Studies on the effect of environmental stressors on physiological activities of mangrove bacteria
Received: 03-Nov-2017 Accepted Date: Jan 11, 2018; Published: 15-Jan-2018
Citation: Saha S. Studies on the effect of environmental stressors on physiological activities of mangrove bacteria. J Microbiol Biotechnol Rep. 2018;2(1):5-13.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The effect of selected environmental stressors (pH, temperature, Ultra Violet exposure, PAH and heavy metals) on important physiological activities of mangrove bacteria was assessed. Cellulose degradation and phosphate solubilisation are two such important physiological activities mainly regulated by mangrove bacteria. Cellulase is a crucial enzyme required for converting waste cellulosic materials into foods and cleans the lignocellulosic waste present in the environment. Along with cellulases, phosphatases are also physiologically necessary enzymes required for maintaining environmental balance. Phosphatases are essential enzymes required for degradation of toxic agricultural waste containing organophosphates, like parathion, paraoxon, diazinon, coumaphos, and Malathion etc. For analysing the effects of environmental stressors on phosphatase and cellulose activity, a previously isolated bacterial culture designated as NE3B02 from Bhitarkanika mangrove, Odisha, India (stored as glycerol stock at -80°C) was screened for phosphate solubilisation and cellulose degradation potential. The mangrove bacterium efficiently solubilizes phosphate and utilizes cellulose at normal environmental conditions [pH=7, temperature (25°C to 37°C)]. Any alterations in environmental conditions interfere with phosphatase and cellulase production ability. The phosphate solubilisation activity of isolate increased with decrease in pH (from pH 7.4 to 7.1). Similarly the cellulose utilization ability of isolate NE3B02 also increased with decrease in pH (pH 7.4–pH 7.1). With increased temperature and time of UV exposure (short UV rays) the phosphate solubilisation and cellulose utilization potential of the isolate decreases significantly. PAH pollutant significantly elevates phosphate solubilisation and cellulose utilization ability of NE3B02. Heavy metal cadmium declines the activities of phosphatase and cellulose, whereas the phosphatase and cellulose activities were completely inhibited in the presence of heavy metal lead. The conclusions were drawn on the basis of calculation of solubilisation index for phosphatase activity and diameter of cellulose clearance zone was considered for cellulose activity.
The term “mangrove microbe” includes a wide range of microorganisms, which includes microalgae, bacteria and archaea, protozoa, fungi, and viruses. These organisms are extremely small. Millions of them reside in just one millilitre of sea water. Microbes are known to be the Earth’s processing factories of different interactions such as biological, geological, and chemical (biogeochemical). These organisms have the ability to keep themselves alive in any environment and renewable energy from a variety of sources, ranging from chemosynthesis to solar radiation. These microbes play many distinct roles in the mangrove environment by controlling the flow of energy and nutrients and also maintaining the health of the ocean as well as the earth surface [1]. Due to the vast diversity of mangrove microbes it is not possible to study on each and every species of microbes for which in this project we considered to work on bacterial population. The relationship of bacteria to the chemical cycles of the soil it is necessary to study their activities as a part of the vital energy cycles such as phosphorus cycle, sulphur cycle, nitrogen cycle, and carbon cycle. Since few years we are in touch with how ecosystem is undergoing changes in various parameters due to our Earth’s environment deterioration. Still anyhow each species trends to tolerant to different environmental conditions despite of changement in gene level [2]. To understand the alterations in microbial population for environment deterioration it is highly advisable to analyse and comprehend the reasons for environment imbalance which includes bio magnification effect of heavy metals and PAHs, ocean acidification, the ongoing decrease in the pH of the Earth’s oceans, global warming the ongoing increase in temperature [3]. Mangrove ecosystems are one of the most threatened tropical environments. Environmental pollution due to trace metals, Polycyclic Aromatic Hydrocarbons (PAHs), Persistent Organic Pollutants (POPs), Pharmaceuticals and Personal Care Products (PPCPs) and Endocrine Disrupters Compounds (EDCs) could be significantly contributing to the degradation of mangroves [4]. Sediment microorganisms play important role in the mangrove ecosystems producing organic carbon well in excess of the ecosystem requirements and contributing significantly to the global carbon cycle. Several biological processes with mutual relationships between mangroves take place including N-fixation, sulphate reduction and phosphate solubilisation and methanogenesis. Decomposition of the mangrove vegetation takes place through microorganisms via the use of enzymes such as cellulose, pectinase, protease and amylase [5]. However, environmental pollutants contribute as stressors for the mangrove microbial flora affecting the ecosystem and biodiversity. In the present study, the effect of selected environmental stressors viz., pH, temperature, UV exposure, PAHs and heavy metals on important physiological activities of mangrove bacteria was assessed. Cellulose degradation and phosphate solubilisation are two such important physiological activities mainly regulated by mangrove bacteria. Cellulose a polysaccharide consisting of a linear chain of several glucose residues with β-1, 4-glycosidic linkages form a crystalline structure. Cellulose is the most important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some different species of bacteria secrete it to form biofilms. Cellulose the organic polymer is available abundantly on earth. Cellulose can be converted to glucose with the help of cellulolytic system which consists of three classes of extracellular enzymes: 1, 4-β-endoglucanase, 1, 4-β-exoglucanase, and β-glucosidase [6]. Because of many different applications in industries of starch processing, grain alcohol fermentation, malting and brewing, extraction of fruit and vegetable juices, pulp and paper industry, and textile industry researchers have strong interests in cellulases. For random cleavage of β-1, 4-glycosidic bonds along a cellulose chain endoglucanase is responsible and for cleavage of the non-reducing end of a cellulose chain whereas exoglucanase is souly responsible as well as for hydrolysing of cellobiose and water-soluble cello dextrin to glucose β-glucosidase is responsible. The above three enzymes is believed to be the components for complete cellulose hydrolysis to glucose. In the world of microbes many researchers have dedicatedly spared a long period of time to get desire results from several bacterial strains which can help to obtain a clear idea about the cellulosic activities taking place in the strains. Cellulolytic bacterial species include Trichonympha, Clostridium, Actinomycetes, Bacteroides succinogenes, Butyrivibrio fibrisolvens, Ruminococcus albus and Methanobrevibacter ruminantium [7]. As we all have previously studied that for successful carrying out of any enzymatic activity in a microbe some specific enzymes are actively involved so same as there is involvement of cellulolytic enzymes of different specificities, which act together in synergism in cellulosic activities (degradation of cellulose). Phosphorus as compared to other nutrients is less available to plants. H2PO4- and HPO42- are the forms of phosphates which are used by the plants in abundant for their growth. Phosphorus get involved for other plant processes such as energy transfer reactions, development of reproductive structures, maturity of crop, root growth and synthesis of protein. To evade phosphorus deficiency, phosphate-solubilizing microorganisms (PSMs) play an important role in supplying phosphate to plants as well as increase the fertility of the soil [8]. Microorganisms are also actively taking part in the process that affects the transformation of soil. Phosphorus is integral component of the soil ‘Phosphorous’ cycle. Mechanisms like lowering of soil pH by acid production, ion chelation, and exchange reactions in the growth is another site for phosphate solubilisation by PSMs. Phosphate-Solubilizing Bacteria (PSB) is slowly emerging as important organisms for the soil improvement. Different bacterial species like Pseudomonas, Bacillus, Rhizobium, Burkholderia, Achromobacter, Agrobacterium, Micrococcus, Aereobacter, Flavobacterium and Erwinia, both aerobic and anaerobic strains, has the ability to solubilize insoluble inorganic phosphate compounds, like tricalcium phosphate, dicalcium phosphate, hydroxyapatite, and rock phosphate (through their hydroxyl and carboxyl groups chelate the cation bound to phosphate, the latter being converted to soluble forms) [9]. Moreover Phosphate solubilization is a complicated phenomenon, which depends on several factors such as nutritional, physiological and growth conditions of the bacterial culture.
