Stress cardiomyopathy in patients with COVID-19 infection
2 Division of Cardiology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
3 Department of Internal Medicine, University of Arkansas for Medical Sciences, Little Rock, AR, USA, Email: Mehtajl@uams.edu
Received: 10-Jan-2021 Accepted Date: Mar 10, 2021; Published: 17-Mar-2021
Citation: Sharma T, Mansour M, Al-Emam AR, et al. Stress cardiomyopathy in patients with COVID-19 infection. Clin Cardiol J. 2021;5(2):1-3.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: COVID-19 pandemic has been the most widespread pandemic in the last century. 8% of COVID-19 patients have some sort of cardiac injury. Only sporadic case reports and case series have been published addressing stress cardiomyopathy in this patient population. We reviewed the clinical presentation, pathogenesis, and outcomes of stress cardiomyopathy in patients with COVID-19 infection.
Methods: We did a key-word search through PubMed and MEDLINE for stress cardiomyopathy and COVID-19 published between December 1, 2019 and November 1, 2020. We included all case reports and case series of stress cardiomyopathy in patients who tested positive for COVID-19.
Results: We found 37 patients, 43.25% were males. 21.6% presented with chest pain and 90% had elevated troponin during the illness. ECG changes included T wave inversion in 37.4% and ST segment elevation in 35% of patients. Coronary angiography when pursued was negative for obstructive coronary artery disease. The diagnosis was confirmed by decreased left ventricular systolic function (</=45%) and wall motion abnormalities on echocardiography or ventriculography. 68% had the typical Takotsubo pattern, while reverse-Takotsubo pattern was observed in 19% of patients. 8% patients had global/ biventricular hypokinesia and 5% were found to have a mid-ventricular variant. 73% of reported patients recovered completely from COVID-19.
Conclusion: Stress cardiomyopathy can be important consequence of COVID-19 infection. High index of suspicion is crucial for prompt diagnosis and management.
Keywords
Stress cardiomyopathy; Takotsubo cardiomyopathy; COVID-19; SARSCoV- 2
Introduction
COVID-19 infection was first described in December 2019 as acute hypoxemic respiratory failure due to a novel Coronavirus, later named SARS-CoV-2. While predominantly identified as a respiratory syndrome, a sizeable proportion of patient population also had concomitant cardiac involvement. Multiple independent case reports have identified myocarditis, heart failure, arrhythmias and thromboembolic disease as common cardiovascular manifestations of COVID-19. According to a meta-analysis involving 1527 individuals, around 8% of patients were seen to have cardiac injury [1].
Stress cardiomyopathy has been identified as an important differential diagnosis in patients with COVID-19 infection presenting with elevated troponin levels, electrocardiographic (ECG) changes and myocardial dysfunction in the absence of obstructive coronary lesions. In a global online survey with echocardiographic information of over 1200 patients with COVID-19, about 2% patients showed patterns consistent with stress cardiomyopathy or Takotsubo physiology [2]. We found 37 reported cases of stress cardiomyopathy in patients with COVID-19 infection. With this review, we attempt to summarize the findings noted in these cases and elucidate the pathophysiology of stress cardiomyopathy in COVID-19.
Literature Review
We did a key-word search through electronic medical databases including PubMed and MEDLINE for all case reports published in English language (or for which translation could be found online) including the keywords COVID-19 and stress cardiomyopathy, Takotsubo cardiomyopathy or Takotsubo syndrome. We additionally searched the references of all articles retrieved. As of November 1, 2020, we found 26 results, two reports were excluded as the patient remained negative for COVID-19 through-out the course of the illness. We review 20 case reports and 4 case series with a total of 37 patients presenting with stress cardiomyopathy [3-26].
