Shoulder dislocation in athletes: overview and update on treatment
2 Orthopaedic Surgery Department, Infanta Sofia Hospital, Madrid, Spain
3 European University of Madrid, Spain
Received: 05-Feb-2018 Accepted Date: Feb 09, 2018; Published: 16-Feb-2018
Citation: Lopez-Franco M, Blanco-Diaz D, Murciano-Anton MA. Shoulder dislocation in athletes: overview and update on treatment. J Clin Diag Treat 2018;1[1]: 13-22.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Shoulder dislocation result in a significant amount of lost practice and game time, and has physical and psychological consequences for the athlete. It is paramount to identify the correct pathogenesis of instability so that treatment can be appropriately tailored to the patient’s needs. When medical literature is analyzed searching for the most suitable treatment in a patient with recurrent shoulder dislocation, we must evaluate the overall outcomes using the different procedures of treatment, but we should choose a scoring system reliable, valid, and responsive in patients with instability. Nonoperative treatment may be useful for the in-season athlete looking to complete the season and then undergo off-season stabilization. Primary surgical treatment after first-time traumatic anterior shoulder dislocation may be considered to avoid secondary damage to articular structures. Open or current arthroscopic repair techniques have shown equivalent results. In considering a surgical procedure involving bone defects, the surgeons always have to measure the length and take into account the location of the injury. Isolated soft tissue repair is generally not sufficient for the surgical management of patients with large bone defects. Latarjet procedure provides a glenoid bony augmentation and the transferred conjoined tendon creates a dynamic belt that reinforces the weak anteroinferior capsule. Remplissage arthroscopic technique can be useful in engaging Hill-Sachs lesions. Arthroscopically aided anterior capsular reinforcement can be used when there is insufficient quantity and quality of the anterior capsuloligamentous tissue.
Several athletes require increased shoulder mobility to meet their functional demands in addition to enough stability to prevent joint subluxation. Wilk and Arrigo have referred to this contradictory relationship of a shoulder being hypermobile enough to perform overhead activity yet stable enough to prevent joint subluxation as the throwers paradox [1]. Furthermore, the wide range of shoulder motion makes it difficult to clearly determine the differences between normal motion, asymptomatic hypermobility or laxity, or symptomatic pathologic instability.
Laxity is the asymptomatic passive translation of the humeral head on the glenoid and may be essential to athletic performance. In hyperlaxity, range of joint motion and joint distractibility are increased without loss of function. Glenohumeral instability is defined as excessive translation of the humeral head on the glenoid associated with a functional deficit [2]. In a complete dislocation, the humeral head remains in a dislocation position until it is reduced.
There exists a wide range of shoulder instabilities, from subtle subluxations [as seen in overhead athletes] to gross instability with recurrent shoulder dislocation events. In the compensated athlete, static soft tissue and bony deficiency are often counteracted by advanced neuromuscular control. Symptomatic instability results from acute or chronic deterioration in the compensatory dynamic stabilizers of the glenohumeral joint or a frank traumatic event [3]. Lephart and co-authors reported that proprioception of the affected shoulder was altered in patients with glenohumeral instability compared with the asymptomatic extremity [4].
The cause of shoulder instability is complex and multifactorial. Although several classification systems have been suggested, there is no one that adequately serves as a guide to treatment, predicts outcome, or facilitates communication between clinicians. Two typical groups of individuals who develop glenohumeral instability have been described based on the cardinal features of their condition [5]. The acronym TUBS describes patients with traumatic instability, who characteristically have unidirectional instability [traumatic, unilateral, with a Bankart lesion generally requiring surgical treatment]. The acronym AMBRI describes instability that is typically atraumatic, multidirectional, bilateral, responds to rehabilitation, and occasionally requires an inferior capsular shift. However, this system has been recognized over-simplistic [3], because some patients with hyperlaxity have reported unidirectional or bidirectional instability without a traumatic precipitant [6], traumatic unidirectional instability occurs bilaterally in a quarter of patients with excessive capsular elastin [implying an element of inherited predisposition] [7] and some patients are able to voluntarily dislocate their shoulder. Therefore, these 2 classical groups represent extremes in a spectrum of pathologic conditions with many patients exhibiting overlapping traits. The concept of instability being caused by a combination of structural [traumatic and atraumatic] and neurologic system disturbances has led to a classification of instability as a continuum of pathologies that can be displayed graphically as a triangle [the Stanmore classification] [8]. In considering the most appropriate treatment in shoulder instability in the athlete, it is required to take into account the clinical manifestation of instability, the underlying pathological findings, the athletic needs of the patient and the timing within the athletic calendar. This article reviews the different treatment options in shoulder instability in the athlete.
Epidemiology
The incidence of traumatic dislocation ranges from 11.2 to 23.9 per 100,000 person-years [9-13]. Shoulder dislocations are more common in men, ages 20 to 29 years, with almost half occurring during sports [9-11]. Owens et al. reported an injury rate of 0.12 per 1000 exposures, with the highest risk sports being ice hockey, football, and wrestling [14]. The injuries resulted in a significant amount of time lost, with at least 10 days being missed in 45% of cases. Posterior instability is less usually associated with a frank dislocation, but more commonly seen as recurrent transient subluxation events, resulting in pain and an inability to perform at the athlete’s desired level [15].
Three broad etiologic categories have been implicated in instability of the shoulder: repetitive microtrauma to the shoulder, acute traumatic events, and purely atraumatic causes. It is crucial to identify the correct pathogenesis of instability so that treatment can be appropriately tailored to the patient’s needs [3].
Traumatic anterior shoulder dislocation in the athlete usually occurs with a posteriorly directed force applied to the anterior aspect of an abducted, externally rotated arm. In this scenario, the humeral head is driven forward, producing a spectrum of soft tissue and bony lesions that are implicated in the pathogenesis of recurrent instability. Sports have been classified by degree of contact and athletes involve in collision or contact sports are more likely to have a traumatic shoulder dislocation [16].
Pathoanatomy of Traumatic Anterior Instability
The underlying cause of anterior recurrent shoulder dislocation is multifactorial. We can find soft tissue and bony lesions implicated and the plastic deformation of the inferior glenohumeral ligament complex [IGHLC] regarded central to development of recurrent anterior instability [17].
Soft tissue lesions
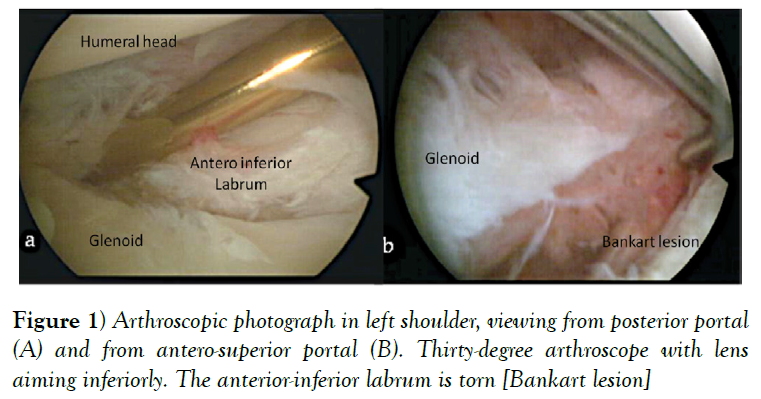
The anteroinferior labrum detachment with its attached IGHLC or Bankart lesion, has been traditionally described as the “essential lesion” of anterior traumatic dislocation of the shoulder occurring in 90% of anterior instability cases [3].
Because the union between the collagen fibers of the IGHLC and the glenoid labrum is stronger than the union between the labrum and the glenoid rim, failure typically manifests as a labral avulsion from the glenoid and a Bankart or Bankart variant lesion [18-20]. Young athletes and those who participate in contact sports are most prone to developing recurrent dislocation or subluxation events after an initial traumatic dislocation. These events most commonly result in anterior inferior “Bankart” labral tears [21,22].
According to Murray and co-authors although the Bankart lesion (Figure 1) is almost always present in patients with traumatic instability it does not produce recurrent anterior instability in isolation [3]. Plastic deformation of the IGHLC has been considered paramount to develop recurrent anterior instability [17]. In a cadaveric study, IGHLC specimens were loaded in tension to failure and 3 types of failure were observed: at the site of the glenoid insertion [40%], in the mid-substance of the ligament [35%], and at the site of the humeral insertion [25%].
Even when failure occurred at the site of the glenoid insertion, it occurred only after significant elongation of the IGHLC [23]. Clinical studies have reinforced that capsular stretching can occur simultaneously with a Bankart lesion during an anterior dislocation, with an abnormal capsular redundancy reported in up to 28% of patients with recurrent anterior instability [24].
In addition to Bankart lesion, we can find other capsulolabral injuries after an anterior shoulder dislocation. Habermayer developed a classification system of lesions types in anteroinferior shoulder instability [20]. It might be useful to better understanding the underlying pathology. The Perthes lesion, for example, represents a detachment of the labrum-ligament complex without detachment of the periostal ligament insertion.