Review of Literature
Microbial diversity is one of the difficult areas of biodiversity research, extensive exploration is required for understanding the biogeography, community assembly and ecological processes which will for isolating and identifying new and potential microorganisms having high specificity for recalcitrant compounds [10]. Potential useful microbes include bacteria (nitrogen fixing, phosphate solubilizing, cellulose degrading, surface reducing, photosynthetic anoxygenic, methanogenic, enzyme producing bacteria) fungi, actinomycetes and other useful microbes. The development of microbial communities that effect the degradation of cellulose in environment is one of the hallmarks of evolution. The ecology of cellulose degradation in environment is very complex; it involves numerous, varied interactions of metabolically diverse microorganisms whose activities are influenced by a wide range of environmental factors [11]. Despite of its simple chemical composition, cellulose exists in a number of crystalline and amorphous topologies. Its insolubility and heterogeneity makes native cellulose a recalcitrant substrate for enzymatic hydrolysis. Microorganisms meet this challenge with the aid of a multi-enzyme system. Aerobic bacteria produce numerous individual, extracellular enzymes with binding modules for different cellulose conformations. Specific enzymes act in synergy to elicit effective hydrolysis [12]. The diversity of cellulases may result from the extreme diversity of their natural substrates, plant cell walls. Individual cellulases have very low activity on crystalline cellulose but they have a very high catalytic enhancement due to the very long half-life of crystalline cellulose.
Enzyme mechanisms generally depend on single molecules fitting in their substrate pocket. However, this simplified model has been later modified in the light of new findings. Many cellulases from a wide range of organisms have been cloned and characterized biochemically. All cellulases hydrolyse the β -1, 4-glucosidic bond between glucosyl moieties by a general acid catalysis, requiring a proton donor and a nucleophile/base. They release the products either by overall retention or inversion of the anomeric configuration at C1. All cellulolytic microorganisms possess genes for more than one β-glucanase and also for more than one GH family. Although many more than 600 cellulase genes are known, a detailed biochemical characterization has been undertaken for only a very limited number of endo and exoglucanases. Wilson [13] stated that there are several different mechanisms that are used by cellulolytic microorganisms to degrade cellulose, although cellulases are used in all of them. Cellulases are the most diverse enzymes that catalyse a single reaction, which is hydrolysis of the β-1, 4 linkage joining two glucose molecules in a cellulose molecule. There are at least eleven cellulase families based on the similarities of their amino acid sequence and structural studies of the different families show that cellulases have eight different protein folds. Cellulases are very different from most enzymes, as they degrade an insoluble substrate. This requires that the enzyme diffuses to the substrate and then it has to move a segment of a cellulose molecule from the insoluble particle into its active site, whereas soluble substrates diffuse to the enzyme and bind into the active site by themselves. Almost all enzymes that degrade insoluble substrates contain a substrate binding domain, which is usually joined to the catalytic domain (cd) by a flexible linker peptide. In the case of cellulases, where this type of domain was first discovered, the domain was originally called a cellulose binding domain and then the name was changed to carbohydrate binding module (CBM), so as to include the many other types of polysaccharide binding domains. Shoseyov et al. [14] reviewed the subject on carbohydrate binding module (CBM). It is clear that one role of the CBM is to bind the enzyme to the cellulose so that the cd spends less time away from the substrate and it also gives the cd time to move the chain into its active site before the enzyme diffuses away from the cellulose particle. It is still not clear whether the CBM also can modify cellulose or otherwise assists cellulose hydrolysis by the catalytic domain.
Schülein [15] summarized the enzyme mechanisms involved. The enzymes have a complex molecular architecture, comprising discrete modules: the catalytic domains are joined to non-catalytic modules, originally called substrate-binding domains or cellulose-binding domains, by linker regions [commonly proline/threonine/serine- (PTS)]. Now they began to be more generally called carbohydrate binding modules (CBM). Other non-catalytic modules found in extracellular β-glucanases are fibronectin-3-like modules, surface-layer homologous modules (SLH), and modules of unknown function. An example of a highly cellulolytic bacterium is Thermobifida fusca (recently renamed from Thermomonospora fusca). Six β -glucanase genes, named E1–E6, were cloned and biochemically characterized [16]. No single enzyme component was able to hydrolyze crystalline cellulose substantially. However, significant increases in the rate and extent of it was accepted that cellulolysis is performed by the synergistic action of three types of enzymatic activities: Cellulose can be converted to glucose with the help of cellulolytic system which consists of three classes of extracellular enzymes: 1, 4-β-endoglucanase, 1, 4-β-exoglucanase, and β-glucosidase. For random cleavage of β-1, 4-glycosidic bonds along a cellulose chain endoglucanase is responsible and for cleavage of the non-reducing end of a cellulose chain exoglucanase is souly responsible. For hydrolysing of cellobiose and watersoluble cello dextrin to glucose, β-glucosidase is responsible. The above three enzymes are believed to be the components required for complete cellulose hydrolysis to glucose. Schwarz [12] reported that clostridia and ruminococci have developed the cellulosome, an optimized multi-enzyme complex, which displays intra-molecular synergism by connecting all the necessary components in the correct ratio and order. It contains a non-catalytic scaffolding protein, which captures a number of hydrolytic components by specific protein–protein interaction. Major catalytic components of the cellulosome are two cellobiohydrolases with different directions of processing (GHF 48 and 9). Endoglucanases also play an important role in hydrolysis. C. thermocellum, so far the most thermophilic cellulosome producer, has the largest cellulosome with the greatest complexity and a genetic organization of the genes which is different from the mesophilic clostridia. The cellulosome components of Ruminococcus differ in their modular pattern. Successful exploitation of cellulose as a source of food and fuel could be achieved only through understanding the enzyme systems involved and the mechanism of action of the relevant components individually and jointly. The investigations on structure/function relationships that have contributed to our current understanding of the subject include affinity chromatographic isolation of “pure” enzyme preparations, the use of artificial substrates in specificity and ligand binding studies, active site modification and site-directed mutagenesis, partial proteolysis, recombinant DNA technology, electron microscopy and X-ray scattering. Coughlan et al. [17] has briefly reviewed these studies. Coughlan [17] studied the mechanisms of cellulose degradation by fungi and bacteria and reported that two general enzyme-catalysed mechanisms are utilized for cellulose conversion. The aerobic fungi catalyse by the synergistic action of endo-glucanases and exo-cellohiohydrolases that exist extracellularly as free entities. Other hydrolytic enzymes, such as fl-glucosidases, and oxidative enzymes participate in the overall process. Some aerobic bacteria possess a similar system. By contrast, in anaerobic bacterial systems, cellulose degradation is brought about by the action of large calcium- and thioldependent multicomponent complexes (cellulosomes) located, at least in the early stages of cultivation, on the cell surface. In Clostridium thermocellum cellulosomes, the paracrystalline arrangement of the individual components is such as to allow the complete conversion of cellulose to cellobiose. The latter is taken into the cell where it is metabolized further by either cellobiose phosphorylase or fl-glucosidase, or both. The cellulose systems of some aerobic bacteria and of anaerobic fungi, under some growth conditions, also exist as cell-bound complexes. The structure of cellulose was reviewed and described in detail [18]. Many reviews appeared in the literature describing the mode of action