Results
Amongst the 37 patients reported, 43.25% (16) were males, with a median age of 67 years. At presentation, dyspnea, cough or fever were reported by the vast majority of the patients. Only 8 patients complained of chest pain during the illness [5,6,11,12,17,18,21,23]. One patient presented with syncope and one presented with neurological symptoms concerning for stroke [12,13]. Out of 36 patients, 32 (90%) had elevated troponin. Brain natriuretic peptide (BNP or pro-BNP) levels were reported as elevated in 27 patients. Approximately, 35% (n=13) had significant ST segment elevations and 37.4% (n=14) had T wave inversions on ECG. The ECG changes were not limited to a specific anatomical distribution and 54% (n=13) patients had diffuse changes. Echocardiogram, when mentioned was significant for reduced ejection fraction (EF) less than/equal to 45% in all but one of the reported cases of stress cardiomyopathy due to COVID-19 [16]. About, 68% (n=25) patients had apical hypokinesis and basal hyperkinesis, or the typical Takotsubo pattern on echocardiogram or ventriculography, while 19% (n=7) had basal hypokinesis or reverse-Takotsubo pattern. Two cases presented with midventricular hypokinesia with apical sparing and three cases had biventricular or global hypokinesis. Eleven patients underwent coronary angiography, and one underwent CT coronary angiography. Only one patient had a significant obstruction in left anterior descending and diagonal arteries for which she underwent percutaneous coronary intervention, but the pattern of wall motion abnormality on ventriculography was not consistent with the obstructive disease [13]. Twelve patients had repeat imaging prior to discharge (6-62 days after initial diagnosis) with improvement in ventricular function. Amongst the 37 patients, outcome data was reported for 36 patients. Nine patients died due to progressive respiratory and/or circulatory decompensation. About, 50% (n=18) patients required mechanical ventilation and 2 patients [10,12] were reported to require mechanical circulatory support in the form of extracorporeal membrane oxygenation (ECMO).
Discussion
Stress cardiomyopathy or Takotsubo syndrome is an acute reversible heart failure syndrome characterized by impaired left ventricular systolic function and regional wall motion abnormalities that may extend beyond a single coronary vascular bed [27,28]. The etiology is not completely understood but emotional or physical stress is described as a trigger for stress cardiomyopathy [29]. Physical stress includes acute critical illness, sepsis, surgery or severe pain. Association of COVID-19 and stress cardiomyopathy has been noted in multiple independent case series and individual case reports. Secco et al. described a series of eleven patients with history suspicious for acute coronary syndrome. On coronary angiography, three patients were found to have non obstructive coronary disease and apical ballooning on ventriculogram, typical of stress cardiomyopathy [30].
The diagnosis of stress cardiomyopathy is based on the fulfilment of certain diagnostic criteria. The criteria devised by Heart Failure Association of the European Society of Cardiology defines stress cardiomyopathy as the presence of (i) transient regional wall motion abnormalities of left ventricle or right ventricle myocardium, which are frequently, but not always, preceded by a stressful trigger (emotional or physical), (ii) regional wall motion abnormalities usually extending beyond a single epicardial vascular distribution, and often resulting in circumferential dysfunction of the ventricular segments involved, (iii) absence of culprit atherosclerotic coronary artery disease or other pathological conditions to explain the pattern of temporary left ventricular (LV) dysfunction observed (e.g., hypertrophic cardiomyopathy, viral myocarditis), (iv) new and reversible electrocardiography abnormalities (ST-segment elevation, ST-segment depression, left bundle branch block, T-wave inversion, and/or QTc prolongation) during the acute phase (3 months), (v) significantly elevated serum natriuretic peptide (BNP or NTproBNP) during the acute phase, (vi) positive but relatively small elevation in cardiac troponin measured with conventional assay and (vii) recovery of ventricular systolic function on cardiac imaging at follow-up (3 to 6 months) [31].
Risk factors
Stress cardiomyopathy is traditionally seen more commonly in postmenopausal women. Psychiatric comorbidities like anxiety and panic disorders are commonly pre-existent [32]. Diabetes mellitus is also described as a risk factor, as is asthma [29]. Hyperlipidemia has also been recognized as a pre-disposing factor [33]. Only 26 patients had reported comorbidities, among the 26 patients 13 had history of hyperlipidemia, 14 with Diabetes, 21 with hypertension, one had generalized anxiety disorder and two with previously diagnosed schizophrenia. Presence of other psychiatric comorbidities was not reported.