The anterior labral periosteal sleeve avulsion [ALPSA] is a lesion characterized by medial displacement, and inferior rotation of the anteroinferior labrum along with the anterior IGHLC, with medial stripping but without complete disruption of the scapular periosteum. We can also find a glenolabral articular disruption [GLAD] lesion that consists of a superficial tear of the anteroinferior labrum in combination with an articular cartilage injury of the adjacent glenoid. Sometimes, an infrequent but important injury occurs in the humeral insertion of the IGHLC: The humeral avulsion of glenohumeral ligaments [HAGL] lesion.
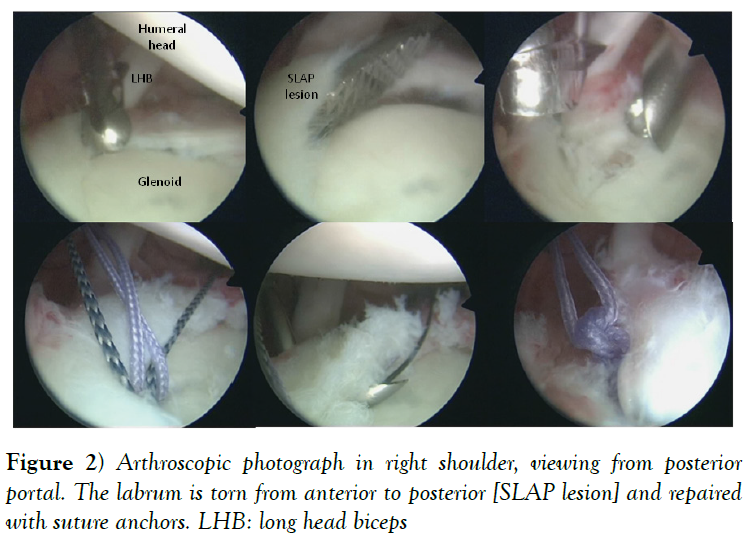
On the other hand, we can find labral injuries in the superior labrum [superior labrum from anterior to posterior-SLAP- lesions] (Figure 2), more common in throwing athletes perhaps because of the eccentric loads on the biceps anchor during the deceleration phase of throwing (25,26) or rotator cuff tears more likely in athletes older than 40 years [27].
Figure 2: Arthroscopic photograph in right shoulder, viewing from posterior portal. The labrum is torn from anterior to posterior [SLAP lesion] and repaired with suture anchors. LHB: long head biceps
When a traumatic anterior shoulder dislocation occurs, the humeral head can damage the anteroinferior aspect of the glenoid. It may produce a bony Bankart lesion, a compression fracture or wear of the glenoid rim. These injuries predispose to instability because of a decrease of antero-posterior size of the inferior glenoid, loss of the glenoid concavity and compromise the static shoulder restraints [28]. Biomechanical studies have demonstrated an inverse relationship between the size of the glenoid defect and joint stability [29]. The normal glenoid surface is pear-shaped; the inferior aspect of the glenoid takes the shape of a true circle and the superior aspect of the glenoid is 20% narrower [30]. Burkhart and DeBeer coined the term “inverted-pear” for significant bone defects on the glenoid. In these patients, the normally pear-shaped glenoid had lost enough anterior-inferior bone to assume the shape of an inverted pear and the recurrence rate after an arthroscopic Bankart repair for contact athletes was reported to be significantly higher [31].
Bigliani et al. developed a classification system of the glenoid rim lesions associated to anterior shoulder instability into three types and a fourth type was lately added [32,33]. They also analyzed the relationship between the bone glenoid lesions and the outcome of surgeries.
Recurrent anterior shoulder dislocation is often associated with a humeral head defect. When the humeral head impacts on the anterior glenoid, an impression fracture is created on the posterolateral aspect of the humeral head. Hill and Sachs performed a radiologic analysis of humeral head compression fractures, which is known as a Hill-Sachs lesion and was found in 74% of patients in their series [34].
Most lesions are small to moderate in size and do not influences shoulder stability but the larger defects could prone to recurrent anterior shoulder dislocation. Rowe and co-authors classified humeral head lesions into 3 categories according to defect size [length and depth]: mild lesions represented defects that were 2 × 0.3 cm, moderate 4 × 0.5 cm and severe 4 × 1 cm or larger [35]. Bigliani and co-authors classified Hill-Sachs defects according to the percentage of head involvement using CT or MRI axial images: less than 20% [mild defect], between 20% and 45% [moderate defect] and greater than 45% [severe defect] [35,36]. Burkhart and DeBeer reported a high failure rate of soft tissue stabilization procedures when there was an “engaging” Hill-Sachs lesion of the humerus, in which the orientation of the Hill-Sachs lesion was such that it engaged the anterior glenoid with the shoulder in abduction and external rotation [31].
Recently, there has been increased awareness of the significance of humeral head defects on shoulder instability, with the acceptance that bone loss is a bipolar problem. In addition to defect of the humeral head, not only is the size of the lesion important but also the amount of articular surface involved, location of the defect relative to the glenoid track, and presence of associated glenoid bone loss [37]. The glenoid track is a contact zone of the glenoid on the humeral head with the arm at the end range of motion, e.g., in various degrees of elevation with the arm in maximum external rotation and maximum horizontal extension. This end range of motion is critical for anterior dislocation because the anterior soft tissue structures become tight and prevent the anterior translation of the humeral head in this position. In this position, those patients with recurrent anterior dislocation of the shoulder feel anterior apprehension. If the Hill-Sachs lesion is always covered by the glenoid at this end range of motion, or in other words, if the Hill- Sachs lesion stays within the glenoid track, the lesion does no harm, because it is always covered by the glenoid even at the end range of motion. On the other hand, if the lesion comes out of the glenoid coverage, it engages with the anterior rim of the glenoid and causes a dislocation. Clarifying the exact location of this contact zone or the glenoid track enables us to evaluate any Hill-Sachs lesion for its risk of engagement. According to glenoid track concept, a small defect with a significant articular cartilage involvement that creates an engaging lesion with the glenoid rim is more important than a larger defect out of the glenoid track.
Pathoanatomy of Traumatic Posterior Instability
The most frequent cause of recurrent posterior shoulder instability in the athlete is repetitive microtrauma to the posterior shoulder complex. In contrast to anterior instability, acute dislocation is not usually the most common initial presentation of posterior instability [38]. Several soft tissue and bony pathologies are encountered in a traumatic posterior instability, which nature depends on the cause of the instability.
Soft tissue lesions
Tears of the posteroinferior aspect of the capsulolabral complex [reverse Bankart lesion] involving the posterior band of the inferior glenohumeral ligament resulting of posterior load, but lesions of the posterior labrum, frequently accompanied by stretching of the posteroinferior aspect of the capsule are more common when there has been a discrete injury to the shoulder [39-42]. After recurrent posteroinferior subluxation events, the capsule undergoes plastic deformation producing a patulous posteroinferior capsular pouch and increased joint volume. The excessive capsular laxity and large capsular recess can be a cause of posterior instability [42-44].
Several variations from the typical pattern of capsulolabral pathoanatomy have been described in patients suffering posterior shoulder dislocation. Kim and colleagues described an incomplete and concealed avulsion of the posteroinferior aspect of the labrum than may be associated with unidirectional or posteroinferior instability and it is known as Kim’s lesion. In the posterior labrocapsular periosteal sleeve avulsion [POLPSA], the posterior labrum and the intact posterior scapular periosteum are stripped from the glenoid, producing a redundant recess that communicates with the joint space [45,46]. Bennett lesion is an extra-articular curvilinear calcification along the posteroinferior glenoid near to the attachment of the posterior band of the inferior glenohumeral ligament [47]. It has been hypothesized that POLPSA may represent the acute stage of a Bennett lesion [46]. Posterior dislocation of the shoulder may also result in failure of the posterior capsule at its humeral insertion, either as a humeral avulsion of the posterior band of the inferior glenohumeral ligament [IGHL] or a humeral avulsion of the posterior capsule above the level of the posterior band [PHAG], with or without extension inferiorly to involve the posterior band of the IGHL [RHAGL] [48]. These acronyms are frequently used interchangeably in the radiologic and surgical literature. A muscle strain injury or partial-thickness tear of the tendon of the teres minor muscle has been described in association with these posterior capsular injuries, and the presence of such an injury should prompt a search for either a PHAGL or RHAGL lesion [48].
Bony lesions
Acute traumatic dislocation or erosions, as a result of localized glenoid hypoplasia or repeated subluxations, damages posterior stabilizers and may present as posterior rim fractures [reverse bony Bankart lesions] [43,49,50]. There is a relationship between the extent of glenoid erosion seen on CT and posterior recurrent instability [51].
Posterior dislocation often results in an osteochondral fracture of the anterior humeral head medial to the lesser tuberosity, in the region of the anatomic neck from impaction on the posterior glenoid rim [a reverse Hill-Sachs lesion]. This may extend into the contact zone between the humeral head and the glenoid during flexion, adduction, and internal rotation, producing subsequent engagement and subjective instability or dislocation [52].
Pathoanatomy of Atraumatic Instability
Patients with multidirectional instability [MDI] tend to have generalized increase in joint volume with posterior, inferior, and anterior capsular redundancy [6]. Bony abnormalities are not generally present; however pathologic findings may be present when a traumatic dislocation is superimposed in the setting of MDI.