of enzymes on the crystalline cellulose surface [19-21], and the structure of cellulolytic multi-enzyme complexes [22-24]. Schwarz [12] reviewed the cellulosome and cellulose degradation by anaerobic bacteria in which he describes the advances made in the understanding of the bacterial hydrolysis of crystalline cellulose, with emphasis on the cellulosome. Cellulose exists in a number of crystalline and amorphous topologies. It is insoluble and heterogeneous. This property makes native cellulose a difficult substrate for enzymatic hydrolysis. Microorganisms meet this challenge with the aid of a multi-enzyme system. Aerobic bacteria produce numerous individual, extra-cellular enzymes with binding modules for different cellulose conformations. Specific enzymes act in synergy to elicit effective hydrolysis. In contrast, anaerobic bacteria possess a unique extracellular multi-enzyme complex, called cellulosome. Up to 11 different enzymes are aligned on the non-catalytic scaffolding protein and thus ensure a high local concentration, together with the correct ratio and order of the components. These multi-enzyme complexes attach both to the cell envelope and to the substrate, mediating the proximity of the cells to the cellulose. Binding to the scaffolding stimulates the activity of each individual component towards the crystalline substrate. The most complex and best investigated cellulosome is that of the thermophilic bacterium Clostridium thermocellum, but a scheme for the cellulosomes of the mesophilic clostridia and the ruminococci emerges. Wilson [25] reported that many aerobic microorganism use the free cellulase mechanism in which they secrete a set of individual cellulases, most of which contain a carbohydrate binding module (CBM) joined by a flexible linker to one end of the catalytic domain. The cellulases in the mixture act synergistically to degrade crystalline cellulose. Irwin et al. [26] are of the opinion that cellulase synergism can result in increases in the specific activity of appropriate mixtures that are up to fifteen fold higher than that of any individual cellulase. Ding et al. [27] reviewed cellulosomes and reported that that many anaerobic microorganisms use cellulosomes, large multienzyme complexes (multimillion molecular weight), to degrade cellulose. Guillén et al. [28] stated that some aerobic fungi degrade cellulose but not lignin, such as Trichoderma reesei, use the free enzyme mechanism while true brown rot fungi secrete both cellulases and peroxidases. The peroxide and OH–radicals produced by the peroxidases and iron partially oxidize the cellulose, making it much easier for the cellulases to degrade it. Therefore brown rot fungi are able to use a set of cellulases that lack both CBMs and processive cellulases, which are needed to degrade unmodified crystalline cellulose. Wilson [29] reviewed the microbial diversity of cellulose hydrolysis and reported that the enzymatic hydrolysis of cellulose by microorganisms is a key step in the global carbon cycle. In-spite of the vast variety of microbes in the universe, only a small percentage of microorganisms can degrade cellulose, probably because it is present in recalcitrant cell walls. There are at least five distinct mechanisms used by different microorganisms to degrade cellulose all of which involve cellulases. Cellulolytic organisms and cellulases are extremely diverse possibly because their natural substrates, plant cell walls, are very diverse. Wang et al. [30] considered that Phosphorus (P) is one of the most essential plant nutrients which profoundly affect the overall growth of plants. It was reported that P influences various key metabolic processes such as cell division and development, energy transport, signal transduction, macromolecular biosynthesis, photosynthesis and respiration of plants [31-35]. The source of P is largely the primary and secondary minerals and/or organic compounds and is not in free form. Broadly, there are three categories of P compounds: (i) inorganic compounds, (ii) organic compounds of the soil humus and (iii) organic and inorganic P compounds associated with the cells of living matter. Mineral compounds of P usually contain aluminium (Al), iron (Fe), manganese (Mn) and calcium (Ca) and vary from soils to soils. For example, P forms a complex with Al, Fe and Mn in acidic soils, while in alkaline soils it reacts very strongly with Ca. However, under all conditions, the types of soil P compounds are determined mainly by soil pH and by the type and concentrations of soil minerals. Richardson [36] reported that out of the total soil P pool, about 50 % is in the organic forms. Yadav and Verma [37] reported that the organic forms of P vary between 4% and 90% in most soils. The organic P in plants includes (i) inositol phosphate (10–50% in soil) which represents a series of phosphate esters ranging from monophosphates up to hexaphosphates. Phytic acid (inositol hexakisphosphate) is the main compound that plants use to store P in seeds to support early seedling growth following germination. Phytin (Ca–Mg salt of phytic acid) is the most abundant of the known organophosphorus compounds in soils. Other organic P in soils occur as sugar phosphates, nucleotides (0.2–2.5 %), phosphoprotein (trace), phosphonates and as phospholipids (1–5 %) [37]. Of the various forms of P, plants take up only negatively charged primary and secondary orthophosphate ion as nutrient. Indeed, the amount of plant available P is very low relative to the total soil P. Moreover, majority of the soil P is fixed, and only a small fraction of P is available for uptake by plants. The insoluble and inaccessible forms of P are hydrolysed to soluble and available forms through the process of solubilisation (inorganic P)/mineralization (organic P). The immobilization in contrast is the reverse reaction of mineralization. During immobilization, microorganisms convert inorganic forms to organic phosphate. Khan and Zaidi [38] reviewed mechanism of phosphate solubilisation by microorganisms. The insoluble forms of P such as tricalcium phosphate (Ca3PO4)2 , aluminium phosphate (Al3PO4), iron phosphate (Fe3PO4), etc. may be converted to soluble P by P-solubilizing organisms inhabiting different soil ecosystems [39,40]. Soil microorganisms in this regard have generally been found more effective in making P available to plants from both inorganic and organic sources by solubilizing [41] and mineralizing complex P compounds [42] respectively. Several workers have documented their findings in order to better understand as to how the microbial populations cause the solubilisation of insoluble P [43]. Of the various strategies adopted by microbes, the involvement of low molecular mass organic acids (OA) secreted by microorganisms has been a wellrecognized and widely accepted theory as a principal means of P-solubilisation., and various studies have identified and quantified organic acids and defined their role in the solubilisation process [44,45]. Consequently, the acidification of microbial cells and their surrounding leads to the release of P-ions from the P-mineral by H+ substitution for Ca2+ [46,47]. Tarafdar and Claasssen [48] reported that many soil microbes or rhizosphere microflora can transform organic P into soluble forms. This process is mediated by the enzymes released by the soil microbes especially phosphatases and phytases [49,50]. Beech et al. [51] stated that phosphatases (acid and alkaline phosphatases) released extracellularly (exo-enzymes) use organic P as a substrate to convert it into inorganic form. RodrıÌÂguez and Fraga [52] reported that of the two phosphatases, acid phosphatases are considered as the principal mechanism for mineralization of soil organic P. It catalyses the release of inorganic P from organic P compounds such as inositol hexaphosphates [53]. P-dissolving enzymes mineralize soil organic P through phytase degradation mediated by phytase, which specifically causes release of P from phytic acid. Richardson [54] and Vassilev et al. [55] reported an increase utilization of inositol P by plants in the presence of microbial communities including P-solubilizing fungus (Aspergillus niger) capable of producing phytase is reported. Phosphonatases and C–P lyases are the other enzymes that cleave the C–P of organophosphates [56,57]. Once the inorganic or organic P compound is changed to soluble P, it can easily be used up as P nutrient by plants, algae, cyanobacteria and autotrophic bacteria and thereafter could be immobilized into organic cellular macromolecules, for example, DNA, RNA and ATP.
Objectives
1. Screening for cellulose degrading and phosphatase solubilizing mangrove bacteria.
2. To study the effect of environmental stressors on the cellulose degradation and phosphate solubilisation ability of a mangrove bacterium.
