Sarcoidosis and tuberculosis: Small fiber neuropathy in quality life of patients.
2 St. Petersburg Scientific Research Institute, St. Petersburg, Russia, Email: starshinova_777@mail.ru
3 Department of Research Management, Sechenov University, Moscow, Russia
4 Institute of Immunology, Moscow, Russia
5 National Medical Research Center for Oncology, St. Petersburg, Russia
6 City Tuberculosis Hospital, St. Petersburg, Russia
7 Zabludowicz Center for Autoimmune Diseases, Sheba Medical Center, Tel HaShomer, Israel
Received: 22-Oct-2021 Accepted Date: Nov 05, 2021; Published: 12-Nov-2021
Citation: Yu GN, Anna S, Yu SZ, et al. Sarcoidosis and tuberculosis: Small fiber neuropathy in quality life of patients. J Histol Histopathol Res.2021;5(4):1-6.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Sarcoidosis (SC) is the granulomatous disease of an unknown origin, where the differential diagnosis with Tuberculosis (TB) is challenging and vital for patients’ prognosis. The common neurological complication in SC is a Small Fiber Neuropathy (SFN) that is considered to be the result of the chronic inflammation, and remains significantly understudied. Aim: To identify the clinical and histological correlates of the small fiber neuropathy in sarcoidosis and tuberculosis patients.
The study was performed in 2018-2019 years and included 71 patients with pulmonary sarcoidosis (n=25), pulmonary tuberculosis (n=21), and healthy subjects (n=25). For the clinical verification of the SFN, the “Small fiber neuropathy screening list” (SFN-SL) was used. A punch biopsy of the skin was performed followed by the enzyme immunoassay analysis with PGP 9.5 antibodies. Up to 60% of sarcoidosis patients and 19% tuberculosis patients report the presence of at least one complaint, which may be associated with SFN. The most frequent complaints included dysfunctions of the cardiovascular, musculoskeletal system and gastrointestinal tract. A negative, statistically significant correlation between the Intra-epidermal Nerve Fiber Density (IEND) and SFN-SL score was revealed in both groups.
Keywords
Sarcoidosis; Tuberculosis; Autoimmune inflammation; Polyneuropathy; Small fiber neuropathy; Autoimmunity
Introduction
In patients with sarcoidosis, the development of systemic inflammation and internal organ dysfunction are observed, which significantly reduces the quality of life and worsens the prognosis of the patients. One of the most common complication is considered to be a Small Fiber Neuropathy (SFN), that remains significantly understudied [1-4]. Quite often, patients with lung sarcoidosis complain of a number of non-specific symptoms, such as weakness, sleep disorders, etc., which can significantly affect the patient’s quality of life, even in the absence of significant deviations from the pulmonary system [5-11].
The prevalence of sarcoidosis varies throughout the world. In Japan there is 1 case per 100,000 people, while in Scandinavian countries the prevalence of sarcoidosis is as much as 63 cases per 100,000 people, In Russian Federation sarcoidosis is described with the prevalence from 22 to 47 cases per 100,000 people, depending on the region [12-15]. The prevalence of SFN may also vary because it presents not only with neuropathic pains and paresthesias, but also with various symptoms of autonomic dysfunction, which may not be recognized as a neurologic complication [16-20].
The development of SFN is considered to be the result of a cytokine-mediated inflammation, which is typical for various autoimmune diseases, including sarcoidosis [5-8]. Small nerve fiber damage is also observed in systemic lupus erythematosus, Sjogren’s syndrome, and fibromyalgia [9,10]. Considering the significant role of the genetic predisposition and the possible provocative role of exogenous triggers in the development of this complication, SFN in patients with sarcoidosis can be considered as a part of the Autoimmune/ inflammatory Syndrome Induced by Adjuvants (ASIA) [11]. Several cases of the proven SFN were also observed in patients, suffering from the bacterial inflammation, e.g., Lime disease and leprosy [1-4].