Outcome Measurement System
Various scoring systems for functional assessment of the shoulder have been reported [53]. Unfortunately, these scores have concentrated on range of motion, pain, functional limitation, or strength, while the primary complaint in the patient with shoulder instability. Nevertheless, sometimes the only complaint is apprehension or avoidance of activity. Because most instruments have not focused on apprehension, dichotomy has occurred in many recent studies between postoperative recurrence of instability and reporting of high shoulder scores.
When medical literature is analyzed searching for the most suitable treatment in a patient with recurrent shoulder instability, we must evaluate the overall outcomes using the different procedures of treatment. A standard, universally accepted shoulder scoring system for assessing the functional state of a normal, diseased, or postoperative shoulder does not exist. With the multitude of scoring systems used in clinical trials, a direct comparison between studies is difficult to perform and what is tested may not even be an accurate measure of shoulder instability outcomes. Hence, we should choose a scoring system reliable, valid, and responsive in patients with instability. Plancher and Lipnick analyzed several shoulder scoring systems and recommend using both the Melbourne Instability Shoulder Score [MISS] and the Western Ontario Shoulder Instability Index [WOSI] to evaluate patients with instability to make clinical decisions [54].
Treatment
Management of shoulder instability is multifactorial. Nonoperative treatment may allow return to sport, even at high levels, at a much faster rate than operative treatment [55]. The indication for surgical intervention in patients with microinstability, according to Chambers and Altchek is debilitating shoulder pain and instability despite 3 to 6 month course of suitable conservative measures [56].
Due to the fact that shoulder recurrent instability has physical and psychological consequences for the athlete, the soft tissue and bony injuries will be more severe after new dislocation events, the time away from sports participation greater and the negative impact on quality of life, surgery is generally recommended in contact athletes suffering recurrent anterior shoulder instability [57-60].
Nonoperative Treatment
Rehabilitation plays a variable role depending on the cause of the instability. Success of rehabilitation is higher in multidirectional instability and athletes with microinstability or disabled throwing shoulder than in post-traumatic instability [61].
Various braces and slings have been used to immobilize the shoulder after a first-time dislocation until pain has subsided. Individuals older than 29 years old are usually immobilized for 2 to 4 weeks to allow scarring of the injured capsule. However, no long-term benefits regarding recurrence rates and immobilization have been observed in younger patients between the ages of 17 and 29 [62-64]. Traditionally the shoulder has been immobilized with the arm close to the body in internal rotation [64]. Itoi and co-authors developed a study with use of MRI and observed the labral lesion is rolled up and away from the glenoid in the conventional immobilization position of internal rotation which is a suboptimal situation for anatomic healing, nevertheless in external rotation there was a higher rate of anatomic reduction of the labral lesion [65]. Later, Itoi and co-authors developed a prospective randomized study comparing immobilization in external rotation after an initial shoulder dislocation and reported that this position reduces the risk of recurrence compared with that associated with the conventional method of immobilization in internal rotation [66]. They also thought that his treatment method appears to be particularly beneficial for patients who are thirty years old or younger. The major limitation to the use of an external rotation brace is patient compliance but several investigators have reported no improved stability and outcomes with immobilization in external rotation; thus the current consensus as stated by Paterson and co-authors [67-69] is that there seems to be no benefit in immobilizing the shoulder in external rotation following anterior dislocation. Furthermore, potential complications with immobilization may include a decrease in joint proprioception, muscle disuse and atrophy, and a loss of range of motion in specific age groups. They also recommend a short period of immobilization for young active individuals for one week in an internal rotation traditional sling. Conversely for patients older than 30, especially if an anterior labral periosteal sleeve avulsion [ALPSA] lesion is identified, immobilization for 3 weeks in a traditional internal rotation sling is recommended.
Nonoperative treatment may be useful for the in-season athlete looking to complete the season and then undergo off-season stabilization. It may also be the most appropriate option in lower-demand patients who rejected surgery, with multidirectional instability and without a traumatic cause. In patients that fail nonoperative measures elective surgery is indicated [55].
First-Time Dislocation
The main complication associated with conservative management of traumatic first-time anterior shoulder dislocation is the risk of recurrent instability. This risk is significant and although Cordischi and co-authors found this risk to be 21%, most reports document this risk is 60% or greater, mainly in adolescents [70-77].
Every time we are considering the most suitable treatment after a first-time shoulder dislocation we have to take into account the patient’s age, direction of dislocation, kind of sport and the skill level of the patient. Kuroda and coauthors studied patients with atraumatic shoulder instability for several years with conservative treatment and noted spontaneous recovery of stability in 50 of 450 shoulders [9%] and that spontaneous recovery were statistically more likely if patients were willing to switch to nonoverhead athletics [78]. Maybe these patients still had symptoms but avoided frank dislocation because of activity modification.
Regarding the age, several authors advise surgical treatment in the youngest patients in order to reduce the risk of recurrence and facilitate patients’ return to their physical activities [79]. Rowe co-authors reported a recurrence rate after a shoulder dislocation event in patients younger than 10 years to be 100%, and 94% if between 10 and 20 years of age [71]. Postacchini and co-authors found a recurrence rate of 92% in patients between the ages of 14 and 17 years after traumatic dislocation and Hovelius and co-authors found 55% of patients younger than 22 years of age had 2 or more recurrences of dislocation at 5-year follow-up [74,76]. The rate of recurrent dislocation decreases with increasing age, with a 56% recurrence rate in those 23 to 29 years old [80], 27% rate in those older than the age of 30 and 14% to 22% rate of recurrence in those older than 60 [80,81].
Taking into account that recurrences are more likely in the early period after the first event, primary surgical treatment after first-time traumatic anterior shoulder dislocation may be considered to avoid secondary damage to articular structures. Robinson and co-authors studied a prospective cohort with 252 patients ranging from fifteen to thirty-five years old sustained an anterior glenohumeral dislocation and treated with sling immobilization. They observed more than 55% of the patients who developed recurrent dislocation did so within the first 2 years after the index event. An additional 11% developed recurrence between years 2 and 5, implying that recurrence is most likely in the early period after the primary event [82].
These factors are in favor of primary surgical treatment of first-time dislocations in young active individuals, given the risk of recurrence in these younger populations, and if patients and their families are unable or unwilling to modify the children’s activities and sports. But every case is individual and the indications and prognosis should be discussed with the patient.
Surgical Treatment
The goal of surgical treatment of recurrent dislocation in contact athletes is to achieve a stable shoulder, allowing early return to sports participation without recurrence and with minimal risk of complications [60]. In considering the most appropriate treatment, it is required to take into account the clinical manifestation of instability, the underlying pathoanatomical findings, the athletic needs of the patient and the timing within the athletic calendar. Last but not least, we have always reminded we treat patients not disease and of course we should never treat MRI.
Soft tissue procedures
As we said above, Bankart lesion is the most frequent pathoanatomical lesion after a shoulder dislocation event, occurring in 90% of cases of anterior dislocation [3].
Conventional open Bankart repair historically was the gold standard for stabilization in athletes because of low recurrence rates and high rates of return to play (24,83,84). Rowe and co-authors reported a rate of recurrent dislocation of 3.5% with 97% of athletes returning to sports after the open Bankart repair [24]. Pagnani and Dome reported a 3.4% recurrence rate in American football players, with return to play in all of the college and professional players studied [83]. Rhee and co-authors reported a recurrence rate of 12.5% and 90% returned to sports at complete or near- complete preinjury levels of performance after undergoing an open Bankart repair [84].
With the advent of arthroscopy, Bankart arthroscopic repair very quickly become the desired method of primary treatment of traumatic shoulder instability. However, with long-term follow-up the initial success rates were quite poor with recurrence rates near 50% [84-89]. Improving techniques and implants have influenced a paradigm shift toward arthroscopic repair, and now most surgeons recommend arthroscopic Bankart repair for the athlete with instability, citing equivalent results to open repair [90-98]. A meta-analysis of 26 studies of long-term outcomes in 1781 patients after Bankart repair showed an 11% recurrence rate after arthroscopic Bankart repair compared with 8% after open repair. When only the arthroscopic suture anchor technique was considered, the rate of recurrence was 8.5%. The overall rate of return to sport was 87% for arthroscopic suture anchor Bankart repair and 89% for open Bankart repair [96].
Boileau and co-authors believed that a minimum of three double- loaded suture anchors had to be used in order to obtain a satisfactory capsular shift [28]. However, a recent study reported that one to two anchors could be enough [99]. This depends on the type of injury, and one must take caution with the position of the anchors.
The Perthes and ALPSA lesions are variants of the Bankart lesion. An ALPSA lesion is better identified from the anterosuperior portal, and it must be mobilized and appropriately tensioned on the face of the glenoid [98].
Patients with posterior dislocation generally have some component of anterior and/or inferior instability [multidirectional instability] that may also need to be surgically addressed at the same time. The most commonly described soft tissue procedures involve tightening the redundant posterior capsule [i.e., vertical posterior capsular shift], reverse Bankart repair, or tendon tenodesis [i.e., reverse Putti-Platt, but this procedure is not recommended in athletes because it often causes a significant loss of internal rotation]. Thermal capsulorrhaphy has shown poor outcomes and is no longer considered as an acceptable treatment option [100,101]. Although open and arthroscopic procedures have shown positive outcomes, arthroscopic techniques are becoming more favorable [102,103]. The most commonly used arthroscopic technique involves the use of suture anchor fixation of the posterior labrum with or without capsular plication to tighten the posterior capsule.
