Survey cytotoxicity and genotoxicity of hydroalcoholic extract of Stevia rebaudiana in breast cancer cell line (MCF7) and human fetal lung fibroblasts (MRC-5)
2 Faculty of Pharmacy, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Mazandaran, Iran, Email: rajabali.farnaz@yahoo.com
3 Department of Pharmacognosy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Mazandaran, Iran, Email: emran.habibi@gmail.com
4 Pharmaceutical Sciences Research Centre, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Mazandaran, Iran, Email: mona.modanloo@gmail.com
Received: 25-Oct-2018 Accepted Date: Nov 16, 2018; Published: 20-Nov-2018
Citation: Shokrzadeh M, Rajabali F, Habibi E, et al. Survey cytotoxicity and genotoxicity of hydroalcoholic extract of Stevia rebaudiana in breast cancer cell line (MCF7) and human fetal lung fibroblasts (MRC-5). J Can Res Metastasis. 2018;1(2):12-7.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Breast cancer is the most commonly diagnosed cancer in women and is the second leading cause of cancer death among them. Chemotherapy is one of the commonly used strategies in treatment. This therapy is usually associated with adverse side effects. Isolating and identifying potent anti- Tumor Compounds from Medicinal Plants motivated researchers to find plant species for anti- Tumor Effects. Therefore, finding natural compounds from plants may provide an alternative cancer treatment. Stevia rebaudiana is an important medicinal plant in the world.
Keywords
Micro-nucleus assay; MTT; Hydro-alcoholic extract of Stevia rebaudiana
Introduction
C ancer remains one of the world’s most devastating diseases, with more than 10 million new cases every year [1]. There are many reports that hazardous chemicals can cause genotoxic and carcinogenic effects on the breast. The main mechanism is the production of free radicals that cause damage to macromolecules such as DNA vital to promote chronic diseases such as cancer [2]. Conventional treatments for cancer have many types, which are intended to kill tumor cells or prevent their proliferation. Current cancer treatments include surgical intervention, radiation and chemotherapy drugs, which often also kill healthy cells and cause toxicity to the patient [3]. Methotrexate (MTX) is an anti-folic acid agent due to its antagonistic effect on folic acid metabolism [4]. MTX is also a mitotic inhibitor that arrests the cell cycle in interphase and leads to prolongation of meta-phase [5]. The genotoxic effects of MTX have already been reported in the somatic cells employing chromosome aberration and micronucleus test as the end points of evaluation [6-8]. The genotoxic potential of MTX disposed the induction of MN in the oral mucosa sweeps of rheumatoid arthritis (RA) patients as well as the sister chromatic exchanges (SCEs) in oncology nurses [9,10]. Also, cisplatin is a commonly used anti-neoplastic agent with acceptable efficacy against a variety of human malignancies [11]. Interaction of cisplatin with DNA is the most important cytotoxic effect [12]. Since it is important for World Health Organization (WHO) to take advantage from traditional medicine, the approach based largely on the use of medicinal herbs. Recently, there is a lot of interest in finding plant-derived compounds that can reduce toxic effects of chemotherapy on normal cells without damaging their antineoplastic activity [13]. Consumption of vegetables and fruits has been an effective strategy in reducing the genotoxic and carcinogenic effects induced by hazardous chemicals [14,15]. The preventive effects of natural products are caused by their antioxidant and free radical scavenging activities, which can affect cellular signaling pathways [16,17]. Many natural compounds in plants have anti-mutagenic or anti-carcinogenic effects [2]. On the other hand, the use of cell cultures in the field of toxicology has proven significant in assessing cytotoxicity of chemicals, pharmaceuticals, pesticides, and in vitro examination of all natural and synthetic compounds [18].