3. To analyse the effect of different environmental stressors on cellulase and phosphatase activity.
Materials and Methods
Strain retrieval
After isolation, the strain was designated as NE3B02 and maintained at -80°C as a glycerol stock. The NE3B02 strain was revived from the glycerol stock on Luria Bertani (LB) agar plate. Subsequently, a single isolated colony was inoculated in 5 ml of LB broth at 180 rpm for 12 h at 37˚C. This culture was used for further studies.
Media
The following media were used for different purposes as mentioned below:
i. Luria Bertani broth (LB) and Luria Bertani agar (LBA) for the propagation of NE3B02 culture.
ii. Pikovskaya agar (PA) to detect the phosphate solubilizing effect.
iii. Carboxymethyl cellulose (CMC) agar for detection of cellulose degradation effect.
Composition of media
1. LB Broth:
a) 1 gram tryptone,
b) 0.5 gram yeast extract,
c) 1 gram sodium chloride (NaCl),
d) 100 millilitres distilled water.
2. Pikovskaya Agar:
a) 1 gram glucose,
b) 0.5 gram tricalcium phosphate [Ca3(PO4)2],
c) 0.05 gram ammonium sulphate [(NH4)2SO4],
d) 0.02 gram potassium chloride (KCl),
e) 0.01 gram magnesium sulphate (MgSO4),
f) 0.01 gram manganese sulphate (MnSO4),
g) 0.01 gram ferrous sulphate (FeSO4),
h) 1.5 gram yeast extract,
i) 1.5 gram agar
j) 100 millilitres distilled water.
3. Carboxymethyl cellulose agar (CMC agar):
a) 0.2 gram sodium nitrate (NaNO3),
b) 0.1 gram di potassium phosphate (K2HPO4),
c) 0.05 gram magnesium sulphate (MgSO4),
d) 0.05 gram potassium chloride (KCl),
e) 0.20 gram CMC salt,
f) 0.02 gram peptone,
g) 1.5 gram agar
h) 100 millilitres distilled water.
Physical parameters
The physical parameters studied include pH, effect by increasing temperature, UV radiation, PAH, and heavy metals (Lead, Cadmium) on phosphate solubilisation and cellulose degradation.
Screening of phosphate solubilisation bacteria
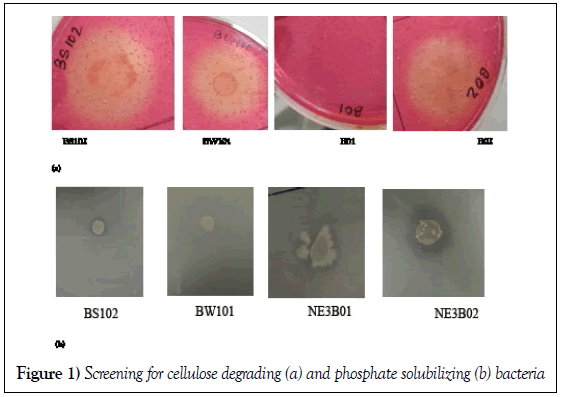
Four different bacterial strains (BS102, BW 101, NE3B02 and B01) were examined for their ability to solubilise phosphate. For this each of the strains viz., BS102, BW 101, NE3B02 and B01 was separately inoculated into LB agar plates and incubated overnight at 37°C. After incubation a single isolated colony of each strain was inoculated in 5 mL of LB broth and incubated overnight at 37°C. Further, 3 μL of inoculum of overnight LB broth cultures was dropped as a single drop on Pikovskaya agar plate and was incubated for 7 days at 37°C. The bacterial strains BS102, BW101 and B01 did not show any halo zone formation after 7 days of incubation indicating lack of phosphate solubilisation ability. A halo zone was observed on Pikovskaya agar plate inoculated with bacterial strain, NE3B02. Therefore NE3B02 strain was utilized for carrying out all further experiments. Moreover the solubilizing efficiency (E) of NE3B02 was calculated [58]:-

Effect of different environmental stressors on phosphate solubilisation ability of NE3B02
pH
The effect of four different levels of pH, viz., 7.1, 7.2, 7.3, and 7.4 were examined. Twenty milliliters of Pikovskaya agar was distributed into each of the four falcon tubes so that four different scale of pH can be adjusted. After the adjustment of pH the falcon tubes as indicated above were autoclaved at 121°C for 15 min. Four different PA plates were made into which 3 μL of fresh LB cultured NE3B02 was inoculated drop wise. The plates were incubated for 7 days at 37°C and observed for halo zone formation. Solubilizing efficiency was calculated.
UV radiation
3 μL of overnight grown culture of NE3B02 was inoculated on the PA plate. NE3B02 inoculated PA plates were exposed to UV radiation for different time duration i.e. 5 min, 10 min and 15 min. The plates were then incubated at 37°C for 7 days and solubilizing efficiency was calculated for all the plates. One plated not exposed to any UV radiation was used as a control.
Temperature
The effect of two different temperatures was examined viz., 39°C and 41°C on phosphate solubilisation ability of NE3B02. 3 μL of overnight grown culture of NE3B02 was inoculated on PA plate and was incubated for 7 days, NE3B02 inoculated PA plates were incubated at 39°C and 41°C. After the incubation solubilizing efficiency of the plates were calculated.
PAH
100 μL of 100 ppm of naphthalene was added to the PA plates, 3 μL of overnight grown culture of NE3B02 was inoculated and was incubated for 7 days at 37°C. Lastly its solubilizing efficiency was calculated.
Heavy metals
Lead and Cadmium was selected to determine the effect of heavy metals. 100 μL of 100 ppm of lead and cadmium was added to the PA plates. 3 μL of overnight grown culture of NE3B02 was inoculated in the plates and was incubated for 7 days at 37°C. Lastly solubilizing efficiency was calculated.
Enzyme extraction
To measure the enzyme activity of NE3B02 after exposing to various stressors, enzymes were extracted from overnight grown cultures of the strain NE3B02 after various treatments like pH, heavy metals (Cadmium, and Lead), temperature and UV treatment and PAH . For this, 200 ml of LB broth was prepared and was distributed into 13 falcon tubes (15 ml each). One was kept as control (stress free).
The pHs of LB broth of individual Falcon tubes were adjusted to pH 7.1, pH 7.2, pH 7.3 and pH 7.4 in each of the tubes separately. The pH adjusted LB broth falcon tubes were autoclaved for 15 min at 121°C. The tubes were allowed to cool down. To each of falcon tubes containing LB broth, 100 μL of 100 ppm each of PAH (Naphthalene), Cadmium and Lead was added separately. Each tube was separately inoculated with 100 ml of LB broth grown fresh cultures of NE3B02.
Effect of UV radiation was studied using three separate overnight grown cultures by exposing to UV radiation (254 nm) at different time duration viz., 5, 10 and 15 min. Effect of varying temperatures of growth was examined by incubating inoculated culture tubes at two different temperatures Viz., 39°C and at 41°C for 24 h. After 24 h. of incubation all the tubes were centrifuged at 4°C at 7500 rpm for 15 min. The supernatant was discarded and the pellets were suspended in 2 ml of PBS. The suspended pellets were transferred to 2 mL centrifuged tubes and sonicated for 6 min. The tubes were centrifuged for 20 min at 4°C at 13500 rpm. After centrifugation the supernatant was obtained and pallets were discarded. The supernatant contained the enzymes of NE3B02.
Enzymatic assay of phosphatase
One ml of each supernatant was taken and to it one ml of distilled water was added along with one ml of potassium phosphate, 3 drops of ammonium molybdate and 2 drops of stannous chloride was added. The absorbance of the resultant colour was read at 690 nm at 0th hour against blank (PBS) in UV/visible spectrophotometer. The same was done and absorbance was measure after 15 min of incubation.