Previously, there were no studies on clinical manifestations of small fiber neuropathy, as well as on detecting the reduction of the small nerve fibers in patients with tuberculosis. Among the lesions of the peripheral nervous system in tuberculosis, drug-induced lesions (rifampicin, streptomycin, and ethambutol), rare cases of autoimmune neuropathies, direct nerve damage due to surgery, or the development of granulomatous inflammation are more often described? Though this data may contribute to a better understanding of the pathogenesis of different types of inflammation, as well as to determine the contribution to the course of the disease, the need to correct the clinical manifestations of SFN in patients with tuberculosis.
Currently, there are no generally accepted criteria for the diagnosis of SFN [21-23]. The presence of a small nerve fibers dysfunction in a patient usually is based mainly on clinical criteria, such as neurological examination and validated scales (for example, small fiber neuropathy screening list) [19,24- 26]. In addition, electroneuromyography can be performed mainly to exclude damage to large nerve fibers. The “gold standard” for diagnostics is immunofluorescence or immunohistochemistry of skin biopsy with the calculation of the density of intraepidermal nerve fibers. This technique requires special training and equipment, and is significantly time consuming [27-33]. Preliminary clinical diagnosis of SFN in patients with sarcoidosis is important due to the low awareness of healthcare practitioners about this complication and the need for a quick assessment of neuropathy signs for the further skin biopsy performance. Aim of the study is to identify the clinical and histological patterns and correlates of the small fiber neuropathy in lung sarcoidosis and lung tuberculosis patients.
Materials and Methods
A prospective comparative study was performed in 2018-2019 years at St. Petersburg Research Institute of Phthisiopulmonology and St. Petersburg City Public Health Institution “City Multi-disciplinary Hospital No. 2”. The study was approved by the independent ethics committee of the St. Petersburg Research Institute of Phthisiopulmonology (extract from protocol No. 46.1 of 04/20/2018). All study participants signed an informed consent.
The study entailed 119 patients with lung sarcoidosis and lung tuberculosis, and healthy subjects. Among them were 42 men and 56 women, average age was 38.4 ± 7.2 years. The first group consisted of patients with lung sarcoidosis (n=25, average age 33.4 ± 8.5 years), the second group- patients with verified lung tuberculosis before initiating specific therapy (n=21, average age 36.6 ± 9.3 years), the third group included healthy subjects (n=25, average age 43.2 ± 11.7 years). There were no statistically significant differences in gender and age among the patient groups. The inclusion criteria were age from 18 to 65 years and signing informed consent to participate in the study. The patients from the group 1 were also diagnosed with sarcoidosis, stage I-II, for group 2-focal and disseminated lung tuberculosis in the pre-treatment period.
Exclusion criteria were: systemic glucocorticoid therapy, the presence of Löfgren’s syndrome and a chronic course of the disease (for patients with sarcoidosis), the presence of other infectious diseases (HIV, hepatitis C), the history of cancer and their treatment using chemotherapy, the presence of other diseases (diabetes, hypothyroidism, renal failure, vitamin deficiency or overdose), medications (metronidazole, nitrofurantoin, linezolid, flecainide, statins), as well as the medical history of autoimmune diseases.
According to the design of the study, patients with sarcoidosis and pulmonary tuberculosis underwent a standard examination using X-ray, morphological, bacteriological and molecular genetic methods. A neurological examination with an assessment of the superficial and deep pain sensitivity, muscle strength and muscle-tonic reflexes was performed. For clinical verification of the SFN, the validated questionnaire for the detection of small fiber neuropathy was used (Small fiber neuropathy screening list, SFN-SL, (19, Appendix 1). The questionnaire consists of 2 parts and 21 questions, from 0 to 4 points each, evaluating both the frequency of development of symptoms and their intensity. A moderate likelihood of neuropathy is established when the diagnostic threshold is reached at 22 points, high-at 48 points. With scores less than 11, sensitivity is 100%, specificity is 31.0%. With scores of more than 48-sensitivity-19.0%, specificity-100%.
13 patients with lung sarcoidosis and 10 patients with lung tuberculosis agreed to undergo a punch biopsy of the skin (10 cm proximal to the external malleolus) followed by the fixation of the specimen in Zamboni solution and the performance of enzyme immunoassay with primary PGP 9.5 antibodies (Abcam) and secondary AlexaFluor goat-anti rabbit antibodies 488 (Abcam). The results were compared with the normal values obtained in the worldwide normative reference study [23].