Stevia rebaudiana (Bert.), Bertoni is an herbaceous perennial plant of the Asteraceae family [19]. It is native to Paraguay. The leaf extract of S. rebaudiana used traditionally in treatment of diabetes. The main sweet component in the leaves of S. rebaudiana is stevioside [20]. This plant used from 16th century and nowadays can be replaced by synthetic sweetening agents [21]. The Stevia genus comprises at least 110 species that S. rebaudiana Bertoni is being the sweetest of all [22]. Stevia sweetener extractives exert beneficial effects on human health, including anti- hypertensive [23-25], anti-hyperglycemic non-cariogenic, anti-human Rota virus [26] and antimicrobial activities [27- 29]. Aqueous extract of S. rebaudiana dried leaves induce systemic and renal vasodilation, causing hypotension and diuresis in rats [30]. Also, physiological and pharmacological researches reveal its anti-tumor effect [31,32]. Thus, we decided to study the IC50 and cytotoxic effects of hydro-alcoholic extract of Stevia rebaudiana on human fetal lung fibroblasts (MRC-5) and human breast cancer cell lines (MCF7) and compare the parameters with the results concluded from cisplatin cytotoxic effect of on theses cell lines. In spite of the fact that some researches declare Genotoxic risk of Stevia rebaudiana to human cells should be discussed [33], this study was undertaken to assess genotoxicity of hydro-alcoholic extract of Stevia rebaudiana determining the micro-nuclei (MN) frequencies in peripheral blood erythrocytes and compare the parameters with the result concluded from methotrexate using the micronucleus test. However, further studies are required to determine their exact mechanism of action.
Material and Methods
Cytotoxicity assay chemicals and reagents
26 to 31 cell passages in dulbecco’s modified eagle medium DMEM medium with 10% fetal bovine serum (FBS), 100 mg sodium pyruvate, 1.5 g/l sodium bicarbonate and 1% penicillin- streptomycin antibiotic in BINDER incubator, USA) at 37°C and sufficient humidity and 5% carbon dioxide were used. MTX (CAS 59-05 2) was obtained as gift sample from GlaxoSmithKline Pharmaceuticals Limited, Mumbai, India.
Cell culture
In this study, human breast cancer cell lines (MCF7) and human fetal lung fibroblasts (MRC-5) were used. Cell lines were purchased from the Tehran Pasteur Institute according to the ATCC number and cultured in the cell culture laboratory of Sari Pharmacy Faculty. For different tests, cells with at least 70% of growth were isolated by trypsin-ethylene diamine tetra acetic acid (EDTA) from the flask and centrifuged at 1500 rpm for 5 minutes. Cellular sedimentation was prepared as a suspension in 1 cc of DMEM and the percentage of cell survival in the cell suspension was determined by mixing the equal ratio of trypan blue with a hemocytometric Lumina and by optical microscopy. After ensuring non-contamination of the cells, cells with a viability greater than 90% were used for testing [34-36].
Cytotoxicity assay
In order to investigate the effect of methanol-aqueous extract of Stevia rebaudiana on growth and proliferation of cancer cells, the colorimetric method (3-(4,5-dimethylthiazol-2-yl) -2,5- diphenyltetrazolium bromide) MTT was used [32]. This method is a competitive metabolic test and is based on the breakdown of tetrazolium salt by mitochondrial succinate dehydrogenase enzyme.
MTT assay
In MTT assay, 20 μl of DMEM/F12 including 1×105 cells were added to 3 wells for each concentration of the extract and Cisplatin. Then, they incubated for 72 hours until the cells reach their logarithmic growth stage. During incubation, the cells are examined every two days to ensure the growth and non-contamination of the cells. After incubation sample solutions were added at concentrations ranging from 5 to 1000 μg/ml to each well and incubated for 72 h; So that the cells are adequately exposed to the extracts and Cisplatin drug. DMSO (0.5%) treated cells served as the solvent control, and then washed using sterile normal saline 0.09%. After this period, the contents of wells were excluded; The cells were dyed by 30 μg of MTT (Methyl thiotetrazolium) and incubated for 4 hours. Then, MTT solution was excluded from wells, to each house, a plate of 20 μl of dilute DMSO was added to dissolve purple crystalline of formazan and the 96 well/ plates were shaken for 15 minutes [37,38]. At the end, the absorbance of colored colonies, Healthy cells that are in contact with the compounds have not been degraded by membranes and the color has penetrated into them, was determined by ELISA reader (λ max=490 and 630 nm).