Screening for cellulose degradation bacteria
Four different bacterial trains (BS102, BW 101, NE3B02 and B01) were examined for their ability to solubilise phosphate. For this each of the strains viz., BS102, BW 101, NE3B02 and B01 was separately inoculated into LB agar plates and incubated overnight at 37°C. After incubation a single isolated colony of each strain was inoculated in 5 mL of LB broth and incubated overnight at 37°C. Carboxy methyl cellulose (CMC) agar plates were inoculated 3 μL of overnight grown cultures followed by incubation for 3 days at 37°C. After 3 days the plates were washed with 1% Congo red and then with 1 Molar NaCl for 30 mins.
Effect of different environmental stressors on cellulose degradation ability of NE3B02
pH
The effect of four different pH, viz., 7.1, 7.2, 7.3, and 7.4 on cellulose degradation was examined. 20 mL of CMC agar was distributed in four falcon tubes so that four different scales of pH can be adjusted. 3 μL of overnight grown culture of NE3B02 was inoculated drop wise. The plates were incubated for 3 days at 37°C. The plates were washed with 1% Congo red and then with 1 Molar NaCl for 30 mins.
UV radiation
3 μL of overnight grown culture of NE3B02. Keeping one plate as control rest of the three plates were exposed to UV radiation (254 nm) at different length of exposure time i.e. 5 min, 10 min and 15 min, after which the plates were incubated at 37°C for 3 days. The plates were washed with 1% Congo red and then with 1 M NaCl for 30 mins.
Temperature
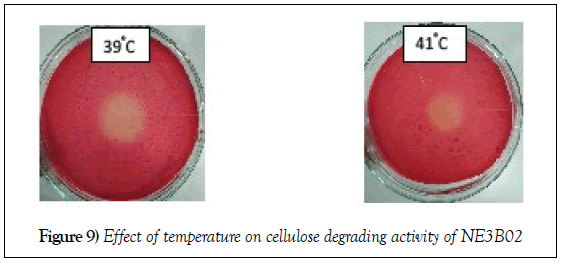
Effect of varying temperatures of growth was examined by incubating inoculated cultures at two different temperatures viz., 39°C and at 41°C for 24 h. 3 μL of overnight grown culture of NE3B02 was inoculated drop wise and was incubated for 3 days. The plates were incubated at 39°C and 41°C. Lastly the plates were washed with 1% Congo red and then with 1M NaCl for 30 min.
PAH
100 μL of 100 ppm Naphthalene was added to the CMC plates. 3 μL of overnight grown culture of NE3B02 was inoculated and was incubated for 3 days at 37°C. The plates were washed with 1% Congo red and then with 1 M NaCl for 30 min.
Heavy metals
Lead and Cadmium were selected to determine the effect on cellulose degradation. 100 μL of 100 ppm lead and cadmium was added to CMC plates respectively. 3 μL of overnight grown culture of NE3B02 was incubated for 3 days at 37°C and the plates were washed with 1% Congo red and then with 1 M NaCl for 30 min.
Enzymatic assay of cellulose
The cellulase (exo-β 1, 4 glucanase) activity of the isolate was determined according to the method of (Miller). A reaction mixture composed of 0.2 mL of crude enzyme was taken in 13 test tubes of different parameters (Control, pH 7.1, pH 7.2, pH 7.3, pH 7.4, Ultra Violet treatment for 5 min, 10 min, 15 min, 39°C, 41°C, PAH, Cadmium and Lead) to these 1.8 mL of 0.5% carboxymethyl cellulose and 1 mL of PBS was added and incubated at 37°C in a shaking water bath for 30 mins. The reaction was terminated by adding 3 ml of DNS reagent. The colour was developed by boiling the mixture for 5 mins. Optical Density was measured at 575 nm against a blank containing all the reagents minus the crude enzyme using spectrophotometer.
Results
Screening for phosphate and cellulose degrading bacteria solubilizing of phosphate by NE3B02
Four different bacterial strains (BS102, BW101, NE3B02 and B01) were examined for their ability to degrade cellulose as well as to solubilize phosphate. Out of these four only NE3B02 had the capability to degrade cellulose and solubilize phosphate (Figure 1a and 1b).
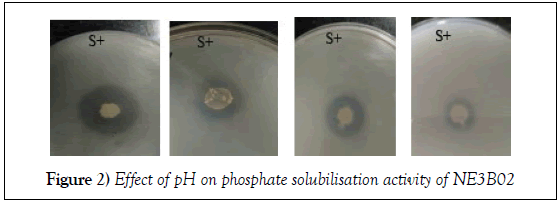
Effect of pH on phosphate solubilisation activity of NE3B02
The effect of four different pH (7.1, 7.2, 7.3 and pH 7.4) was evaluated on the phosphate solubilisation efficiency of NE3B02. The halo zone formation decreases with increase in pH indicating that the phosphate solubilisation efficiency increases with decrease in pH towards neutral range (Figure 2). Solubilizing efficiency was calculated for each pH (Table 1).
Table 1: Phosphate solubilization efficiency of NE3B02 at different pH
| pH | Control | pH 7.1 | pH 7.2 | pH 7.3 | pH 7.4 |
|---|---|---|---|---|---|
| Solubilizing efficiency | 362.5 | 350 | 325 | 287.5 | 225 |
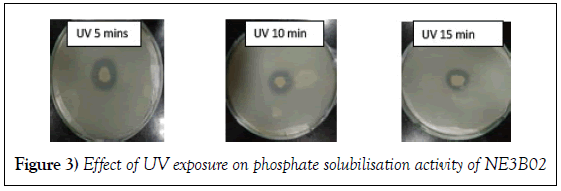
Effect of Ultra violet ray exposure
The phosphate solubilisation efficiency determined in terms of diameter of the halo zone formed decreased with the increase of time of Ultra Violet exposure, in other words the Ultra Violet rays treatment for high time duration alleviated the power of NE3B02 to solubilize phosphate (Figure 3). The solubilisation efficiency calculated is depicted in Table 2.
Table 2: Phosphate solubilization efficiency of NE3B02 after UV exposure
| UV time duration | Control | 5 min | 10 min | 15 min |
|---|---|---|---|---|
| Solubilizing efficiency | 362.5 | 212.5 | 187.5 | 162.5 |
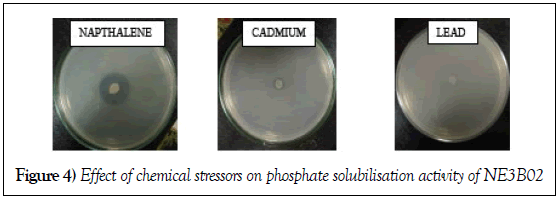
Effect of Heavy metals and PAH
Three PA plates were made containing heavy metals lead, cadmium and PAH (Naphthalene). Strain was inoculated in the plates and incubated for 7 days at 28°C. After the incubation the diameter of the halo zone was measured and we drew the conclusion that PAH (Naphthalene) increases phosphate solubilization efficiency of NE3B02 whereas, cadmium and lead inhibited the phosphate solubilization potential of NE3B02 (Figure 4). The phosphate solubilization efficiency in presence of different stressors is depicted in Table 3.
Table 3: Phosphate solubilization efficiency of NE3B02 in presence of different environmental stressors
| Stressors | Control | Napthalene | Cadmium | Lead |
|---|---|---|---|---|
| Solubilizing efficiency | 362.5 | 400 | 300 | 37.5 |
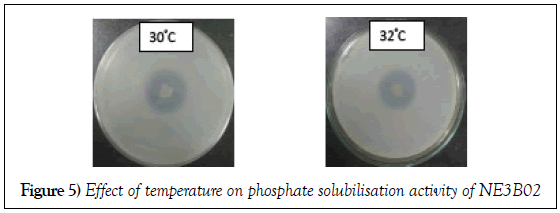
Effect of increase in temperature
The phosphate solubilization efficiency decreased with increase in temperature. The diameter of the halo zole reduces from 2.6 cm to 2.5 cm (Figure 5). The phosphate solubilization efficiency in different temperature is depicted in Table 4.