Savoie and co-authors described 3 different arthroscopic techniques for repairing the posterior capsule and labrum [103]. They recommended using a suture anchor technique in the presence of damage to the labrum and/ or capsule. A suture capsulorrhaphy was recommended for patients with more extensive damage to the posterior inferior capsulolabral complex, those with an absent posterior capsule from prior surgery, or those with posterior inferior capsule tears near the labrum. They also described a combined tendon/capsule plication or “mini-open technique” for patients with extensive damage to the posterior capsule and the infraspinatus and teres minor tendons [as seen in competitive weightlifters].
Glenoid defects procedures
Isolated Bankart repair is generally not sufficient in the surgical management of the patients with significant glenoid defects. However, the glenoid bone defects are not always the same.
Fracture should be differentiated by the erosion bone loss. In case of anterior glenoid bone erosion with a deficiency of <20% of entire surface, without an engaging or off-track Hill–Sachs lesion, a simple Bankart repair could be still a suitable option [98]. In case of bone defect with glenoid bony Bankart or fracture, an all-arthroscopic repair of a bony Bankart lesion with incorporation of the bony fragment in the repair has had acceptable results [104]. Park and co-authors reported that following arthroscopic fixation of the glenoid fracture in its anatomic position, fragments unite and survive without resorption at 1 year [105].
In the setting of recurrent dislocations for longer than 6 months, the glenoid may remodel completely [32,106]. So, we should avoid recurrent events of shoulder dislocation and treat the patients before larger defects develop as well as to perform an accurate of study all pathoanatomical lesions before choosing the most suitable technique.
Cadaveric studies report that glenoid lesions measuring more than half of the glenoid length reduce dislocation resistance by more than 30%; and defects wider than 20% glenoid length predispose to recurrence despite Bankart repair [29,107]. When glenoid defects are significant and assume the shape of an inverted pear, the recurrence rate after an arthroscopic Bankart repair for contact athletes is significantly high. The incidence of inverted pear glenoid is significantly greater in high degree contact sports. For instance, Burkhart and co-authors reported greater incidence of inverted pear in rugby versus football players [31].
Because of both biomechanical and clinical studies have shown that when bone loss approaches a critical threshold Bankart repairs have a high failure rate when performed alone, the highest priority for selection of surgical technique in the contact or collision athlete is accurate determination of the amount of glenoid bone loss [29,28,31,32,35,55,107].
Balg and Boileau developed the Instability Severity Index Score [ISIS] to determine which patients would benefit from an open bony reconstruction and proposed a Latarjet procedure if the athlete scores high on this test [108,109]. Patients with ISIS greater than or equal to six had at least a 70% risk of recurrence with arthroscopic Bankart repair and therefore they proposed undergo a bony stabilization surgery for these patients [55].
Several procedures have been developed for glenoid bone augmentation. Bristow procedure, Latarjet procedure, autogenous iliac crest grafting [the Eden-Hybenette procedure] and fresh or fresh-frozen osteochondral allograft are the most commonly described glenoid bone augmentation procedures.
There are several studies demonstrating well to excellent clinical outcomes of the Latarjet procedure in the setting of bone loss. Burkhart and co-authors studied 47 patients who underwent an open Latarjet procedure for shoulder instability with an inverted-pear glenoid, with or without an associated engaging Hill-Sachs lesion, and reported only a 4.9% recurrence rate [109]. Schmid and co-authors studied 49 patients after failed stabilization surgery other than Latarjet procedure, revised with a coracoid transfer as described by Latarjet [110]. They reported no shoulder redislocation with forty-three shoulders [88%] subjectively graded as excellent or good. In a prospective study of 118 shoulders following a Bristow-Latarjet procedure, Hovelius and co-authors reported three shoulders redislocated and subluxations in eleven shoulders [111]. They concluded that the overall clinical results, with a satisfaction rate of 98% using the Rowe score fifteen years after the Bristow- Latarjet procedure, were good. But they only recommended this procedure for failed previous surgery and to surgeons familiar with this technique because of they observed moderate to severe joint arthropathy with longerterm follow-up. Attention must be paid to avoid positioning the graft lateral with the glenoid joint line.
A technical modification was proposed by Patte and Debeyre to address both bony and soft tissue deficiencies in patients suffering shoulder dislocation with a “triple-blocking” effect [112]. First, the coracoid graft provides a glenoid bony augmentation and restores the width of the glenoid surface, thereby increasing stability and preventing an otherwise engaging Hill-Sachs lesion from levering on a deficient anteroinferior glenoid rim [109,113]. Second, the transferred conjoined tendon creates a dynamic belt that reinforces the weak anteroinferior capsule by lowering the inferior part of the subscapularis when the arm is abducted and externally rotated [seat belt effect] [113]. Third, the labral repair to the stump of the coracoacromial ligament recreates the anterior bumper and protects the humeral head from direct contact with the coracoid bone graft [bumper effect] and completes the triple-blocking effect.
An alternative option to coracoid transfer for glenoid bone augmentation is autogenous iliac crest grafting [Eden-Hybenette procedure]. This procedure can be more suitable when a large glenoid defect is found [114]. Alternative donor sites have been described, such as the distal clavicle.
The Latarjet procedure results in a nonanatomic repair of the glenoid defect, nonanatomic capsular repair, and a lack of a traditional chondral surface in the region of the transferred coracoid graft [115]. By contrast, fresh osteochondral allograft confers the benefit of introducing potentially viable cartilage matrix and chondrocytes into the area of glenoid bone loss. Osteochondral graft can be shaped from various described donor sites including fresh- frozen glenoid, humerus, and the distal tibial plafond. Proposed disadvantages associated with fresh osteochondral allografting include a potential increase in risk of infection and pathogen transmission, increased expense, limited graft availability, and less predictable graft healing and incorporation.
Arthroscopic modifications of Latarjet procedure have been reported [113,116,117]. These procedures combine the advantages of the open procedure with those of arthroscopic stabilization. However, these procedures are technically difficult and potentially dangerous because of the proximity of the brachial plexus and axillary vessels. Furthermore, the much higher direct costs of the arthroscopic procedure [double in comparison to open surgery] do not seem, nowadays, to be justified by a benefit to the patient [118].
Posterior instability caused by posterior glenoid deficiency from an osteochondral fracture, hypoplasia, and/or excessive retroversion can be corrected using either a posterior iliac bone block or a posterior glenoid opening wedge osteotomy. Both of these posterior glenoplasty procedures aim to reinforce posterior glenoid rim deficiency, but they can also be used in patients who have had a previous capsular plication or shift that failed to prevent recurrent dislocation [119-124]. Servien and co-authors reviewed twenty-one shoulders that had undergone a posterior bone block procedure in the treatment of recurrent posterior shoulder instability and reported a low rate of recurrent dislocation following this procedure. Fifteen patients returned to sports at their pre-injury level, three patients were considered clinical failures [one with a recurrent posterior dislocation and two with substantial posterior apprehension] and two shoulders had glenohumeral arthritis on radiographs at the latest follow-up [119]. Barbier and co-authors analyzed eight patients who underwent an iliac bone-block autograft for posterior shoulder instability [120]. They did not report recurrences but only four patients were able to return to their preoperative sports activity level.
Humeral head defects procedures
Most cases of shoulder dislocation with humeral bone loss can be successfully managed with soft tissue procedures or treating glenoid defects. But large humeral defects or smaller defects involving the glenoid track in presence of associated glenoid bone loss need to be treated to prevent engagement [37].
Surgical options available for the management of humeral-sided bone loss include Hill-Sachs remplissage, autogenous or allograft resurfacing of a Hill- Sachs lesion and Hill-Sachs disimpaction. All these procedures attempt to fill the humeral defect. Depending on the extent of bone loss, athletic demands, and surgeon experience, arthroscopic or open surgical options can provide shoulder stability and return athletes to their prior level of activity.
Although unusual, sometimes humeral bone loss may be the unique lesion in shoulder instability. These patients need to recover the humeral head diameter to restore the normal glenoid track. Typically, defects between 20% and 40% are needed to justify humeral-sided bone grafting. Iliac crest bone graft or osteochondral allograft can be shaped to match the contour of the humeral head defect. Bulk grafts can be fixed with screws placed outside of the articular region or by using headless screws buried to the level of the subchondral bone. Alternatively, osteochondral plugs can be placed using a mosaicoplasty technique [125].
A series of 18 size-matched osteoarticular allograft transplantations were performed by Miniaci and Gish in patients who have previously undergone and failed repair of anterior structures of the glenoid, labrum, and/or capsule with symtomatic anterior instability [126]. The size of the defect was more than 25% of the humeral head measured by CT reconstruction. The success rate for their population studied was 100% with no recurrences of dislocation during an average 50-month follow-up. Diklic and co-authors reported that 12 of 13 patients in their series had stable shoulders after undergoing fresh-frozen femoral head allograft for humeral head defects at more than 4 years postoperatively [127].