Statistical analysis was performed using the Statistica 8.0 software package. The distribution of patients into groups was carried out in accordance with the presence of verified diagnoses of lung sarcoidosis, lung tuberculosis or in the absence of verified somatic diseases (for healthy subjects).
All data collected during the study were analyzed using methods of descriptive statistics. The data with the normal distribution are presented in the form of the “mean ± standard deviation” formula. Data that were not normally distributed are presented in the form of the “median (interquartile range 25- 75 quartiles)”, where the confidence interval was also calculated. Normality testing was performed using the Shapiro-Wilk test [33-35].
All data were analyzed using methods of parametric and nonparametric statistics, Chi-square test and statistical comparison methods for two (Mann- Whitney U-test) and three (Kruskal-Wallis test) groups were performed, correlations were carried out using the Spearman coefficient.
Differences or association rates were considered statistically significant at a p<0.05.
Results
The average score of the SFN Screening List scale (SFN-SL) in patients with sarcoidosis was 2.0 (0.0; 7.0), with tuberculosis-0 (0;0), and in healthy volunteers-0 (0;0) points (Table 1).
| Group | % (n/N) | SFN-SL scale points, median (IQR) | CI 95% |
|---|---|---|---|
| Sarcoidosis (n=25) | 60.0*; 15/25 | 2.0 (0.0;7.0) | 1.836-2.748 |
| Tuberculosis (n=21) | 19.1*; 4/21 | 0 (0;0) | 0.00-0.00 |
| Healthy volunteers (n=25) | 8.0, 2/25 | 0 (0;0) | 0.00-0.00 |
*Significant differences were found in the results of patients with sarcoidosis and tuberculosis (p=0.0012, the Mann-Whitney U-test), in patients with sarcoidosis and healthy subjects (p<0.0001), as well as in patients with tuberculosis and healthy subjects (p=0.03).
Table 1: The results of the “Small Fiber Neuropathy Screening list” (SFNSL) testing in patients with pulmonary sarcoidosis, pulmonary tuberculosis and healthy volunteers
Thus, in the first group, a higher average SFN-SL score was observed than in the second group, while the results of the 1 and 2 groups exceeded those of healthy individuals. The main clinical symptoms of the SFN were: pain, changes in body temperature, impaired motility of the gastrointestinal tract and urinary system, heart palpitations (Table 2).
| Sarcoidosis, (n=25), n (%) | CI 95% | Tuberculosis, (n=21), n (%) | CI 95% | Р1 | Healthy volunteers, (n=25), n (%) | CI 95% | P2 | |
|---|---|---|---|---|---|---|---|---|
| Cardiovascular disorders | 9 (36.0) | 34.5-38.0 | 0 | 0 | 0.005 | 0 | 0 | 0.005 |
| Gastrointestinal disordes | 3 (12.0) | 11.8-12.3 | 2 (9.5%) | 8.7-9.9 | 0.837 | 1 (0.04) | 0.8-1.9 | 0.084 |
| Urinary disorders | 0 | 0 | 1 (4.8%) | 4.1-5.2 | 0.93 | 0 | 0 | - |
| Musculoskeletal disorders | 4 (16.0) | 15.5-16.7 | 2 (9.5%) | 9.0-10.1 | 0.834 | 1 (0.04) | 0.7-1.8 | 0.02 |
| Skin and mucous membranes disorders | 7 (28.0) | 27.6-29.0 | 0 | 0 | 0.261 | 0 | 0 | - |
| Ophtalmologic disorders | 5 (20.0) | 19.4-20.9 | 0 | 0 | 0.091 | 0 | 0 | 0.005 |
Note: p1 is the difference between the results of groups 1 and 2, the Chi-square test with Yates correction. p2-the difference between the results of groups 1 and 3, Chi-square criterion with Yates correction.