Pathophysiology
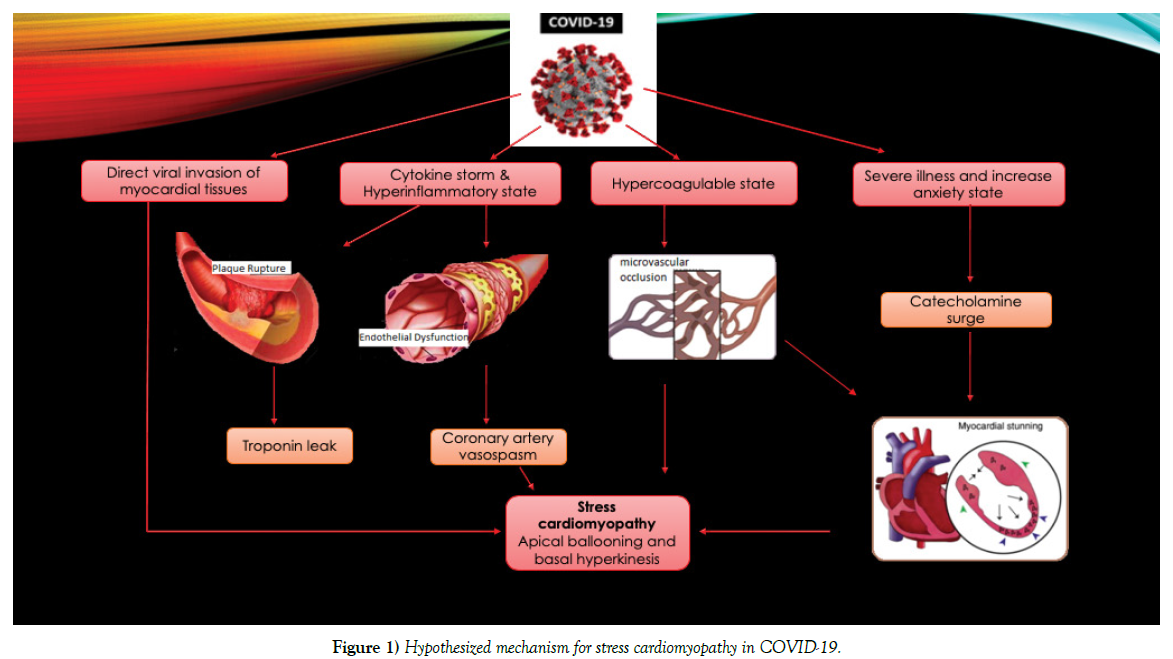
Stress induced cardiomyopathy is far more prevalent in women. In our cohort of COVID-19 patients, although stress cardiomyopathy is more common in female patients (43.25% vs 56.75%) we observed increasing numbers of males with stress cardiomyopathy causing decrease in the gap between females and males. This is probably because male patients are more likely to be infected with the novel coronavirus and more likely to have severe illness. The pathophysiology of stress cardiomyopathy is only partially understood. A triggering event is present in about three-quarters of the affected patients. Emotional triggers are identified more commonly in women [32,34,35]. Only one patient in our review was identified to have a recent emotional stressor in the form of a sudden demise of spouse to SARS-COV-2 infection. While initially identified to be triggered by intense emotional states instilling a sense of doom, danger or desperation; physical stress is noted to be a more common trigger of stress cardiomyopathy overall [32]. While bacterial sepsis is a common cause of stress cardiomyopathy, cases attributed to viral illness are rare [36]. Acute respiratory failure due to exacerbation of chronic obstructive pulmonary disease and pneumonia have also been implicated. Some association between acute viral illnesses and stress cardiomyopathy has been noted in the form of isolated case reports with dengue fever and influenza viruses. It was noted that stress cardiomyopathy is more relevant with COVID-19 infection due to the presence of a florid inflammatory response [37]. Stress adrenergic discharge, catecholamine induced myocardial stunning, coronary endothelial dysfunction, plaque rupture, and microvascular vasoconstriction and dysfunction could be involved in the pathogenesis of stress cardiomyopathy in COVID-19 [38]. This has been summarized in Figure 1.
Figure 1: Hypothesized mechanism for stress cardiomyopathy in COVID-19.
Of note, a higher rate of incidence of stress cardiomyopathy has been noted even in the non-infected population since the onset of the pandemic, reiterating the association of the pathology with anxiety [39]. Chadha et al. report a case of 85-year old female presenting with chest pain, and anxiety which was worse due to the pandemic. She did not report any respiratory symptoms and made an uneventful recovery [40].
Clinical features and investigations
Patients with stress cardiomyopathy typically present with chest pain (75.9%), dyspnea (46.9%) or syncope (7.7%) [32]. In our patients with COVID-19 stress cardiomyopathy, only 21.6% (n=8) reported chest pain. Dyspnea which is also a prominent feature of COVID-19 infection was present in 62% (n=23) patients and possibly masked other symptoms of stress induced cardiomyopathy. Physical examination in stress cardiomyopathy typically reveals tachycardia, hypotension, signs of hypoperfusion, S3 gallop, jugular venous distension, basilar pulmonary crackles, and rarely a systolic ejection murmur due to left ventricular outflow tract obstruction due to wall motion abnormalities [29]. Similar exam findings were reported in COVID-19 patients, predominantly respiratory distress, rhonchi and crackles. Added heart sounds or murmurs were not reported in any of the cases.