Re and co-authors reported no instability or other complications after a transhumeral head plasty for Hill-Sachs lesion plus either the Bankart or Latarjet technique in four patients [128]. This procedure has the advantage of restoring the humeral head to a near native topography without transpositioning soft tissue structures or using a rotational osteotomy of the humeral head. However, it does not address the problem of any osteochondral defects that could be present, and it is limited to moderate-sized defects.
Remplissage, translated from French as “to fill,” is an arthroscopic procedure coined by Eugene Wolf that has been advocated as an option for the management of moderately sized humeral head defects [129]. The procedure is an arthroscopic capsulotenodesis whereby the posterior capsule and overlying infraspinatus tendon is secured into a moderately sized Hill- Sachs lesion using one or multiple suture anchors. It can be coupled with Bankart repair when there is little glenoid-sided bone loss. The effect of the remplissage procedure has been described as a check rein whereby the humeral lesion is obscured by both the interposed soft tissue and the anterior translation, and subsequent engagement is prevented because of the capsulotenodesis effect [125]. Purchase and co-authors used this technique in 24 patients and reported 7% recurrence rate secondary to traumatic events, with full restoration of motion [129]. However, range of motion, particularly external rotation, can be potentially diminished after using this technique, which may be of concern for certain athletes [130]. Boileau and co-authors also reported a mean deficit in external rotation [8º ± 7º with the arm at the side of the trunk and 9º ± 7º in abduction at the time of the last follow up] in a retrospective study of 459 patients undergoing Bankart repair plus remplissage for recurrent shoulder dislocation, but 37 of 41 athletes in their group returned to their previous sport practice following the procedure [131]. Giles and co-authors compared remplissage, allograft reconstruction, and partial resurfacing in a biomechanical study and demonstrated improved stability with all 3 techniques, with reduction in range of motion noted in the remplissage group [130]. Comparing three different remplissage techniques, medial suture placement has been reported in the greatest joint stiffness values and mean restriction in motion regard anchors in the defect valley or anchors in humeral head rim suture placement [132].
Surgical management is usually recommended in patients with a reverse Hill-Sachs defect [i.e., McLaughlin lesion] experiencing persistent posterior shoulder instability/dislocations or impression fractures affecting more than 25% to 30% of the humeral articular morphology [133]. These lesions can be corrected using either anatomic or non-anatomic techniques, but they are very rare injuries in sports.
McLaughlin procedure [134] involves transferring the subscapularis into the humeral head defect to limit maximal internal rotation and prevent the edges of reverse Hill-Sachs defects from dropping behind the posterior glenoid rim. Neer modification of McLaughlin procedure is another nonanatomic surgical technique that involves transferring the subscapularis tendon in continuity with an osteotomized lesser tuberosity [135]. A rotational osteotomy of the proximal part of the humerus could be another solution, but this is not widely used because it is technically challenging and poses a risk of humeral head devascularization [136].
On the other hand, we can choose anatomic techniques using autogenous bone grafts from the iliac crest to fill smaller defects [<25% of the humeral head articular surface]; however, allogenous osteochondral bone grafts are more commonly recommended for larger defects sizing from 40% to 50% of the articular surface [135,137,138]. These procedures have been mainly used for the treatment of chronic locked posterior dislocation and they are unusual in sport.
Capsular reinforcement
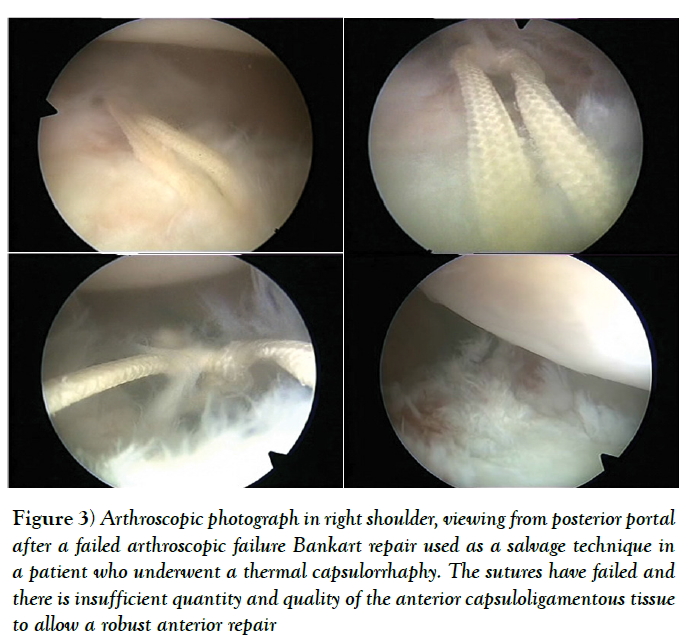
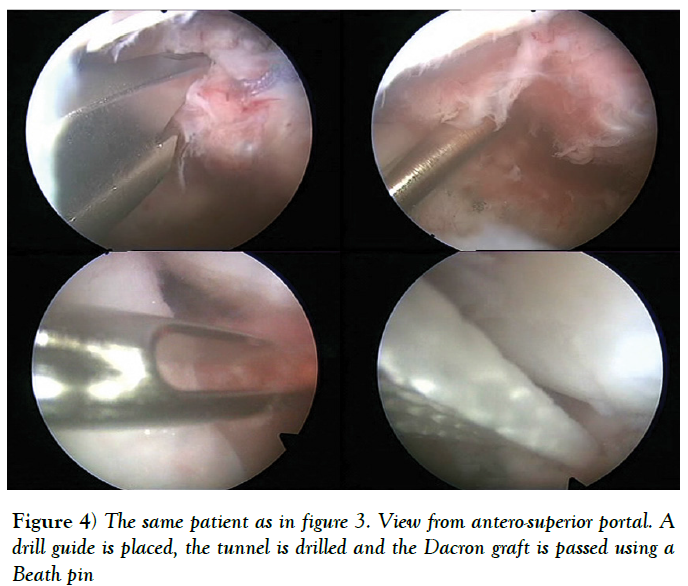
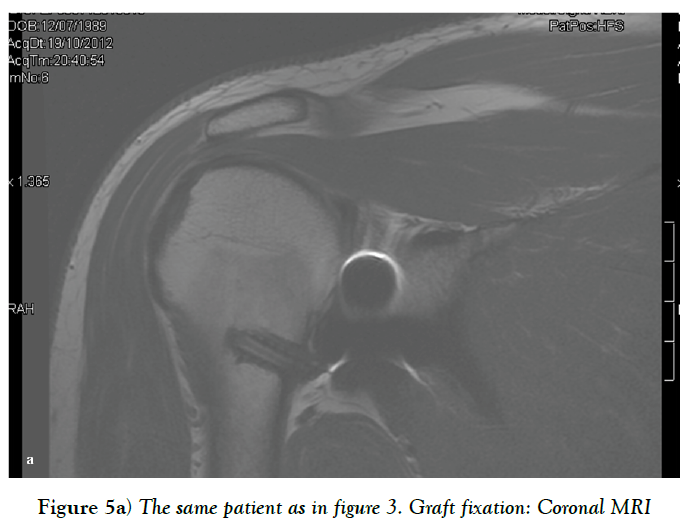
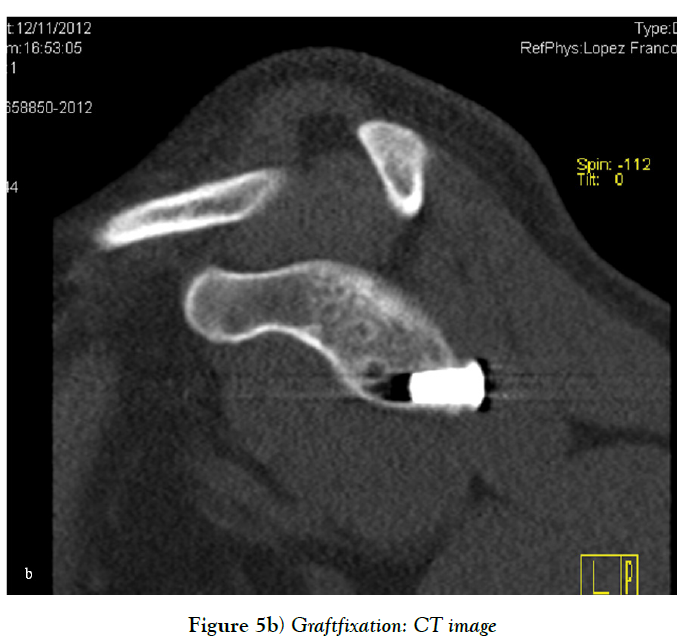
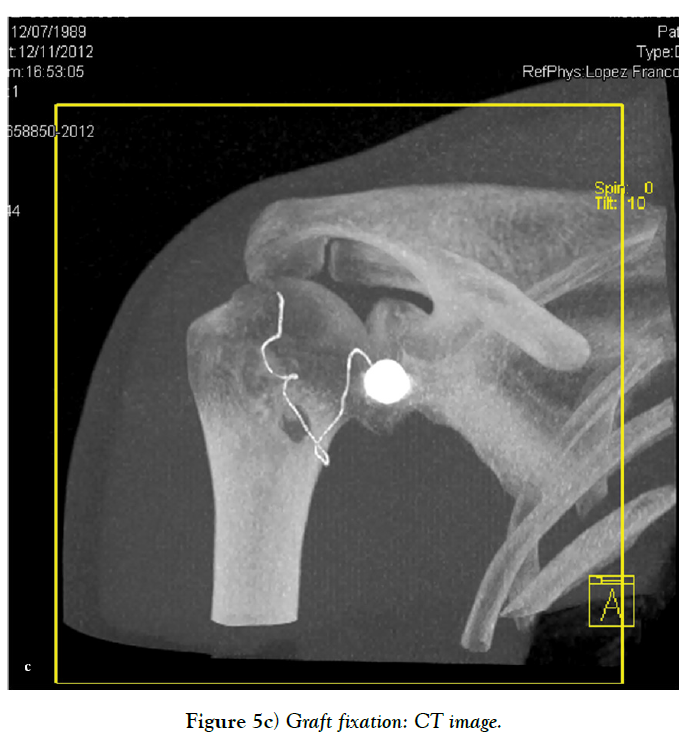
Sometimes, there is insufficient quantity and quality of the anterior capsuloligamentous tissue to allow for a robust anterior repair and a capsular reinforcement is needed. Gallie and Le Mesurier first reported a ligamentoplasty using fascia lata autograft for the treatment of anterior shoulder instability [139]. Caspari reported 8% of recurrences with an arthroscopic assisted ligamentoplasty freeze-dried using fascia lata in 50 patients [140]. Sanchez and co-authors developed an arthroscopically aided operative technique for the stabilization of the shoulder by means of an anterior capsular reinforcement [141,142]. It carried out a multicenter study group on 168 shoulders reporting a 3.57% of recurrence rate and recommended this surgical technique specially in laxity patients or patients with a failed shoulder instability procedure [142]. Lopez-Franco and coauthors reported a case of failed shoulder instability surgical treatment after thermal capsulorrhaphy using Dacron graft (Figures 3-5) [143].