Table 2: Clinical symptoms of small fiber neuropathy in patients with pulmonary sarcoidosis, pulmonary tuberculosis and healthy volunteers
In patients with sarcoidosis, the most frequent clinical symptoms include impaired cardiovascular regulation (palpitations, dizziness), pain in the chest or in the extremities, and blurred vision. It is important to note that in 76% of patients with sarcoidosis, clinical symptoms had a severity of no more than 1 point on the SFN-SL scale and, in most cases, did not bother patients or lead to a decrease in the quality of life. Among patients with tuberculosis, ingeneral, fewer clinical manifestations were noted, the main of which were dysfunction of the gastrointestinal tract, muscle cramps and chest pain (Table 3).
| Clinical manifestation of SFN | Sarcoidosis, (n=25), n (%) | Tuberculosis, (n=21), n (%) | Healthy volunteers, (n=25), n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Points | Points | Points | ||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Cardiovascular disorders | 4(16.0) | 2(8.0) | 3(12.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal disordes | 2 (8.0) | 0 | 1(4.0) | 0 | 1(4.8) | 1(4.8) | 0 | 0 | 0 | 1(0.08) | 0 | 0 |
| Urinary disorders | 0 | 0 | 0 | 0 | 1(4.8) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscular spasms | 2(8.0) | 2(8.0) | 0 | 0 | 2(9.5) | 0 | 0 | 0 | 1(0.08) | 0 | 0 | 0 |
| Pain syndrome | 3 (12.0) | 1(4.0) | 0 | 2(8.0) | 2(9.5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Temperature dysfunction | 1 (4.0) | 0 | 0 | 1(4.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Skin discoloration | 3 (12.0) | 0 | 0 | 1(4.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paresthesias | 1 (4.0) | 0 | 1(4.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blurred vision | 2 (8.0) | 2(8.0) | 0 | 1 (4.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dry mucous membranes, change in skin moisture | 1(4.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Results | 19(76.0) | 7(28.0) | 5(20.0) | 5(20.0) | 6 (28.6) | 1(4.8) | 0 | 0 | 1(1.4) | 1(0.08) | 0 | 0 |
| CI 95% | 75.6-77.4 | 27.6-29.0 | 19.5-21.1 | 18.3-20.8 | 26.5-29.3 | 4.3-5.2 | 0 | 0 | 0.9-1.9 | 0.9-1.9 | 0 | 0 |
Table 3: Clinical manifestations of small fiber neuropathy in patients with sarcoidosis, tuberculosis and healthy individuals
| Group | IEND in 1 mm, median (Q1; Q3) | Normal values, -median | Normal values, - 0.05 quantile |
|---|---|---|---|
| Pulmonary sarcoidosis (n=13) | 7.68 (7.02;8.34) | 12.4 | 7.1 |
| Pulmonary tuberculosis (n=10) | 10.1 (9.44:10.21) | 12.4 | 7.1 |
Table 4: Intraepidermal Nerve Fiber Density (IEND) in patients with sarcoidosis and tuberculosis
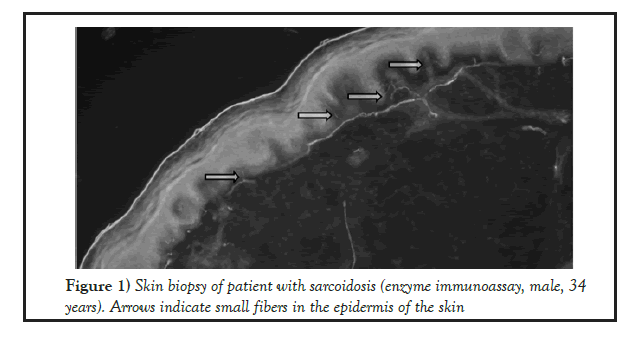
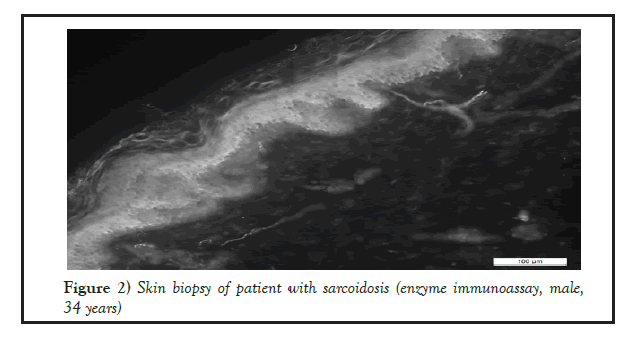
23 biopsy specimens have been obtained in patients with sarcoidosis and tuberculosis, where the intraepidermal density (IEFD) of small nerve fibers was calculated (Figures 1 and 2).