ECG changes and raised biomarkers are typical of stress cardiomyopathy. Ischemic ST-T segment changes specifically ST segment elevations, and deep T wave inversions are common findings. ST segment depression is uncommon and present in <10% of patients and its presence should prompt the consideration of an alternate diagnosis [41]. QT prolongation is also an important and clinically relevant finding, predisposing to torsade de pointes and ventricular fibrillation and prompts careful monitoring when present. In COVID-19 cases, 91% (n=34) showed ECG changes suggestive of stress cardiomyopathy.
Troponin elevation was seen in 88.9% (n=32) of COVID-19 positive patients, with 2 patients exhibiting a delayed peak during the illness. The degree of elevation could not be commented on as the values were not consistently reported and due to the lack of a standardized measure. In the general population, around 87% patients present with troponin elevation. Troponin elevation is generally low grade, when compared to patients with acute coronary syndrome (1.8-fold vs 6-fold) [32]. BNP elevation is more consistent in stress cardiomyopathy and correlates with degree of ventricular dysfunction [42].
Echocardiography is usually the preferred non-invasive imaging modality for the diagnosis of stress cardiomyopathy. Circumferential regional wall motion abnormalities extending beyond a single epicardial vascular distribution are typical. Apical ballooning, the typical Takotsubo cardiomyopathy is the most common anatomical variant, identified in >80% of patients, the midventricular form was found in 11-14%, and the basal variant is found in <5% of patients [43,44]. In our review, 25 patients presented with a clear pattern of apical hypokinesis and ballooning with and basal hyperkinesis, 7 patients presented with basal hypokinesis with normal to increased apical contractility on echocardiography and only 2 patients presented with the midventricular form. One patient [6] presented with infero-lateral hypokinesis, however the diagnosis of reverse Takotsubo with hypokinesia of the mid and basal left ventricular segments with normal apical contraction, was made on dynamic 3D volume rendering reconstruction with CT imaging.
Cardiac magnetic resonance (CMR) imaging is a useful mode of imaging in stress cardiomyopathy, especially to differentiate from other cardiac pathologies, including myocarditis. During the acute phase of stress cardiomyopathy, T2 weighed images show myocardial edema corresponding to the areas of wall motion abnormalities. Absence of delayed gadolinium enhancement (DGE) rules out myocardial scarring from myocarditis or myocardial infarction in suspicious cases [29,43]. CMR was not usually performed in COVID-19 positive patients due to the consideration of infection prevention. Sala et al. reported CMR findings of their patient on day 7 which showed a recovery of cardiac function with mild hypokinesia of basal and mid left ventricular segments, consistent with their diagnosis of reverse anatomical variant of stress cardiomyopathy [6]. Bernardi et al. reported CMR findings where T2-weighted images (short tau inversion recovery and T2 mapping) showed myocardial edema in the mid-apical segments of the left ventricle. After gadolinium administration, no areas of late enhancement were found [18].
Coronary angiography is not necessary for the diagnosis of stress induced cardiomyopathy but often pursued given the risk vs benefit consideration of missing acute coronary syndrome. The presence of underlying nonobstructive coronary artery disease does not rule out the presence of stress cardiomyopathy as these conditions may coexist [41-44]. Presence of regional wall motion abnormalities, not limited to the territory of the artery implicated in coronary artery disease further supports the diagnosis of stress cardiomyopathy, as seen in one of the patients with COVID-19 [13]. Ventriculography shows apical ballooning in typical cases. However, it is especially important in cases with equivocal findings on echocardiography, frequently seen with the mid-ventricular form [32].
Endomyocardial biopsy is usually not done but can provide important information about pathogenesis of stress cardiomyopathy. It was only pursued in one of our cases [6] and showed diffuse T-lymphocytic inflammatory infiltrate with interstitial edema and limited foci of necrosis and no evidence of fibrosis. The absence of the SARS-CoV-2 genome within the myocardium was demonstrated by molecular analysis, indicating no direct viral infection as an etiology.
Outcomes and complications
Overall prognosis of stress cardiomyopathy is favorable. Most patients recover completely [32]. However, it is important to monitor for complications including acute heart failure, left ventricular outlet tract obstruction, arrhythmias, formation of intra-cardiac thrombus and intramyocardial hemorrhage and rupture [29]. Management is centered around improving left heart function with pre-load and after-load reduction and beta-blockers. Inotropic support and mechanical circulatory support may be needed in cases of cardiogenic shock. Presence of left ventricular outlet tract obstruction is an important consideration before initiating inotropes as increased basal contractility may worsen the outflow obstruction.
