by blockade of reninangiotensin system. Am J Physiol Heart Circ Physiol 2005;288:H2637-46.
- Shao Q, Ren B, Saini HK, Netticadan T, Takeda N, Dhalla NS. Sarcoplasmic reticulum Ca2+-transport and gene expression in congestive heart failure are modified by imidapril treatment. Am J Physiol Heart Circ Physiol 2005;288:H1674-82.
- Wang J, Liu X, Ren B, Rupp H, Takeda N, Dhalla NS. Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. J Mol Cell Cardiol 2002;34:847-57.
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertension 2000;18:655-73.
- Dhalla NS, Elmoselhi AB, Hata T, Makino N. Status of myocardial antioxidants in ischemia reperfusion injury. Cardiovasc Res 2000;47:446-56.
- Dhalla NS, Saini HK, Tappia PS, Sethi R, Mengi SA, Gupta SK. Potential role and mechanisms of subcellular remodeling in cardiac dysfunction due to ischemic heart disease. J Cardiovasc Med 2007;8:238-50.
- Zimmerman AN, Daems W, Hulsmann WC, Sinjder J, Wisse E, Durrer D. Morphological changes of heart muscle caused by successive perfusion with calcium-free and calcium-containing solutions (calcium paradox). Cardiovasc Res 1967;1:201-9.
- Yates JC, Dhalla NS. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca++-free medium. J Mol Cell Cardiol 1975;7:91-103.
- Chapman RA, Suleiman MS, Rodrigo GC, Tunstall J. The calcium paradox: A role for [Na]i, a cellular or tissue basis, a property unique to the Langendorff perfused heart? A bundle of contradictions. J Mol Cell Cardiol 1991;23:773-7.
- Dhalla NS, Singh JN, McNamara DB, Bernatsky A, Singh A, Harrow JA. Energy production and utilization in contractile failure due to intracellular calcium overload. Adv Exp Med Biol 1983;161:305-16.
- Persad S, Vrbanova A, Meij JT, Panagia V, Dhalla NS. Possible role of phospholipase C in the induction of Ca2+-paradox in rat heart. Mol Cell Biochem 1993;121:181-90.
- Dhalla NS, Alto LE, Singal PK. Role of Na+-Ca2+ exchange in the development of cardiac abnormalities due to calcium paradox. Eur Heart J 1983;4(Suppl):51-6.
- Alto LE, Dhalla NS. Myocardial cation contents during induction of the calcium paradox. Am J Physiol 1979;237:H713-9.
- Persad S, Gupta KK, Dhalla NS. Status of Ca2+ channels in hearts perfused with Ca2+ free medium as well as upon reperfusion (Ca2+-paradox). J Mol Cell Cardiol 1995;27:513-22.
- Gurinieri JJ. Decrease in the transmembrane sodium activity gradient in ferret papillary muscle as a prerequisite to the calcium paradox. J Clin Invest 1988;81:1938-44.
- Makino N, Panagia V, Gupta MP, Dhalla NS. Defects in sarcolemmal Ca2+ transport in hearts due to induction of calcium paradox. Circ Res 1988;63:313-21.
- Lamers JMJ, Ruigrok TJ. Diminished Na+/K+ and Ca2+ pump activities in the Ca2+ depleted heart: Possible role in the development of Ca2+ overload during Ca2+ paradox. Eur Heart J 1983;4 (Suppl H):73-9.
- Alto LE, Dhalla NS. Role of changes in microsomal calcium uptake in the effects of reperfusion of Ca2+-deprived rat hearts. Circ Res 1981;48:17-24.
- Alto LE, Elimban V, Dhalla NS. Alterations in sarcolemmal Ca2+/Mg2+ ecto-ATPase activity in hearts subjected to calcium paradox. Exp Clin Cardiol 1999;4:29-34.
- Alto LE, Elimban V, Lukas A, Dhalla NS. Modification of heart sarcolemmal Na+-K+ ATPase activity during development of the calcium paradox. Mol Cell Biochem 2000;207:87-94.
- Zhang M, Xu Y-J, Saini HK, Turan B, Liu PP, Dhalla NS. TNF-α as a potential mediator of cardiac dysfunction due to intracellular Ca2+-overload. Biochem Biophys Res Commun 2005;327:57-63.
- Kawabata K, Netticadan T, Osada M, Tamura K, Dhalla NS. Mechanisms of ischemic preconditioning effects on Ca2+-paradox induced changes in the heart. Am J Physiol Heart Circ Physiol 2000;278:H1008-15.
- Makazan Z, Saini-Chohan HK, Dhalla NS. Mitochondrial oxidative phosphorylation in hearts subjected to Ca2+-depletion and Ca2+-repletion. Can J Physiol Pharmacol 2009;87:789-97.
- Müller AL, Dhalla NS. Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 2012;17:395-409.
- Müller AL, Hryshko LV, Dhalla NS. Extracellular and intracellular proteases in cardiac dysfunction due to ischemia-reperfusion injury. Int J Cardiol 2013;164:39-47.
- Singh RB, Dhalla NS. Ischemia reperfusion-induced changes insarcolemmal Na+-K+ ATPase are due to the activation of calpain in the heart. Can J Physiol Pharmacol 2010;88:388-97.
- Singh RB, Hryshko L, Freed D, Dhalla NS. Activation of proteolytic enzymes and depression of the sarcolemmal Na+-K+ ATPase in ischemia-reperfused heart may be mediated through oxidative stress. Can J Physiol Pharmacol 2012;90:249-60.
- Müller AL, Freed D, Dhalla NS. Activation of proteases and changes in Na+-K+ ATPase subunits in hearts subjected to ischemia-reperfusion. J Appl Physiol 2013;114:351-60.
- Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemicreperfused heart. J Mol Cell Cardiol 2004;37:101-10.
- Maddika S, Elimban V, Chapman D, Dhalla NS. Role of oxidative stress in ischemia-reperfusion induced alterations in myofibrillar ATPase activities and gene expression in the heart. Can J Physiol Pharmacol 2009;87:120-9.
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987;162:156-9.
- Temsah RM, Netticadan T, Chapman D, Takeda S, Mochizuki S, Dhalla NS. Alterations in sarcoplasmic reticulum function and gene expression in ischemia-reperfused rat heart. Am J Physiol 1999;277:H584-94.
- Ostadal P, Elmoselhi AB, Zdobnicka I, Lukas A, Chapman D, Dhalla NS. Ischemia-reperfusion alters gene expression of Na+-K+ ATPase isoforms in rat heart. Biochem Biophys Res Commun 2003;306:457-62.
- Singal T, Dhalla NS, Tappia PS. Regulation of c-Fos and c-Jun gene expression by phospholipase C in adult cardiomyocytes. Mol Cell Biochem 2009;327:229-39.
- Saini HK, Dhalla NS. Defective calcium handling in cardiomyocytes isolated from hearts subjected to ischemiareperfusion. Am J Physiol Heart Circ Physiol 2005;288:H2260-70.
- Saini HK, Xu Y-J, Zhang M, Liu PP, Kirshenbaum LA, Dhalla NS. Role of tumor necrosis factor-alpha and other cytokines in ischemia-reperfusion induced injury in the heart. Exp Clin Cardiol 2005;10:213-22.
- Condorelli G, Latronico MVG, Cavarretta E. MicroRNAs in cardiovascular diseases. J Am Coll Cardiol 2014;63:2177-87.