Figure 1: Skin biopsy of patient with sarcoidosis (enzyme immunoassay, male, 34 years). Arrows indicate small fibers in the epidermis of the skin
Figure 2: Skin biopsy of patient with sarcoidosis (enzyme immunoassay, male,34 years)
Of the 23 biopsies, 13 were obtained in patients with sarcoidosis, 10-in patients with tuberculosis in Table 4. The calculations of intraepidermal density of the small fibers were performed in accordance with the values from the worldwide normative reference study [23].
Thus, in all examined patients, the number of small nerve fibers was within normal limits, but below average values. There is a tendency to a higher density of small nerve fibers in patients with tuberculosis compared with sarcoidosis (Mann-Whitney test, p=0.0047). A negative, statistically significant correlation was revealed between IEND results and SFN-SL score in patients with sarcoidosis (Spearman’s nonparametric rank correlation coefficient, r=-0.3508, p=0.0102).
A negative, statistically significant correlation between IEND results and SFN-SL score was observed in patients with p tuberculosis (Spearman’s nonparametric rank correlation coefficient, r=-0.7382, p=0.0064).
Thus, a decrease in the density of small nerve fibers in patients with sarcoidosis was noted, compared with the results of patients with tuberculosis (Mann- Whitney test, p=0.0047). A negative, statistically significant correlation between the IEND and SFN-SL score was described in both groups (Spearman coefficient, r=-0.3508, p=0.0102, and r=-0.7382, p=0.0064).
Discussion
In our study, complaints, typical for the small fiber neuropathy were described in 60% of patients with sarcoidosis and 19.1% of patients with tuberculosis. Small fiber neuropathy can manifest with a wide range of symptoms, including autonomic and sensory dysfunction. The most common clinical manifestations observed in our study in patients with sarcoidosis were impaired cardiovascular regulation (36% of cases), e.g. the development of palpitation and dizziness. In 32% of cases, patients noted pain in the chest or limbs, which often accompanied with allodynia, a subjective perception of tactile touch as pain. In some cases, this can result in sleep disturbances due to the pain sensations of bed linen touching the skin. Another symptom that is often noted by patients with sarcoidosis is blurred vision, described in 20% of cases. A physician needs to clarify whether visual impairments are transient or permanent in nature, for the differential diagnosis of ophthalmic pathology. In the neuropathy of small fibers, blurred vision is transient, arising while overworking or physical exertion. Less typical for patients with sarcoidosis are disorders of the gastrointestinal tract. In 12% of the cases, patients complained of impaired intestinal motility with the development of both diarrhea and constipation, which occurred simultaneously with the onset of sarcoidosis. This also includes subjective complaints of swallowing dysfunction, which is associated both with impaired muscle innervation and with the progression of dryness of the mouth.
In the tuberculosis group much less clinical symptoms were observed. The most common symptoms were the dysregulation of the gastrointestinal (9.5%) and urinal tract motility (4.8%) and arthralgia (9.5%). No cardiovascular complaints were observed in this group and the intensity of the symptoms was much less prominent, compared to the sarcoidosis group.
While a negative, statistically significant correlation between the IEND and SFN-SL score was described in both groups (Spearman coefficient, r=-0.3508, p=0.0102, and r=-0.7382, p=0.0064), a decrease in the density of small nerve fibers in patients with pulmonary sarcoidosis was more prominent, compared with the results of patients with pulmonary tuberculosis (Mann- Whitney test, p=0.0047).
A major limitation of this study is that conventional nerve conduction studies and autonomic function tests were not performed. The small sample size also can be considered as a limitation.