Amongst patients with COVID-19 infection, management was reported for 25 patients. Eight patients required vasopressors, and two of them subsequently required ECMO. Another patient in the series reported by Giustino et al. required mechanical circulatory support. Other therapeutic agents used in these patients with COVID-19 infection included hydroxychloroquine (n=12), unspecified antivirals (n=4), steroids (n=9), colchicine (n=2), Tocilizumab (n=2), heparin and heparin analogues (n=6), aspirin (n=2) and dual-antiplatelet therapy (n=2). About, 73% (n=27) patients with COVID-19 and stress cardiomyopathy had favorable outcomes with evidence of recovery of LV function reported in 41% (n=7) patients.
Limitations
This review has some limitations. First, this is a nonrandomized retrospective study, and therefore susceptible to selection and recall bias. Second, case reports and series included in the study lack uniformity and information reported, and not all of the important variables were always reported. Third, we believe the diagnosis of stress cardiomyopathy was missed in a significant number of patients for multiple reasons, including focusing mainly on the respiratory illness or testing restrictions including echocardiography because of lack in personal protective equipment. Most hospitals have policies in place to limit COVID-19 exposure. Fourth, because both illnesses cause shortness of breath, a timeline of cardiac injury after COVID-19 exposure cannot be commented on. Fifth, the use of CMR was limited, limited ability to diagnose myocarditis. Despite all these limitations, we consciously proceeded with this review to summarize all available data addressing this important entity.
Conclusion
Stress cardiomyopathy can be important consequence of COVID-19 infection. High index of suspicion is crucial for prompt diagnosis and management. To the best of our knowledge, this is the first review paper addressing this topic. We reviewed and summarized all available data addressing stress cardiomyopathy in patients with COVID-19 infection. Stress cardiomyopathy should be strongly considered in the appropriate clinical scenarios. LV systolic function recovery has been reported in most patients. Larger future studies are needed for better disease understanding and to establish evidence-based management guidelines.
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531-538.
- Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020.
- Roca E, Lombardi C, Campana M, et al. Takotsubo Syndrome Associated with COVID-19. Eur J Case Rep Intern Med. 2020;7(5):001665.
- Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13(6):236561.
- Moderato L, Monello A, Lazzeroni D, et al. Sindrome Takotsubo in corso di polmonite da SARS-CoV-2: una possibile complicanza cardiovascolare [Takotsubo syndrome during SARS-CoV-2 pneumonia: A possible cardiovascular complication]. G Ital Cardiol (Rome). 2020;21(6):417-420.
- Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41(19):1861-1862.
- Solano-López J, Sánchez-Recalde A, Zamorano JL. SARS-CoV-2, a novel virus with an unusual cardiac feature: inverted takotsubo syndrome. Eur Heart J. 2020;390.
- Pasqualetto MC, Secco E, Nizzetto M, et al. Stress Cardiomyopathy in COVID-19 Disease. Eur J Case Rep Intern Med. 2020;7(6):001718.
- Dabbagh MF, Aurora L, D'Souza P, et al. Cardiac Tamponade Secondary to COVID-19 [published online ahead of print, 2020 Apr 23]. JACC Case Rep. 2020;2(9):1326-1330.
- Minhas AS, Scheel P, Garibaldi B, et al. Takotsubo Syndrome in the Setting of COVID-19 Infection. JACC Case Rep. 2020;2(9):1321-1325.
- Meyer P, Degrauwe S, Van Delden C, et al. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19):1860.
- Giustino G, Croft LB, Oates CP, et al. Takotsubo Cardiomyopathy in COVID-19. J Am Coll Cardiol. 2020;76(5):628-629.
- Nguyen D, Nguyen T, De Bels D, et al. A case of Takotsubo cardiomyopathy with COVID 19. Eur Heart J Cardiovasc Imaging. 2020;152.
- Kariyanna PT, Chandrakumar HP, Jayarangaiah A, et al. Apical Takotsubo Cardiomyopathy in a COVID-19 Patient Presenting with Stroke: A Case Report and Pathophysiologic Insights. Am J Med Case Rep. 2020;8(10):350-357.
- Torabi AJ, Villegas-Galaviz J, Guglin M, et al. Cardiogenic shock following cardiac tamponade and Takotsubo in COVID-19. Future Cardiol. 2020.