Figure 3: Arthroscopic photograph in right shoulder, viewing from posterior portal after a failed arthroscopic failure Bankart repair used as a salvage technique in a patient who underwent a thermal capsulorrhaphy. The sutures have failed and there is insufficient quantity and quality of the anterior capsuloligamentous tissue to allow a robust anterior repair
Given the high risk of recurrent dislocation, young and active patients who seek to return to competitive contact sports should consider surgical stabilization after a first-time shoulder dislocation.
Clinical manifestations of instability, the underlying pathoanatomical findings, the athletic needs of the patient and the timing within the athletic calendar must be taken into account before choosing the most suitable treatment. Different open or arthroscopic techniques have been reported with equivalent overall outcomes. In considering a surgical procedure involving bone defects, the surgeons always have to measure the length and take into account the location of the injury, because isolated soft tissue repair may be not sufficient.
Authors Contributions
Mariano Lopez-Franco, David Blanco-Diaz and Maria Aranzazu Murciano- Anton contributed equally to this work, performed the research and wrote the paper.
Conflict of Interest Statement
The authors did not receive grants or outside funding in support of their research or preparation of this manuscript.
Mariano Lopez-Franco and David Blanco-Díaz have received fees for serving as a speaker for DePuy Synthes Institute. María Aranzazu Murciano-Anton none.
REFERENCES
- Wilk KE, Arrigo C. Current concepts in the rehabilitation of the athletic shoulder. J Orthop Sports Phys Ther 1993;18:365-378.
- Murray IR, Ahmed I, White NJ, et al. Traumatic anterior shoulder instability in the athlete. Scand J Med Sci Sports 2013;23:387-405.
- Murray IR, Goudie EB, Petrigliano FA, et al. Functional anatomy and biomechanics of shoulder stability in the athlete. Clin Sports Med 2013;32:607-624.
- Lephart SM, Warner JJ, Borsa PA, et al. Proprioception of the shoulder joint in healthy, unstable, and surgically repaired shoulders. J Shoulder Elbow Surg 1994;3:371-380.
- Thomas SC, Matsen FA. An approach to the repair of avulsion of the glenohumeral ligaments in the management of traumatic anterior glenohumeral instability. J Bone Joint Surg Am 1989;71:506-513.
- Johnson SM, Robinson CM. Shoulder instability in patients with joint hyperlaxity. J Bone Joint Surg Am 2010;92:1545-1557.
- Dowdy PA, O’Driscoll SW. Shoulder instability. An analysis of family history. J Bone Joint Surg Br 1993;75:782-784.
- Jaggi A, Lambert S. Rehabilitation for shoulder instability. Br J Sports Med 2010;44:333-340.
- Owens BD, Dawson L, Burks R, et al. Incidence of shoulder dislocation in the United States military: demographic considerations from a high-risk population. J Bone Joint Surg Am 2009;91:791-796.
- Simonet WT, Melton LJ, Cofield RH, et al. Incidence of anterior shoulder dislocation in Olmsted County, Minnesota. Clin Orthop Relat Res 1984;186:186-191.
- Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am 2010;92:542-549.
- Nordqvist A, Petersson CJ. Incidence and causes of shoulder girdle injuries in an urban population. J Shoulder Elbow Surg 1995;4:107-112.
- Kroner K, Lind T, Jensen J. The epidemiology of shoulder dislocations. Arch Orthop Trauma Surg 1989;108:288-290.
- Owens BD, Agel J, Mountcastle SB, et al. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med 2009;37:1750-1754.
- Bradley JP, Forsythe B, Mascarenhas R. Arthroscopic management of posterior shoulder instability: diagnosis, indications, and technique. Clin Sports Med 2008;27:649-670.
- Committee on Sports Medicine and Fitness. American Academy of Pediatrics: Medical conditions affecting sports participation. Pediatrics 2001;107: 1205-1209.
- Speer KP, Deng X, Borrero S, et al. Biomechanical evaluation of a simulated Bankart lesion. J Bone Joint Surg Am 1994;76:1819-1826.
- Bencardino JT, Beltran J. MR imaging of the glenohumeral ligaments. Radiol Clin North Am 2006;44:489–502.
- Bankart AS. The pathology and treatment of recurrent instability of the shoulder-joint. Br J Surg 1938;26:23–29.
- Lichtenberg S, Habermeyer P. Operative Arthroskopie des Glenohumeralgelenks. In: Schulterchirurgie. Munchen, Jena, Urban und Fischer, 2002:237-271.
- Owens BD, Nelson BJ, Duffey ML, et al. Pathoanatomy of first-time, traumatic, anterior glenohumeral subluxation events. J Bone Joint Surg Am 2010;92:1605–1611.
- Wang RY, Arciero RA. Treating the athlete with anterior shoulder instability. Clin Sports Med 2008;27:631-648.
- Bigliani LU, Pollock RG, Soslowsky LJ, et al. Tensile properties of the inferior glenohumeral ligament. J Orthop Res 1992;10:187-197.
- Rowe CR, Patel D, Southmayd WW. The Bankart procedure: a long-term end-result study. J Bone Joint Surg Am 1978;60:1-16.
- Digiovine NM, Jobe FW, Pink M, et al. An electromyographic analysis of the upper extremity in pitching. J Shoulder Elbow Surg 1992;1:15-25.
- Glousman R, Jobe F, Tibone J, et al. Dynamic electromyographic analysis of the throwing shoulder with glenohumeral instability. J Bone Joint Surg Am 1988;70:220-226.
- Hodge DK, Safran MR. Sideline management of common dislocations. Curr Sports Med Rep 2002;1:149-155.
- Boileau P, Villalba M, Hery JY, et al. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am 2006;88:1755-1763.
- Itoi E, Lee SB, Berglund LJ, et al. The effect of a glenoid defect on anteroinferior stability of the shoulder after Bankart repair: a cadaveric study. J Bone Joint Surg Am 2000;82:35-46.
- Huysmans PE, Haen PS, Kidd M, et al. The shape of the inferior part of the glenoid: A cadaveric study. J Shoulder Elbow Surg 2006;15:759-763.
- Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic Bankart repairs. Arthroscopy 2000;16:677-694.
- Bigliani LU, Newton PM, Steinmann SP, et al. Glenoid rim lesions associated with recurrent anterior dislocation of the shoulder. Am J Sports Med 1998;26:41-45.
- Bois AJ, Walker RE, Kodali P, et al. Imaging instability in the athlete: the right modality for the right diagnosis. Clin Sports Med 2013;32:653-684.
- Hill HA, Sachs MD. The grooved defect of the humeral head: A frequently unrecognized complication of dislocations of the shoulder joint. Radiology 1940;35:690-700.
- Rowe CR, Zarins B, Ciullo JV. Recurrent anterior dislocation of the shoulder after surgical repair. Apparent causes of failure and treatment. J Bone Joint Surg Am 1984;66:159-168.
- Bigliani LU, Flatow EL, Pollock RG. Fractures of the proximal humerus. In: Fractures in adults. 4th edition. Edited by Rockwood CA, Green DP, Bucholz RW. Philadelphia: Lippincott-Raven; 1996:1055-1107.
- Yamamoto N, Itoi E, Abe H, et al. Contact between the glenoid and the humeral head in abduction, external rotation, and horizontal extension: a new concept of glenoid track. J Shoulder Elbow Surg 2007;16:649-656.
