Sap and sac-mucinous cystic neoplasm -pancreas
Received: 03-Mar-2023, Manuscript No. puljcdt-23-6268; Editor assigned: 05-Mar-2023, Pre QC No. puljcdt-23-6268(PQ); Accepted Date: Mar 26, 2023; Reviewed: 13-Mar-2023 QC No. puljcdt-23-6268(Q); Revised: 22-Mar-2023, Manuscript No. puljcdt-23-6268(R); Published: 30-Mar-2023, DOI: 10.37532/puljcdt.23.5(1).1-3
Citation: Bajaj A. Sap and sac-mucinous cystic neoplasm-Pancreas J. clin. diagn. Treat. 2023; 5(1):1-3
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Mucinous cystic neoplasm pancreas is a benign or low grade, potentially malignant, cystic neoplasm arising from pancreatic epithelial layer constituted of epithelial cells containing intracytoplasmic mucin World Health Organization (WHO) classifies mucinous cystic neoplasm pancreas as. a) Mucinous Cystic Neoplasm (MCN) with low grade dysplasia or adenoma. b) Mucinous Cystic Neoplasm (MCN) with high grade dysplasia or carcinoma in situ. c) Mucinous Cystic Neoplasm (MCN) with invasive carcinoma.
Key Words
Adenocarcinoma, Cholecystitis, Mucinous cystic neoplasm , prediabetes.
Introduction
Mucinous cystic neoplasm is a tumorous lesion which manifests as a precursor to pancreatic adenocarcinoma. The neoplasm may harbour foci of invasive carcinoma. Generally, ovarian type stroma is associated with the tumefaction [1, 2].
Of obscure aetiology, mucinous cystic neoplasm is posited to arise from ectopic ovarian stroma which emerges from primordial ovarian cells discerned within preliminary stages of embryonic development.
The cyst gradually evolves on account of hormones and growth factors secreted by ovarian stroma. Mucinous cystic neoplasm predominantly appears within female subjects (>95%). Mean age of disease emergence is 45 years.
Majority (>95%) of mucinous cystic neoplasms arise within distal pancreas although lesions may be discerned within hepatic parenchyma or gallbladder. Tumour metastases is commonly restricted to abdominal cavity wherein metastases into ovary may simulate primary ovarian tumour [2,3].
In situ or invasive foci of mucinous cystic neoplasm demonstrate KRAS genetic mutations whereas advanced lesions depict inactivating mutations within SMAD4 and TP53 genes. Generally, GNAS genetic mutations are absent. Mucinous cystic neoplasm preponderantly emerges as a singular lesion. Majority of lesions are asymptomatic and gradually progressive. Abdominal pain or acute pancreatitis may appear as representing symptom.
Upon gross examination, an enlarged tumefaction is observed with a mean magnitude of 10 centimetres. Typically, unilocular mega-cysts are encountered which lack communication with pancreatic ductal system. Nevertheless, 15% neoplasms communicate with the main pancreatic duct. Cyst wall may appear as papillary, trabecular or thickened. Cyst contents appear as mucoid or watery. Appropriate tissue sampling of solid areas within the cyst is necessitated [2,3].
Cytological examination of cyst aspirate demonstrates an acellular, viscous, gelatinous or mucoid fluid. Commonly, three dimensional clusters of atypical glandular epithelial cells imbued with hyperchromatic nuclei are discerned, indicative of moderate dysplasia [2,3].
Upon microscopy, mucinous cystic neoplasm delineates an enlarged cyst layered with intestinal, pseudo-pyloric or gastric foveolar type of epithelium with frequent configuration of papillary structures. Characteristically, encompassing stroma is dense and ovarian type. Layering epithelial cells demonstrate variable cellular and nuclear atypia ranging from absent, low grade or high grade atypia. Epithelial cells appear admixed with scattered neuroendocrine cells.
Concurrent invasive adenocarcinoma may or may not be discernible and extensive tissue sampling is mandated for exclusion of an invasive neoplastic component Figure 1 and Figure 2. Foci of calcification are encountered. Additionally, mural nodules enunciating features of giant cell tumour, malignant fibrous histiocytoma or anaplastic carcinoma may be exemplified Table 1 [4,5].
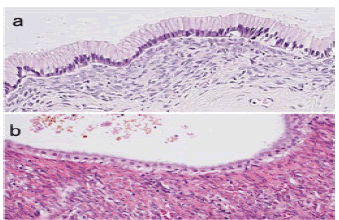
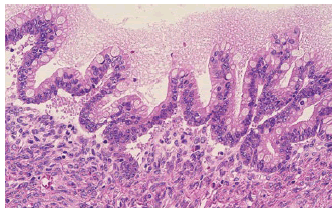
Figure 1: Mucinous cystic neoplasm demonstrating a cystic cavity layered with mucus secreting columnar epithelium intermingled with few neuroendocrine cells and an encompassing dense, ovarian type stroma [6].
Figure 2: Mucinous cystic neoplasm delineating a cystic cavity lined with mucus secreting columnar epithelium with commingled neuroendocrine cells and circumscribing dense, ovarian type stroma. Foci of anaplastic carcinoma appear to concur [7].