- Hegde S, Khan R, Zordok M, Maysky M. Characteristics and outcome of patients with COVID-19 complicated by Takotsubo cardiomyopathy: case series with literature review. Open Heart. 2020;7(2):001360.
- Faqihi F, Alharthy A, Alshaya R, et al. Reverse takotsubo cardiomyopathy in fulminant COVID-19 associated with cytokine release syndrome and resolution following therapeutic plasma exchange: a case-report. BMC Cardiovasc Disord. 2020;20(1):389.
- Bernardi N, Calvi E, Cimino G, et al. COVID-19 Pneumonia, Takotsubo Syndrome, and Left Ventricle Thrombi. JACC Case Rep. 2020;2(9):1359-1364.
- Tsao CW, Strom JB, Chang JD, et al. COVID-19-Associated Stress (Takotsubo) Cardiomyopathy. Circ Cardiovasc Imaging. 2020;13(7):011222.
- Bottiroli M, De Caria D, Belli O, et al. Takotsubo syndrome as a complication in a critically ill COVID-19 patient. ESC Heart Fail. 2020;4.
- Sattar Y, Connerney M, Ullah W, et al. COVID-19 Presenting as Takotsubo Cardiomyopathy Complicated with Atrial Fibrillation. Int J Cardiol Heart Vasc. 2020;29:100580.
- Demertzis ZD, Dagher C, Malette KM, et al. Cardiac sequelae of novel coronavirus disease 2019 (COVID-19): a clinical case series. Eur Heart J Case Rep. 2020;4:1-6.
- Oyarzabal L, Gómez-Hospital JA, Comin-Colet J. Tako-tsubo syndrome associated with COVID-19. Rev Esp Cardiol (Engl Ed). 2020;73(10):846.
- Dave S, Thibodeau JT, Styrvoky K, et al. Takotsubo Cardiomyopathy in a Coronavirus Disease-2019-Positive Patient: A Case Report. Pract. 2020;14(11):01304.
- Chitturi KR, Thacker S, Al-Saadi MA, et al. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: A case report. Eur Heart J Case Rep. 2020;4:1-6.
- Bhattacharyya PJ, Attri PK, Farooqui W. Takotsubo cardiomyopathy in early term pregnancy: a rare cardiac complication of SARS-CoV-2 infection. BMJ Case Rep. 2020;13(9):239104.
- Kurisu S, Sato H, Kawagoe T, et al. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143(3):448-455.
- Hurst RT, Prasad A, Askew JW, et al. Takotsubo cardiomyopathy: a unique cardiomyopathy with variable ventricular morphology. JACC Cardiovasc Imaging. 2010;3(6):641-649.
- Medina de Chazal H, Del-Buono MG, Keyser-Marcus L, et al. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(16):1955-1971.
- Secco GG, Tarantini G, Mazzarotto P, et al. Invasive strategy for COVID patients presenting with acute coronary syndrome: The first multicenter Italian experience. Catheter Cardiovasc Interv. 2020;28959.
- Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18(1):8-27.
- Templin C, Ghadri JR, Diekmann J, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
- Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164(1):66-71.
- Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333-341.
- Summers MR, Prasad A. Takotsubo cardiomyopathy: definition and clinical profile. Heart Fail Clin. 2013;9(2):111-115.
- De Giorgi A, Fabbian F, Pala M, et al. Takotsubo cardiomyopathy and acute infectious diseases: a mini-review of case reports. Angiol. 2015;66(3):257-261.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
- Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352(6):539-548
- Jabri A, Kalra A, Kumar A, et al. Incidence of Stress Cardiomyopathy During the Coronavirus Disease 2019 Pandemic. JAMA Netw Open. 2020;3(7):2014780.
- Chadha S. COVID-19 pandemic' anxiety-induced Takotsubo cardiomyopathy. QJM. 2020;113(7):488-490.
- Ghadri JR, Cammann VL, Jurisic S, et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail. 2017;19(8):1036-1042.
- Madhavan M, Borlaug BA, Lerman A, et al. Stress hormone and circulating biomarker profile of apical ballooning syndrome (Takotsubo cardiomyopathy): insights into the clinical significance of B-type natriuretic peptide and troponin levels. Heart. 2009;95(17):1436-1441.
- Eitel I, Behrendt F, Schindler K, et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29(21):2651-2659.
- Kurisu S, Inoue I, Kawagoe T, et al. Prevalence of incidental coronary artery disease in tako-tsubo cardiomyopathy. Coron Artery Dis. 2009;20(3):214-218.