Table 4: Phosphate solubilization efficiency of NE3B02 at different temperature
| Temperature | Control | 30ºC | 32ºC |
|---|---|---|---|
| Solubilizing efficiency | 362.5 | 325 | 312.5 |
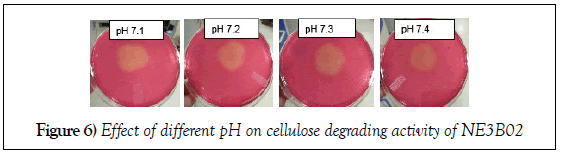
Effect of pH on cellulose degrading activity of NE3B02
Effect of pH
The effect of four different pH (7.1, 7.2, 7.3 and pH 7.4) was evaluated on the cellulose degrading efficiency of NE3B02. The halo zone formation decreased with increase in pH indicating that the cellulose degrading efficiency increases with decrease in pH towards neutral range (Figure 6).
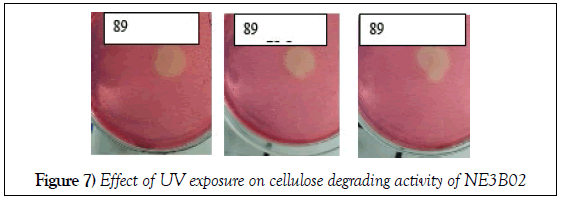
Effect of ultra violet ray treatment
The cellulose degrading efficiency determined in terms of diameter of the halo zone formed decreased with the increase of time of Ultra Violet exposure, in other words the Ultra Violet rays treatment for high time duration alleviated the power of NE3B02 to degrade cellulose (Figure 7).
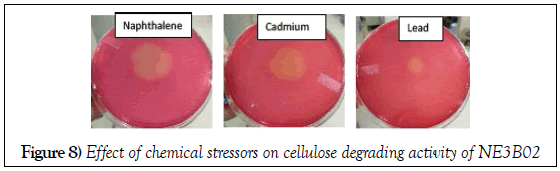
Effect of heavy metals and PAH
The cellulose degrading efficiency was determined in terms of diameter of the halo zone formed. PAH (naphthalene) increases cellulose degrading efficiency of NE3B02 whereas, cadmium and lead inhibited the cellulose degradation potential of NE3B02 (Figure 8).
Effect of increase in temperature
The cellulose degrading efficiency decreased with increase in temperature. The diameter of the halo zole reduces from 1.9 cm to 1.4 cm (Figure 9).
EEnzyme activity calculation
The specific enzyme activities were calculated under different physiochemical conditions and is depicted in Table 5.
Table 5: Specific activities of enzymes involved in phosphate and cellulose degradation by NE3B02
| Conditions | Phosphatase | Cellulase |
|---|---|---|
| pH 7.1 | 56.2 ± 1.3 | 22.8 ± 3.4 |
| pH 7.2 | 50.6 ± 2.9 | 20.3 ± 3.9 |
| pH 7.3 | 49.2 ± 2.3 | 18.6 ± 5.3 |
| pH 7.4 | 33.08 ± 4.7 | 13.8 ± 3.1 |
| Control | 58.9 ± 3.7 | 29.7± 2.7 |
| UV 5 min | 49.33 ± 2.2 | 26.2 ± 2.8 |
| UV 10 min | 41.17 ± 1.8 | 24.4 ± 2.3 |
| UV 15 min | 32.3 ± 1.3 | 21.02 ± 1.7 |
| Temp 30ºC | 51.23 ± 2.3 | - |
| Temp 32ºC | 46.3 ± 2.7 | - |
| Temp 39ºC | - | 24.6 ± 2.2 |
| Temp 41ºC | - | 22.3 ± 1.7 |
| Naphthalene | 62.3± 2.9 | 32.6 ± 3.6 |
| Cadmium | 41.02 ± 2.3 | 18.3 ± 2.2 |
| Lead | 18.02 ± 2.4 | 11.47 ± 3.3 |
Discussion
In contrast to previously published studies on the survival of inoculant strains, the emphasis of this study was to know about the survival capability of NE3B01 after being treated with different environmental stressors. After analysing the results we could draw the conclusion that the NE3B01 strain undergoes different changes in their genetic level due to the external pressure so formed by the changement in different environmental stressors. The increase in pH from 7.1 to 7.4 during the culture conditions the strain decreased its growth rate with the increase in pH in both the cases (i.e., phosphate solubilisation and cellulose degradation). Similarly Abubakar and Oloyede [59], reported that the cellulase activity of Aspergillus niger decreased with increase in pH as well as in accordance with S albidoflavus strain SAMRC-UFH5 showed alkaline tolerance in the production of cellulase. The decrease activity of carboxymethylcellulose, after a limited incubation period of their highest activities it is associated to cumulative effect of cellobiose. Cellobiose, a dimer of glucose which inhibit endoglucanase, also the delignification may produce aromatic water soluble products which is known to repress the cellulolytic action of the enzyme [59]. The decrease may also be due to depletion of mineral/salt other than the energy source. The instability of this enzyme degradation can be also due to the denaturation of protiens. However in contrast to our observation, Das et al. [60] that the highest mean cellulase activity was observed at pH 8.0 and the lowest mean activity was observed at pH 7.0 in some pectinolytic bacteria. Moreover according to Torriani et al. [61] the power of solubilizing phosphate of E. coli is optimum at pH 7.0. Similar results were also observed by Gugi et al. [62] in psychrotrophic bacterium Pseudomonas fluorescence that the ability of the strain to solubilize phosphate decreased with increase in pH value. So the decrease in the potential of the strain to solubilize phosphate indicates that the production of acids responsible for the solubilization lowers. Likewise temperature is another parameter we considered to analyse this effect on the strain. The normal temperature for incubation is 28°C to allow the strain to degrade cellulase [63] and 37°C for phosphate solubilisation [64].
In the present study, we examined the effect of increase in temperature slightly higher than its normal temperature in both the cases. The temperature was 30°C and 32°C for cellulose degradation and 39°C and 41°C for phosphate solubilisation. The results revealed that with the increase in the temperature the strain lost it capability to solubilize phosphate and also to degrade cellulose. On the contradictory Immanuel et al. [65] reported that the cellulolytic enzyme activity was found to be minimum at 20°C and maximum at 50°C by the bacterial strains such as Cellulomonas, Bacillus and Micrococcus species. However similar results were reported by Hasper et al. [66] who stated that higher production of endoglucanase reaches its maximum at 30°C and minimum at 55°C in case of Aspergillus niger. Similarly for phosphatase activity according to Gugi et al. [62] increase in activity was observed at 17.5°C whereas the activity decreased to the minimum at 30°C for both acidic and alkaline phosphatase in psychotropic bacterium Pseudomonas fluorescence. Effect of UV rays was the 3rd parameter to be analysed. On explosure with UV light at different interval of time i.e., 5 min, 10 min and 15 min we drew the conclusion that there was minute decrease in the ability of the strain with increase in time of UV rays explosure. Similar result was also reported in Tank et al. [67] stating that on explosure with UVA and UVB the phosphatase activity was decreased respectively. Seiji and Kikuchi [68] also drew the same conclusion that phosphatase activity showed conspicuous decrease with increase dose of UV rays. On the contradictory Shahbazi et al. [69] reported that on explosure to UV rays the activity of cellulase increased than that of control in Trichoderma reesei. Moreover in Elakkiya and Muralikrishnan [70] it is reported that on explosure of UV for 25 mins the cellulose activity of mutant strain Trichoderma viride increased than that of the untreated strain but when the spores exposed to UV for 35–40 mins they were totally killed. Naphthalene was also used to see the adversed effect on the strain. In the presence of naphthalene the solubilizing and degrading ability of the strain increased from the normal. Same was also reported by Margesin et al. [71] which states that the phosphatase activity was higher than the control in presence of PAH, on contradictory it also states that the cellulose activity in presence of PAH was negligible. The strain was lastly treated with heavy metals such as cadmium and lead, cadmium had more degrading and solubilizing ability than lead i.e. lead was proved to be more toxic for both the cases. Similarly Bielińska and Mocek-Płóciniak [72] showed an adverse effect of Cd and Zn on the activity of phosphatases. But on contradictory Sethi and Gupta [73] and Khan et al. [74] found that cadmium was more toxic for the cellulase enzyme than lead. In addition strong influence of cadmium on the activity of phosphatase was also reported by Wyszkowska and Wyszkowski [75], which again was found to contradict our result.