- Provencher MT, LeClere LE, King S, et al. Posterior instability of the shoulder: diagnosis and management. Am J Sports Med 2011;39:874-886.
- Bigliani LU, Pollock RG, McIlveen SJ, et al. Shift of the posteroinferior aspect of the capsule for recurrent posterior glenohumeral instability. J Bone Joint Surg Am 1995;77:1011-1020.
- Papendick LW, Savoie FH. Anatomy-specific repair techniques for posterior shoulder instability. J South Orthop Assoc 1995;4:169-176.
- Hawkins RJ, Janda DH. Posterior instability of the glenohumeral joint. A technique of repair. Am J Sports Med 1996;24:275-278.
- Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am 2003;85A:1479-1487.
- Fronek J, Warren RF, Bowen M. Posterior subluxation of the glenohumeral joint. J Bone Joint Surg Am 1989;71:205-216.
- Dewing CB, McCormick F, Bell SJ, et al. An analysis of capsular area in patients with anterior, posterior, and multidirectional shoulder instability. Am J Sports Med 2008;36:515-522.
- Kim SH, Ha KI, Yoo JC, et al. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy 2004;20:712-720.
- Yu JS, Ashman CJ, Jones G. The POLPSA lesion: MR imaging findings with arthroscopic correlation in patients with posterior instability. Skeletal Radiol 2002;31:396-399.
- Van Tongel A, Karelse A, Berghs B, et al. Posterior shoulder instability: current concepts review. Knee Surg Sports Traumatol Arthrosc 2011;19:1547-1553.
- Bokor DJ, Fritsch BA. Posterior shoulder instability secondary to a reverse humeral avulsion of the glenohumeral ligament. J Shoulder Elbow Surg 2010;19:853-858.
- Norwood LA, Terry GC. Shoulder posterior subluxation. Am J Sports Med 1984;12:25-30.
- Schwartz E, Warren RF, O’Brien SJ, et al. Posterior shoulder instability. Orthop Clin North Am 1987;18:409-419.
- Weishaupt D, Zanetti M, Nyffeler RW, et al. Posterior glenoid rim deficiency in recurrent (atraumatic) posterior shoulder instability. Skeletal Radiol 2000;29:204-210.
- Goudie EB, Murray IR, Robinson CM. Instability of the shoulder following seizures. J Bone Joint Surg Br 2012;94:721-728.
- Kirkley A, Griffin S, Dainty K. Scoring systems for the functional assessment of the shoulder. Arthroscopy 2003;19:1109-1120.
- Plancher KD, Lipnick SL. Analysis of evidence-based medicine for shoulder instability. Arthroscopy 2009;25:897-908.
- Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med 2013;32:709-730.
- Chambers L, Altchek DW. Microinstability and internal impingement in overhead athletes. Clin Sports Med 2013;32:697-707.
- Owens B, Dickens J, Kilcoyne K, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg 2012;20:518-526.
- Headey J, Brooks J, Kemp S. The epidemiology of shoulder injuries in English professional rugby union. Am J Sports Med 2007;35:1537-1543.
- Meller R, Krettek C, Gosling T, et al. Recurrent shoulder instability among athletes: Changes in quality of life, sports activity, and muscle function following open repair. Knee Surg Sports Traumatol Arthrosc 2007;15:295-304.
- Joshi MA, Young AA, Balestro JC, et al. The Latarjet-Patte procedure for recurrent anterior shoulder instability in contact athletes. Clin Sports Med 2013;32:731-739.
- Bak K, Wiesler ER, Poehling GG. ISAKOS Upper Extremity Committee. Consensus statement on shoulder instability. Arthroscopy 2010;26:249-255.
- Hovelius L, Augustini BG, Fredin H, et al. Primary anterior dislocation of the shoulder in young patients. A ten-year prospective study. J Bone Joint Surg Am 1996;78:1677-1684.
- Kiviluoto O, Pasila M, Jaroma H, et al. Immobilization after primary dislocation of the shoulder. Acta Orthop Scand 1980;51:915-919.
- Wilk KE, Macrina LC. Nonoperative and postoperative rehabilitation for glenohumeral instability. Clin Sports Med 2013;32:865-914.
- Itoi E, Sashi R, Minagawa H, et al. Position of immobilization after dislocation of the glenohumeral joint. A study with use of magnetic resonance imaging. J Bone Joint Surg Am 2001;83:661-667.
- Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence. A randomized controlled trial. J Bone Joint Surg Am 2007;89:2124-2131.
- Finestone A, Milgrom C, Radeva-Petrova DR, et al. Bracing in external rotation for traumatic anterior dislocation of the shoulder. J Bone Joint Surg Br 2009;91:918-921.
- Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am 2011;93:897-904.
- Paterson WH, Throckmorton TW, Koester M, et al. Position and duration of immobilization after primary anterior shoulder dislocation: a systematic review and meta-analysis of the literature. J Bone Joint Surg Am 2010;92:2924-2933.
- Cordischi K, Li X, Busconi B. Intermediate outcomes after primary traumatic anterior shoulder dislocation in skeletally immature patients aged 10 to 13 years. Orthopedics 2009;32.
- Rowe CR. Prognosis in dislocations of the shoulder. J Bone Joint Surg Am 1956;38:957-977.
- Wagner KT, Lyne ED. Adolescent traumatic dislocations of the shoulder with open epiphyses. J Pediatr Orthop 1983;3:61-62.
- Marans HJ, Angel KR, Schemitsch EH, et al. The fate of traumatic anterior dislo- cation of the shoulder in children. J Bone Joint Surg Am 1992;74:1242-1244.
- Hovelius L. Anterior dislocation of the shoulder in teenagers and young adults. Five-year prognosis. J Bone Joint Surg Am 1987;69:393-399.
- Deitch J, Mehlman CT, Foad SL, et al. Traumatic anterior shoulder dislocation in adolescents. Am J Sports Med 2003;31:758-763.
- Postacchini F, Gumina S, Cinotti G. Anterior shoulder dislocation in adolescents. J Shoulder Elbow Surg 2000;9:470-474.
- Hovelius L. The natural history of primary anterior dislocation of the shoulder in the young. J Orthop Sci 1999;4:307-317.
- Kuroda S, Sumiyoshi T, Moriishi J, et al. The natural course of atraumatic shoulder instability. J Shoulder Elbow Surg 2001;10:100-104.
- Milewski MD, Nissen CW. Pediatric and adolescent shoulder instability. Clin Sports Med 2013;32:761-779.
- Hovelius L, Olofsson A, Sandstrom B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am 2008;90:945-952.
- Gumina S, Postacchini F. Anterior dislocation of the shoulder in elderly patients. J Bone Joint Surg Br 1997;79:540-543.
- Robinson CM, Howes J, Murdoch H, et al. Functional outcome and risk of recurrent instability after primary traumatic anterior shoulder dislocation in young patients. J Bone Joint Surg Am 2006;88:2326-2336.
- Gill TJ, Micheli LJ, Gebhard F, et al. Bankart repair for anterior instability of the shoulder. Long-term outcome. J Bone Joint Surg Am 1997;79:850-857.
- Pagnani MJ, Dome DC. Surgical treatment of traumatic anterior shoulder instability in American football players. J Bone Joint Surg Am 2002;84:711-715.
- Walch G, Boileau P, Levigne C, et al. Arthroscopic stabilization for recurrent anterior shoulder dislocation: results of 59 cases. Arthroscopy 1995;1:173-179.
- Guanche CA, Quick DC, Sodergren KM, et al. Arthroscopic versus open reconstruction of the shoulder in patients with isolated Bankart lesions. Am J Sports Med 1996;24:144-148.
- Mologne TS, Lapoint JM, Morin WD, et al. Arthroscopic anterior labral reconstruction using a transglenoid suture technique: results in active duty military patients. Am J Sports Med 1996,24:268-274.
- Manta JP, Organ S, Nirschl RP, et al. Arthroscopic transglenoid suture capsulolabral repair. Am J Sports Med 1997;25:614-618.
- Geiger DF, Hurley JA, Tovey JA, et al. Results of arthroscopic versus open Bankart suture repair. Clin Orthop Relat Res 1997;337:111-117.
- O’Neill DB. Arthroscopic Bankart repair of anterior detachments of the glenoid labrum. A prospective study. J Bone Joint Surg Am 1999;81:1357-1366.
- Cole BJ, L’Insalata J, Irrgang J, et al. Comparison of arthroscopic and open anterior shoulder stabilization. A two to six-year follow-up study. J Bone Joint Surg Am 2000;82:1108-1114.
- Gartsman GM, Roddey TS, Hammerman SM. Arthroscopic treatment of anterior-inferior glenohumeral instability. J Bone Joint Surg 2000;82:991-1003.
- Mishra DK, Fanton GS. Two-year outcome of arthroscopic Bankart repair and electrothermal-assisted capsulor- rhaphy for recurrent traumatic anterior shoulder instability. Arthroscopy 2001;17:844-849.
- Kropf EJ, Tjoumakaris FP, Sekiya JK. Arthroscopic shoulder stabilization: is there ever a need to open? Arthroscopy 2007;23:779-784.