Thus, neuropathy of small fibers seems to be a widespread pathology with the development of multiple organ dysfunctions. Disorders in small fiber neuropathy, along with typical complaints of patients with lung sarcoidosis, such as cough, shortness of breath, can significantly contribute to a decrease in the quality of life. At the same time, focusing on the main complaints, as well as instrumental and laboratory deviations, clinicians often do not attach the necessary importance to the manifestations of SFN. While the identification of the main causes of the decline in the quality of life of patients is necessary to determine the treatment strategy and improve the prognosis of the patient’s disease [34,35].
Given the low awareness of both medical specialists and patients about the development of this complication and the difficulties in diagnostic, further study of this issue is required.
In patients with pulmonary sarcoidosis, small fiber neuropathy may develop as a result of systemic immune-mediated inflammation. Wherein, in tuberculosis patients clinical and histological symptoms of the small fiber neuropathy were subsequently less prominent, which may represent the difference between the autoimmune and bacterial inflammation and will be useful in differential diagnostic with both diseases. The validated questionnaires and histologic verification of the diagnosis help to establish the severity of neuropathy of small fibers, to determine the prognosis and to plan the strategy for treatment and also may allude the possibility for the additional criteria of differential diagnosis between two diseases.
Conclusion
In patients with pulmonary sarcoidosis, small fiber neuropathy may develop as a result of systemic immune-mediated inflammation. The most common symptoms of this complication were dysautonomia and mild sensory dysfunction. Wherein, in tuberculosis patients clinical and histological symptoms of the small fiber neuropathy were subsequently less prominent, which may represent the difference between the autoimmune and bacterial inflammation?
Acknowledgement
This work was supported by the grant from the Government of the Russian Federation (contract No. 14W03.31.0009 dated February 13, 2017) on the allocation of the grant for the state support of scientific researchers conducted under the guidance of leading scientists.
Authors Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Natalia Gavrilova, Anna Malkova and Valeria Shapkina. Patient selection and supervision were performed by Yulia Zinchenko, Maria Pavlova, Ekaterina Belyaeva, Dmitry Kudlay. General supervision was carried out by Anna Starshinova, Piotr Yablonskiy. General research concept, guidance and article review was performed by Yehuda Shoenfeld. All authors read and approved the final manuscript.
Author Information
Gavrilova Natalia, MD, PhD Junior researcher, Laboratory of the Mosaic of Autoimmunity, Saint-Petersburg State University (University Embankment, 7-9, St. Petersburg, 199034, Russia); assistant, Department of Faculty Therapy, Saint-Petersburg State University (University Embankment, 7–9, St. Petersburg, 199034, Russia); Junior researcher, St. Petersburg Scientific Research Institute of Phthisiopulmonology Ministry of Health of the Russian Federation (Ligovskii prospect, 2-4, St. Petersburg, 191036, Russia), e-mail: fromrussiawithlove_nb@mail.ru. ORCID ID: 0000-0002-2957-410X.
Starshinova Anna, Leading Researcher, Laboratory of the Mosaic of Autoimmunity, Saint-Petersburg State University (University Embankment, 7-9, St. Petersburg, 199034, Russia), starshinova_777@mail.ru, ORCID ID: http://orcid.org/0000-0002-9023-6986.
Zinchenko Yulia, MD, PhD Junior researcher, pulmonologist, St. Petersburg Scientific Research Institute of Phthisiopulmonology Ministry of Health of the Russian Federation (Ligovskii prospect, 2-4, St. Petersburg, 191036, Russia), ulia-zinchenko@yandex.ru. ORCID ID: https://orcid.org/0000- 0002-6273-4304.
Kudlay Dmitry Anatolyevich, DMedSci, MD, Professor of the Department of Pharmacology, Institute for Pharmacy, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation. Leading Researcher, Laboratory of Personalized Medicine and Molecular Immunology No. 71, NRC Institute of Immunology FMBA of Russia, 24, Kashirskoye highway, Moscow, 115552, D624254@gmail.com, http : // orcid.org/0000-0003-1878-4467 Scopus AuthorID: 57201653374.