Acknowledgement
I would like to extend my gratitude and sincere thanks to my honorable supervisor Dr. Surajit Das, Assistant Professor, Department of Life Science, NIT Rourkela. I sincerely thank him for his exemplary guidance and encouragement. He is not only a great lecturer with deep vision but also most importantly a kind person. His trust and support inspired me in the most important moments of making right decisions and I am glad to work under his supervision.
I am also extremely thankful to Supriya Kumari (PhD Scholar), Department of Life Science, NIT Rourkela for her constant encouragement, active co-operation, precious suggestion and sincere help. She helped me kindheartedly for everything that I needed to successfully complete my project work.
I too express my heartfelt thanks to Bhakti Patel, Rajanya Banerjee, Shreosi Chatterjee and Suman Dey (PhD scholars) and Aniruddha C. Patil (Technical Assistant), Department of Life Science, NIT Rourkela, for their active cooperation and sincere help. I am genuinely appreciative of all my lab mates for their suggestions and moral support during my project work.
REFERENCES
- Sandilyan S, Kathiresan K. Mangrove conservation: a global perspective. Biodiversity and Conservation. 2012;1-20.
- Gilman EL, Ellison J, Duke NC, et al. Threats to mangroves from climate change and adaptation options: A review. Aquatic Botany. 2008;89(2):237-50.
- Takai K, Nakamura K. Archaeal diversity and community development in deep-sea hydrothermal vents. Current Opinion in Microbiology. 2011;14(3):282-91.
- Bayen S. Occurrence bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environment International. 2012;48:84-101.
- Holguin G, Vazquez P, Bashan Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biology and Fertility of Soils. 2001;33(4):265-28.
- Gupta P, Samant K, Sahu A. Isolation of cellulose-degrading bacteria and determination of their cellulolytic potential. International Journal of Microbiology. 2012.
- Gomashe AV, Gulhane PA, Bezalwar PM. Isolation and screening of cellulose degrading microbes from Nagpur region soil. International Journal of Life Sciences. 2013;1:291-3.
- Roychowdhury D, Paul M, Banerjee SK. Isolation identification and characterization of phosphate solubilising bacteria from soil and the production of biofertilizer. International Journal of Current Microbiology and Applied Sciences. 2015;4(11):808-15.
- Ndung’u-Magiroi KW, Herrmann L, Okalebo JR, et al. Occurrence and genetic diversity of phosphate-solubilizing bacteria in soils of differing chemical characteristics in Kenya. Annals of Microbiology. 2012;1-8.
- Sahoo K, Dhal NK. Potential microbial diversity in mangrove ecosystems: A Review. 2009.
- Leschine SB. Cellulose degradation in anaerobic environments. Annual Reviews in Microbiology. 1995;49(1):399-426.
- Schwarz WH. The cellulosome and cellulose degradation by anaerobic bacteria. Applied Microbiology and Biotechnology. 2001;56(5):634-49.
- Wilson DB. Aerobic microbial cellulase systems. Biomass recalcitrance: Deconstructing the plant cell wall for bioenergy. 2008;374-92.
- Shoseyov O, Shani Z, Levy I. Carbohydrate binding modules: biochemical properties and novel applications. Microbiology and Molecular Biology Reviews. 2006;70(2):283-95.
- Schülein M. Protein engineering of cellulases. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 2000;1543(2):239-52.
- Irwin DC, Zhang S, Wilson DB. Cloning expression and characterization of a family 48 exocellulase Cel48A from Thermobifida fusca. The FEBS Journal. 2000;267(16):4988-97.
- Coughlan MP. Mechanisms of cellulose degradation by fungi and bacteria. Animal Feed Science and Technology. 1991;32(3):77-100.
- Heiner AP, Sugiyama J, Teleman O. Crystalline cellulose Iα and Iβ studied by molecular dynamics simulation. Carbohydrate Research. 1995;273(2):207-23.
- Teeri TT. Crystalline cellulose degradation: new insight into the function of cellobiohydrolases. Trends in Biotechnology. 1997;15(5):160-7.
- Boisset C, Chanzy H, Henrissat B, et al. Digestion of crystalline cellulose substrates by the Clostridium thermocellum cellulosome: structural and morphological aspects. Biochemical Journal. 1999;340(3):829-35.
- Shoham Y, Lamed R, Bayer EA. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends in Microbiology. 1999;7(7):275-81.
- Bégum P, Lemaire M. The cellulosome: an exocellular multiprotein complex specialized in cellulose degradation. Critical Reviews in Biochemistry and Molecular Biology. 1996;31(3):201-36.
- Bayer EA, Chanzy H, Lamed R, et al. Cellulose cellulases and cellulosomes. Current Opinion in Structural Biology. 1998;8(5):548-57.
- Bayer EA, Morag E, Lamed R, et al. Cellulosome structure: four-pronged attack using biochemistry, molecular biology crystallography and bioinformatics. Special Publication-Royal Society of Chemistry. 1998;219:39-65.
- Wilson DB. Three microbial strategies for plant cell wall degradation. Annals of the New York Academy of Sciences. 2008;1125(1):289-97.
- Irwin DC, Spezio M, Walker LP, et al. Activity studies of eight purified cellulases: specificity, synergism, and binding domain effects. Biotechnology and Bioengineering. 1993;42(8):1002-13.
- Ding SY, Xu Q, Crowley M, et al. A biophysical perspective on the cellulosome: new opportunities for biomass conversion. Current Opinion in Biotechnology. 2008;19(3):218-27.
- Guillén F, Martínez MJ, Gutiérrez A, et al. Biodegradation of lignocelluloses: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. International Microbiology. 2005;8:195-204.
- Wilson DB. Microbial diversity of cellulose hydrolysis. Current Opinion in Microbiology. 2011;14(3):259-63.
- Wang X, Wang Y, Tian J, et al. Overexpressing AtPAP15 enhances phosphorus efficiency in soybean. Plant Physiology. 2009;151(1):233-40.
- Shenoy VV, Kalagudi GM . Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnology Advances. 2005;23(7):501-13.
- Ahemad M, Zaidi A, Khan MS, et al. Biological importance of phosphorus and phosphate solubilizing microorganisms—an overview. Phosphate Solubilising Microbes for Crop Improvement. Nova Science Publishers. 2009;1-4.
- Khan MS, Zaidi A. Phosphate solubilizing microbes for crop improvement. Nova Science Publishers. 2009.