- Kartus C, Kartus J, Matis N, et al. Long-term independent evaluation after arthroscopic extra-articular Bankart repair with absorbable tacks. A clinical and radiographic study with a seven to ten-year follow-up. J Bone Joint Surg Am 2007;89:1442-1448.
- Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy 2013;29:920-933.
- Gwathmey FW, Warner JJ. Management of the athlete with a failed shoulder instability procedure. Clin Sports Med 2013;32:833-863.
- Castagna A, Garofalo R, Conti M, et al. Arthroscopic Bankart repair: Have we finally reached a gold standard? Knee Surg Sports Traumatol Arthrosc 2016;24:398-405.
- Witney-Lagen C, Perera N, Rubin S, et al. Fewer anchors achieves successful arthroscopic shoulder stabilization surgery: 114 patients with 4 years of follow-up. J Shoulder Elbow Surg 2014;23:382-387.
- Toth AP, Warren RF, Petrigliano FA, et al. Thermal shrinkage for shoulder instability. HSS J 2011;7:108-114.
- Lubowitz JH, Poehling GG. Glenohumeral thermal capsulorrhaphy is not recommended–shoulder chondrolysis requires additional research. Arthroscopy 2007;23:687.
- Bottoni CR, Franks BR, Moore JH, et al. Operative stabilization of posterior shoulder instability. Am J Sports Med 2005;33:996-1002.
- Savoie FH, Holt MS, Field LD, et al. Arthroscopic management of posterior instability: evolution of technique and results. Arthroscopy 2008;24:389-396.
- Zhu YM, Jiang CY, Lu Y, et al. Clinical results after all arthroscopic reduction and fixation of bony Bankart lesion. Zhonghua Wai Ke Za Zhi 2011;49:603-606.
- Park JY, Lee SJ, Lhee SH, et al. Follow-up computed tomography arthrographic evaluation of bony Bankart lesions after arthroscopic repair. Arthroscopy 2012;28:465-473.
- Sugaya H, Moriishi J, Dohi M, et al. Glenoid rim morphology in recurrent anterior glenohumeral instability. J Bone Joint Surg Am 2003;85:878-884.
- Gerber C, Nyffeler RW. Classification of glenohumeral joint instability. Clin Orthop Relat Res 2002;400:65-76.
- Balg F, Boileau P. The instability severity index score: a simple pre-operative score to select patients for arthroscopic or open shoulder stabilization. J Bone Joint Surg Am 2007;89:1470-1477.
- Burkhart SS, De Beer JF, Barth JR, et al. Results of modified Latarjet reconstruction in patients with anteroinferior instability and significant bone loss. Arthroscopy 2007;23:1033-1041.
- Schmid SL, Farshad M, Catanzaro S, et al. The Latarjet procedure for the treatment of recurrence of anterior instability of the shoulder after operative repair: a retrospective case series of forty-nine consecutive patients. J Bone Joint Surg Am 2012;94:e75.
- Hovelius L, Sandstrom B, Sundgren K, et al. One hundred eighteen Bristow-Latarjet repairs for recurrent anterior dislocation of the shoulder prospectively followed for fifteen years: study I-clinical results. J Shoulder Elbow Surg 2004;13:509-516.
- Patte D, Debeyre J. Luxations recidivantes de l’epaule. Encycl Med Chir Paris- Technique chirurgicale Orthopedie 1980;44265:44-52.
- Lafosse L, Boyle S. Arthroscopic Latarjet procedure. J Shoulder Elbow Surg 2010;19:2-12.
- Warner JJ, Gill TJ, O’Hollerhan JD, et al. Anatomical glenoid reconstruction for recurrent anterior glenohumeral instability with glenoid deficiency using an autogenous tricortical iliac crest bone graft. Am J Sports Med 2006;34:205-212.
- Provencher MT, Detterline AJ, Ghodadra N, et al. Measurement of glenoid bone loss: a comparison of measurement error between 45 degrees and 0 degrees bone loss models and with different posterior arthroscopy portal locations. Am J Sports Med 2008;36:1132-1138.
- Lafosse L, Lejeune E, Bouchard A, et al. The arthroscopic Latarjet procedure for the treatment of anterior shoulder instability. Arthroscopy 2007;23:1242.e1–e5.
- Boileau P, Thelu CÉ, Mercier N, et al. Arthroscopic Bristow-Latarjet combined with Bankart repair restores shoulder stability in patients with glenoid bone loss. Clin Orthop Relat Res 2014;472:2413-2424.
- Randelli P, Fossati C, Stoppani C, et al. Open Latarjet versus arthroscopic Latarjet: clinical results and cost analysis. Knee Surg Sports Traumatol Arthrosc 2016;24:526-532.
- Servien E, Walch G, Cortes ZE, et al. Posterior bone block procedure for posterior shoulder instability. Knee Surg Sports Traumatol Arthrosc 2007;15:1130-1136.
- Barbier O, Ollat D, Marchaland JP, et al. Iliac bone-block autograft for posterior shoulder instability. Orthop Traumatol Surg Res 2009;95:100-107.
- Levigne C, Garret J, Grosclaude S, et al. Surgical technique arthroscopic posterior glenoidplasty for posterosuperior glenoid impingement in throwing athletes. Clin Orthop Relat Res 2012;470:1571-1578.
- Brewer BJ, Wubben RC, Carrera GF. Excessive retroversion of the glenoid cavity. A cause of non-traumatic posterior instability of the shoulder. J Bone Joint Surg Am 1986;68:724-731.
- Millett PJ, Clavert P, Hatch GF, et al. Recurrent posterior shoulder instability. J Am Acad Orthop Surg 2006;14:464-476.
- Tannenbaum EP, Sekiya JK. Posterior shoulder instability in the contact athlete. Clin Sports Med 2013;32:781-796.
- Griffin JW, Brockmeier SF. Shoulder instability with concomitant bone loss in the athlete. Clin Sports Med 2013;32:741-760.
- Miniaci A, Gish M. Management of Anterior Glenohumeral Instability Associated with a Large Hill-Sachs Defect. Techniques in Shoulder and Elbow Surgery 2004;5:170–175.
- Diklic ID, Ganic ZD, Blagojevic ZD, et al. Treatment of locked chronic posterior dislocation of the shoulder by reconstruction of the defect in the humeral head with an allograft. J Bone Joint Surg Br 2010;92:71-76.
- Re P, Gallo RA, Richmond JC. Transhumeral head plasty for large Hill-Sachs lesions. Arthroscopy 2006;22:798e1-4.
- Purchase RJ, Wolf EM, Hobgood ER, et al. Hill-Sachs “remplisage": An arthroscopic solution for the engaging Hill-Sachs lesion. Arthroscopy 2008;24:723-726.
- Giles JW, Elkinson I, Ferreira LM, et al. Moderate to large engaging Hill-Sachs defects: an in vitro biomechanical comparison of the remplissage procedure, allograft humeral head reconstruction, and partial resurfacing arthroplasty. J Shoulder Elbow Surg 2012;21:1142–1151.
- Boileau P, O’Shea K, Vargas P, et al. Anatomical and functional results after arthroscopic Hill-Sachs remplissage. J Bone Joint Surg Am 2012;94:618-626.
- Elkinson I, Giles JW, Boons HW, et al. The shoulder remplissage procedure for Hill-Sachs defects: does technique matter? J Shoulder Elbow Surg 2013;22:835-841.
- Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am 2005;87:639-650.
- McLaughlin H. Posterior dislocation of the shoulder. J Bone Joint Surg Am 1952;24:584-590.
- Hawkins RJ, Neer CS, Pianta RM, et al. Locked posterior dislocation of the shoulder. J Bone Joint Surg Am 1987;69:9-18.
- Keppler P, Holz U, Thielemann FW, et al. Locked posterior dislocation of the shoulder: treatment using rotational osteotomy of the humerus. J Orthop Trauma 1994;8:286-292.
- Gerber C, Lambert SM. Allograft reconstruction of segmental defects of the humeral head for the treatment of chronic locked posterior dislocation of the shoulder. J Bone Joint Surg Am 1996;78:376-382.
- Connor PM, Boatright JR, D’Alessandro DF. Posterior fracture-dislocation of the shoulder: treatment with acute osteochondral grafting. J Shoulder Elbow Surg 1997;6:480-485.
- Gallie WE, Le Mesurier AB. Recurring dislocation of the shoulder. J Bone Joint Surg Br 1948,30:9-18.
- Caspari RB. Arthroscopic reconstruction for anterior shoulder instability. Tech Orthop 1988;3:59-66.
- Sanchez M, Cuellar R, Garcia A, et al. Anterior stabilization of the shoulder by means of an artificial capsular reinforcement and arthroscopy-Part I: Surgical Technique. Journal of Long-Term Effects of Medical Implants 2000;10:187-197.
- Sanchez M, Cuellar R, Garcia A, et al. Anterior stabilization of the shoulder by means of an artificial capsular reinforcement and arthroscopy-Part II: Results. Journal of Long-Term Effects of Medical Implants 2000;10:199-209.
- Lopez-Franco M, Murciano MA, Sanjurjo J, et al. Treatment of a failed surgery shoulder instability using a ligamentoplasty technique]. Noticias SOMACOT 2014;38:6-9.