Shapkina Valeria, National Medical Research Center for Oncology (Leningradskaya str., 68 Pesochiy township, Saint-Petersburg, 197758, Russia. valshapkina@gmail.com ORCID ID: https://orcid.org/0000-0003- 1843-2808.
Malkova Anna, Junior Researcher, Laboratory of the Mosaic of Autoimmunity, Saint-Petersburg State University (University Embankment, 7–9, St. Petersburg, 199034, Russia), anya.malkova.95@mail.ru. ORCID ID: https://orcid.org/0000-0002-3880-1781.
Belyaeva Ekaterina, PhD, Junior Researcher “St. Petersburg Research Institute of Phthisiopulmonology” of the Ministry of Health of the Russian Federation, Head of the Department St. Petersburg City hospital №2., ekaterina_83@bk.ru.
Pavlova Maria, Prof., PhD, Leading Researcher of Phthisiopulmonology department, St. Petersburg Research Institute of Phthisiopulmonology” of the Ministry of Health of the Russian Federation, (Ligovskii prospect, 2-4, St. Petersburg, 191036, Russia), e-mail: mvpavlova2011@mail.ru.
Yablonskiy Piotr, Prof., PhD, MD, Dean, Medical department, St. Petersburg State University (University Embankment, 7–9, St. Petersburg, 199034, Russia). Head, St. Petersburg Scientific Research Institute of Phthisiopulmonology Ministry of Health of the Russian Federation (Ligovskii prospect, 2-4, St. Petersburg, 191036, Russia), e-mail: piotr_yablonskii@mail. ru. ORCID ID: http://orcid.org/0000-0003-4385-9643.
Shoenfeld Yehuda, Prof., MD, FRCP. Head, Zabludowicz Center for Autoimmune Diseases, (Derech Sheba 2, Ramat Gan, Israel), Professor, Sackler Faculty of Medicine, Tel-Aviv University, Israel. Head, Laboratory of the Mosaic of Autoimmunity, St. Petersburg State University (University Embankment, 7-9, St. Petersburg, 199034, Russia) yehuda.shoenfeld@sheba. health.gov.il.
Conflicts of Interest
The authors declare that there is no potential conflict of interest regarding the publication of this article.
Ethics Approval
All human studies have been approved by the independent ethics committee of the St. Petersburg Research Institute of Phthisiopulmonology (extract from protocol No. 46.1 of 04/20/2018) and have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
All study participants signed an informed consent.
REFERENCES
- Abdelrazek MA, Chwalisz B, Oaklander AL, et al. Evidence of Small Fiber Neuropathy (SFN) in two patients with unexplained genital sensory loss and sensory urinary cystopathy. J Neurol Sci. 2017; 380(1):82-84.
- Birnbaum J, Bingham CO. Non-length-dependent and length-dependent small-fiber neuropathies associated with Tumor Necrosis Factor (TNF) inhibitor therapy in patients with rheumatoid arthritis: Expanding the spectrum of neurological disease associated with TNF-inhibitors.Semin Arthritis Rheum. 2014; 43(5) :638-647.
- Blackmore D, Siddiqi ZA. Diagnostic Criteria for Small Fiber Neuropathy. J Clin Neuromuscul Dis. 2017; 18(3): 125-131.
- Brouwer BA, Bakkers M, Hoeijmakers JGJ, et al. Improving assessment in small fiber neuropathy. J Peripher Nerv Syst. 2015;20(3):333-340.
- Bakkers M, Merkies ISJ, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73(14): 1142-1148.
- Patterson KC, Chen ES. The pathogenesis of pulmonary sarcoidosis and implications for treatment. Chest. 2018;153(6): 1432-1442.
- Bindoli S, Dagan A, Torres-Ruiz JJ. Sarcoidosis and autoimmunity: From Genetic background to environmental factors. Isr Med Assoc J. 2016;18(3-4):197-202.
- Starshinova A, Zinchenko Y, Filatov M, et al. Specific features of immune forming complexes in patients with sarcoidosis and pulmonary tuberculosis. Immunol Res. 2018;66(6) 737-743.
- Cazzato D, Lauria G. Small fiber neuropathy. Curr Opin Neurol. 2017;30(5): 490-499.