Table 1) Cystic lesions of pancreas with specific lining epithelium
| Pseudocysts(absence of lining epithelium) | Conventional pseudocyst |
| Para-duodenal wall cyst(cystic dystrophy) | |
| Infection related pseudocyst | |
| Cysts with mucinous epithelium | Intra-ductal papillary mucinous neoplasm and intra-ductal oncocytic papillary neoplasm |
| Mucinous cystic neoplasm | |
| Mucinous non neoplastic cyst, mucocoele, retention cyst | |
| Serous (clear cell) cystic tumours | Serous cystadenoma |
| VHL-associated pancreatic cyst | |
| Serous cystadenocarcinoma | |
| Squamous lined cysts | Lympho-epithelial cyst |
| Epidermoid cyst within intra-pancreatic accessory spleen | |
| Dermoid cyst | |
| Squamoid cyst of pancreatic ducts | |
| Cysts lined by acinar cells | Acinar cell cystadenocarcinoma |
| Acinar cell cystadenomas(cystic acinar transformation) | |
| Endothelial lined cysts | Lymphangiomas |
| Degenerative or necrotic changes in solid tumours | Solid-pseudo-papillary tumour |
| Cystic change in ordinary ductal adenocarcinoma | |
| Cystic pancreatic endocrine neoplasia(islet cell tumours) | |
| Cystic change in other invasive carcinomas | |
| Cystic mesenchymal neoplasms | |
| Other rare cystic lesions | Cystic hamartomas |
| Enterogenous(congenital; duplication) cysts and duodenal diverticula | |
| Endometriotic cyst | |
| Secondary tumours | |
| Congenital or developmental cysts | |
| Others | |
| Unclassified cysts |
Mucinous cystic neoplasm pancreas appears immune reactive to CD10, Estrogen Receptor (ER), Inhibin, Progesterone Receptor (PR), smooth Muscle Actin (SMA), vimentin, Carcino Embryonic Antigen (CEA), CK7, CK8, CK18, CK19, MUC5AC, DPC4 (MADH4, SMAD4), MUC5AC, MUC1, S100P and SF1.
Neoplastic cells can be stained with Alcian blue and mucicarmine. Mucinous cystic neoplasm appears immune non-reactive to MUC1, MUC2, pVHL or DPC4 (MADH4, SMAD4) within foci of tumour invasion [4,5].
Mucinous cystic neoplasm pancreas requires segregation from neoplasms such as intra-ductal papillary mucinous neoplasm, ovarian mucinous tumour, pancreatic ductal adenocarcinoma- large duct variant, pancreatic ductal adenocarcinoma, pancreatic pseudocyst or serous cystadenoma. Besides, conditions such as acute pancreatitis, bile duct neoplasms, cholangitis, cholecystitis, choledochal cyst, chronic pancreatitis, cholelithiasis, gastric carcinoma and peptic ulcer disease necessitate distinction [4,5].
Mucinous cystic neoplasm pancreas can be appropriately discerned with cogent cytological examination and biochemical analysis of pancreatic cyst fluid obtained with Endoscopic Ultrasound Guided Fine Needle Aspiration (EUS/FNA). Additionally, histological assessment of tissue obtained from pancreatic resection specimen can be optimally employed for detecting the neoplasm. Elevated serum Carcinoembryonic Antigen (CEA) levels and occurrence of KRAS genetic mutation within cyst fluid appears indicative of mucinous cystic lesions comprised of mucinous cystic neoplasm pancreas or intra-ductal papillary mucinous neoplasm.
Upon radiography, a singular, thick walled, cystic cavity traversed with intrinsic septa appears confined to body or tail of pancreas. Lesion may demonstrate nodular structures or foci of calcification. Mucinous cystic neoplasm pancreas can be appropriately treated with surgical extermination of neoplasm. Prognostic outcomes are excellent. However, concurrent invasive carcinoma with extracapsular or diffuse, intra-capsular tumour infiltration is accompanied by decimated prognostic outcomes. Features associated with emergence of invasive carcinoma manifest as
1. Enhanced cyst magnitude> 5 centimetres.
2. Intra-cystic papillary nodules exceeding> 1 centimetre magnitude
3. Elevated serum CA19-9 levels.
Generally, < 20% instances demonstrate association with invasive carcinoma [4,5].
References
- Babiker HM, Hoilat GJ and Recio-Boiles A. Mucinous Cystic Pancreatic Neoplasms. Europe PMC. 2017
- Sigel C, Wei XJ, Agaram N, et al. Diagnostic features of low‐and high‐grade mucinous neoplasms in pancreatic cyst FNA cytology. Cancer Cytopath.
- Hu F, Hu Y, Wang D, et al. Cystic neoplasms of the pancreas: differential diagnosis and radiology correlation. Fron. In Onco. 2022; 12.
- Ardeshna DR, Cao T, Rodgers B, et al. Recent advances in the diagnostic evaluation of pancreatic cystic lesions. World J. of gastro. 2022; 28(6):624.
- Shipley LC, Ahmed AM. New and emerging technology in the diagnosis and treatment of pancreatic cysts. Translat. Gastro. & Hepato. 2022; 7.
- Trendowski M. Using cytochalasins to improve current chemotherapeutic approaches. Anti-Cancer Agents in Medicinal Chemistry. 2015; 15(3):327-35.
- Hall B, Holleran G, McNamara D. PillCam COLON 2© as a pan-enteroscopic test in Crohn’s disease. 2015; 7(16):1230.