- Khan MS, Zaidi A, Wani PA, et al. Functional diversity among plant growth-promoting rhizobacteria: current status. In Microbial Strategies for Crop Improvement. 2009;105-32.
- Khan MS, Zaidi A, Musarrat J. Microbial strategies for crop improvement. Berlin: Springer. 2009.
- Richardson AE. Soil microorganisms and phosphorus availability. Soil Biota. 1994;50:35-9.
- Yadav BK, Verma A. Phosphate solubilisation and mobilization in soil through microorganisms under arid ecosystems. In the Functioning of Ecosystems. 2012.
- Khan MS, Zaidi A, Ahmad E. Mechanism of phosphate solubilisation and physiological functions of phosphate-solubilizing microorganisms. In Phosphate Solubilizing Microorganisms. 2014;31-62.
- Gupta N, Sabat J, Parida R, et al. Solubilisation of tricalcium phosphate and rock phosphate by microbes isolated from chromite, iron and manganese mines. Acta Botanica Croatica. 2007;66(2):197-204.
- Khan MS, Ahmad E, Zaidi A, et al. Functional aspect of phosphate-solubilizing bacteria: importance in crop production. In Bacteria in Agrobiology: Crop Productivity. 2013;237-63.
- Toro M. Phosphate solubilizing microorganisms in the rhizosphere of native plants from tropical savannas: An adaptive strategy to acid soils. In First International Meeting on Microbial Phosphate Solubilisation. 2007; 249-52.
- Bishop ML, Chang AC, Lee RWK. Enzymatic mineralization of organic phosphorus in a volcanic soil in Chile Soil Science. 1994;157(4):238-43.
- Illmer P, Schinner F. Solubilisation of inorganic calcium phosphates—solubilization mechanisms. Soil Biology and Biochemistry. 1995;27(3):257-63.
- Khan MS, Zaidi A, Ahemad M, et al. Plant growth promotion by phosphate solubilizing fungi–current perspective. Archives of Agronomy and Soil Science. 2010;56(1):73-98.
- Marciano Marra L, Fonsêca Sousa Soares CR, de Oliveira SM, et al. Biological nitrogen fixation and phosphate solubilisation by bacteria isolated from tropical soils. Plant and Soil. 2012;357(1):289-307.
- Mullen MD. Phosphorus in soils: biological interactions. Encyclopedia of Soils in the Environment. 2005;3: 210-5.
- Trivedi P, Sa T. Pseudomonas corrugata (NRRL B-30409) mutants increased phosphate solubilisation organic acid production, and plant growth at lower temperatures. Current Microbiology. 2008;56(2):140-4.
- Tarafdar JC, Claassen N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biology and Fertility of Soils. 1988;5(4):308-12.
- Yadav RS, Tarafdar JC. Phytase and phosphatase producing fungi in arid and semi-arid soils and their efficiency in hydrolysing different organic P compounds. Soil Biology and Biochemistry. 2003;35(6):745-51.
- Maougal RT, Brauman A, Plassard C, et al. Bacterial capacities to mineralize phytate increase in the rhizosphere of nodulated common bean (Phaseolus vulgaris) under P deficiency. European Journal of Soil Biology. 2014;62:8-14.
- Beech IB, Paiva M, Caus M, et al. Enzymatic activity and within biofilms of sulphate-reducing bacteria. Biofilm Community Interactions: Chance or Necessity. 2001;231-9.
- Rodrı́guez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnology Advances. 1999;17(4):319-339.
- Yadav BK, Tarafdar JC. Penicillium purpurogenum, unique P mobilizers in arid agro-ecosystems. Arid land Research and Management. 2011;25(1):87-99.
- Richardson AE. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Functional Plant Biology. 2001; 28(9):897-906.
- Vassilev N, Vassileva M, Bravo V, et al. Simultaneous phytase production and rock phosphate solubilisation by Aspergillus niger grown on dry olive wastes. Industrial Crops and Products. 2007;26(3):332-6.
- Kumar V, Singh P, Jorquera MA, et al. Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World Journal of Microbiology and Biotechnology. 2013;29(8):1361.
- Salimpour S, Khavazi K, Nadian H, et al. Enhancing Phosphorous Availability to Canola ('Brassica napus' L.) Using P Solubilizing and Sulphur Oxidizing Bacteria. Australian Journal of Crop Science. 2010;4(5):330.
- Seshadri S, Ignacimuthu S. Variations in hetetrophic and phosphate solubilizing bacteria from Chennai, southeast coast of India. 2002.
- Abubakar FA, Oloyede OB. Production and activity of cellulase from Aspergillus niger using rice bran and orange peel as substrates. International Journal of Scientific Research and Management. 2013;1:285-91.
- Das B, Chakraborty A, Ghosh S, et al. Studies on the effect of pH and carbon sources on enzyme activities of some pectinolytic bacteria isolated from jute retting water. Turkish Journal of Biology. 2011;35(6):671-8.
- Torriani-Gorini A, Yagil E, Silver S. Phosphate in microorganisms: cellular and molecular biology. Zondervan. 1994.
- Gügi B, Orange N, Hellio F, et al. Effect of growth temperature on several exported enzyme activities in the psychotropic bacterium Pseudomonas fluorescence. Journal of Bacteriology. 1991;173(12):3814-20.
- Xia L, Cen P. Cellulase production by solid state fermentation on lignocellulosic waste from the xylose industry. Process Biochemistry. 1999;34(9):909-12.
- Pardo AG, Forchiassin F. Influence of temperature and pH on cellulase activity and stability in Nectria catalinensis. Revista Argentina de Microbiologia. 1998;31(1):31-5.
- Immanuel G, Dhanusha R, Prema P, et al. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. International Journal of Environmental Science and Technology. 2006;3(1):25.
- Hasper AA, Dekkers E, van Mil M, et al. EglC a new endoglucanase from Aspergillus niger with major activity towards xyloglucan. Applied and Environmental Microbiology. 2002;68(4):1556-60.
- Tank SE, Xenopoulos MA, Hendzel LL. Effect of ultraviolet radiation on alkaline phosphatase activity and planktonic phosphorus acquisition in Canadian boreal shield lakes. Limnology and Oceanography. 2005;50(5):1345-51.
- Seiji M, Kikuchi A. Acid phosphatase activity in melanosomes. The Journal of Investigative Dermatology. 1969;52(2):212.
- Shahbazi S, Ispareh K, Karimi M, et al. Gamma and UV radiation induced mutagenesis in Trichoderma reesei to enhance cellulases enzyme activity. Int’l J Fam Alli Sci. 2014;3(5):543-54.
- Elakkiya P, Muralikrishnan V. Cellulase production and purification of mutant strain Trichoderma viride. Int J Curr Microbiol App Sci. 2014;3(9):720-7.
- Margesin R, Walder G, Schinner F. The impact of hydrocarbon remediation (diesel oil and polycyclic aromatic hydrocarbons) on enzyme activities and microbial properties of soil. Engineering in Life Sciences. 2000;20(4):313-33.
- Bielińska EJ, Mocek-Płóciniak A. Impact of Eco-chemical soil conditions on selected heavy metals content in garden allotment vegetables. Polish Journal of Environmental Studies. 2010;19(5):895-900.
- Sethi S, Gupta S. Responses of soil enzymes to different heavy metals. Biolife. 2015;3:147-53.
- Khan S, Qing CAO, Hesham AEL, et al. Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. Journal of Environmental Sciences. 2007;19(7):834-40.
- Wyszkowska J, Wyszkowski M. Effect of Cadmium and Magnesium on Enzymatic Activity in Soil. Polish Journal of Environmental Studies. 2003;12(4).