- Chiang MC, Tseng MT ,Pan CL, et al. Progress in the treatment of small fiber peripheral neuropathy. Expert Rev Neurother. 2015;15(3): 305-313.
- Watad A, Quaresma M, Bragazzi NL, et al. The autoimmune/inflammatory syndrome induced by adjuvants (ASIA)//Shoenfeld’s syndrome: descriptive analysis of 300 patients from the international ASIA syndrome registry. Clinical Rheumatol. 2018; 37(2): 483-493.
- Fingerlin TE, Hamzeh N, Maier LA. Genetics of Sarcoidosis. Clin Chest Med. 2015; 36(4):569-584.
- Newman LS, Rose CS, Bresnitz EA, et al. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. American Journal of Respiratory and Critical Care Medicine. 2004;170(12): 1324-1330.
- Kobak S, Sever F, Sivrikoz ON, et al. Sarcoidois: is it only a mimicker of primary rheumatic disease? A single center experience. Ther Adv Musculoskelet Dis. 2014;6(1): 3-7.
- Drori T, Givaty G, Chapman J, et al. Extrapyramidal sings in neurosarcoidosis versus multiple sclerosis: Is TNF alpha the link?. Immunobiology. 2018;223(3):259-263.
- Musaelyan A, Lapin S, Nazarov V, et al. Vimentin as antigenic target in autoimmunity: A comprehensive review. Autoimmun Rev. 2018;17(9):926-934.
- Dori A, Lopate G, Choksi R, et al. Myelinated and unmyelinated endoneurial axon quantitation and clinical correlation. Muscle Nerve.2016;53(2):198–204.
- Dori A, Lopate G, Keeling R, et al. Myovascular innervation: axon loss in small-fiber neuropathies. Muscle Nerve. 2015;51(4): 514-521.
- Hoitsma E, de Vries J, Drent M. The small fiber neuropathy screening list: construction and cross-validation in sarcoidosis. Respir Med.2011;105(1):95-100.
- Hovaguimian А, Gibbons СН. Diagnosis and Treatment of Pain in Small Fiber Neuropathy. Curr Pain Headache Rep. 2011;15(3):193-200.
- Abdulla W, Bragazzi NL, McGregor D, et al. Autoimmune/inflammatory Syndrome Induced by Adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin Immunol. 2019;203(1):1-8.
- Watad A, David P, Brown S, et al. Autoimmune/ inflammatory Syndrome induced by Adjuvants and Thyroid Autoimmunity. Front Endocrinol (Lausanne). 2017;24(7):150.
- Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202-207.
- Lauria G, Cornblath DR, Johansson O, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12(10):747-758.
- Lauria G, Lombardi R, Camozzi F,et al. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology.2009;54(3): 273-285.
- Lauria G, Merkies ISG, Faber CG. Small fiber neuropathy. Curr Opin Neurol. 2012;25(5):542-549.
- Levin TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patient. Clin Rheumatol. 2015; 34(3):413-417.
- McArthur JC. Painful Small Fiber Neuropathies. Continuum (Minneap Minn). 2012;18(1) :106-125.
- McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology.1995;45(10):1848-1855.
- Oaklander AL. Immunotherapy prospects for painful small-fiber sensory neuropathies and ganglionopathies. Neurotherapeutics. 2016;13(1):108-117.
- Peteira MP, Muhl S, Pogatzki-Zahn EM, et al. Intraepidermal nerve fiber density: diagnostic and therapeutic relevance in the management of chronic pruritus: a review. Dermatol Ther (Heidelb). 2016;6(4):509-517.
- Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. A multi-center, multinational age-and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333-338.
- Sene D. Small fiber neuropathy: diagnosis, causes and treatment. Joint Bone Spine. 2018;85(5):553-559.
- Drent M, Lower EE, De Vries J. Sarcoidosis-Associated Fatigue. Eur Respir J. 2012;40(1):255-263
- Saketkoo LA, Russell AM, Jensen K, et al. Health-Related Quality of Life (HRQoL) in Sarcoidosis: Diagnosis, Management, and Health Outcomes. Diagnostics (Basel). 2021;11(6):1089.