Genotoxicity assay micronucleus assay
Blood samples were collected from 5 healthy, no smoking, no alcoholic, no antibiotic treatment in a month, male donors aged between 25-35 years volunteers. To prevent blood coagulation, the syringe is shaken slowly to allow heparin to be uniformly mixed with blood. 0.5 mL of whole blood was added to 4.5mL of Roswell Park Memorial Institute (RPMI) culture medium 1640 supplemented with fetal bovine serum containing L-glutamine, antibiotics and phytohemagglutinin (PHA), and different doses of Stevia rebaudiana (50, 100, and 200 μg/mL) were added. Cytochalasin B (Cyt-B) (Sigma, MO, USA) at the final concentration of 6 μg/mL was added at 44-hour post PHA stimulation. Cyt B prevents complete cytokinesis in mitosis, thus causing an appearance of multi-nuclear cells [39]. The binucleated lymphocytes were harvested 28 hours after adding Cyt-B, they were treated by 6 ml of the hypotonic potassium chloride solution and then centrifuged at about 1000 rpm for 5 minutes. The sample is now found in red due to red blood cell lysis.1 ml of fixative solution (methanol: acetic acid= 6:1), which hemolyses the remaining red blood cells and hemoglobin converts it to hematine was added. For better fixation, samples are stored in the refrigerator for 24 hours. For slide preparation Clean and put it in the freezer until completely cooled, then 2-3 drops of cell suspension were thrown on a clean slide, slowly slide it and dry them in room temperature. The slides were stained with Giemsa solution (4%) for 7-10 mins, washed with distilled water and dried. They were observed at 40× and 100× magnifications using a light microscope to estimate micronuclei frequency (the number of micronuclei in at least 1000 binucleated cells) [40,41]. binucleated cells are lymphocytes that were once divided by mitosis [42]. The experiment was performed twice.
Statistical analysis
The IC50 (inhibition concentration, which produces 50% inhibition) was calculated for each extract using the cytotoxicity of the concentrations used in the extract. The IC50 values were compared using the student’s T-test measuring the effectiveness of a substance to cause cell death or inhibit cell growth. So the lower amount of IC50 represents a higher toxicity of a compound, which leads to death or inhibition of cell growth [38]. Prism ver.3 Software was used to perform statistical tests using nonlinear regression. Standard deviations represent average results of double experiments. Analysis of variance (one-way ANOVA) followed by Tukey test was used to determine the differences among the groups (p<0.05).
Results
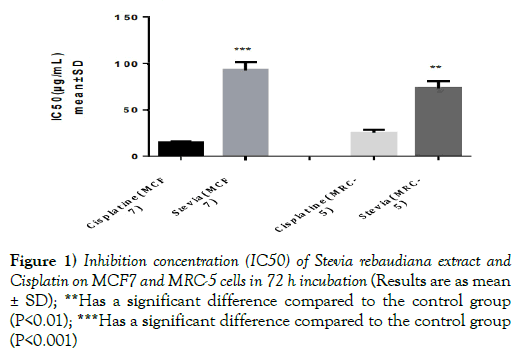
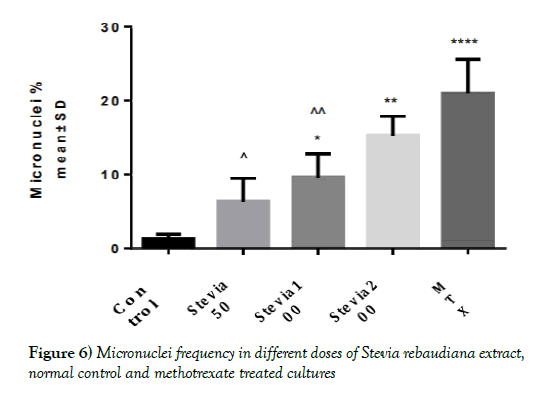
The results showed that IC50 of cisplatin was significantly less than IC50 of Stevia rebaudiana extract on human cancer cell line (MCF7) and human fetal lung fibroblasts (MRC-5) (p<0.01) (Figure 1 and Table 1).
| Control | Stevia 50 | Stevia 100 | Stevia 200 | MTX | |
|---|---|---|---|---|---|
| Mean | 1.333 | 6.333 | 9.667 | 15.33 | 21 |
| Std. Deviation | 0.5744 | 3.055 | 3.055 | 2.517 | 4.583 |
Table 1: Inhibition concentration (IC50) of Stevia rebaudiana extract and Cisplatin on MCF7 and MRC-5 cells in 72 h
Figure 1) Inhibition concentration (IC50) of Stevia rebaudiana extract and Cisplatin on MCF7 and MRC-5 cells in 72 h incubation (Results are as mean ± SD); **Has a significant difference compared to the control group (P<0.01); ***Has a significant difference compared to the control group (P<0.001)
incubation
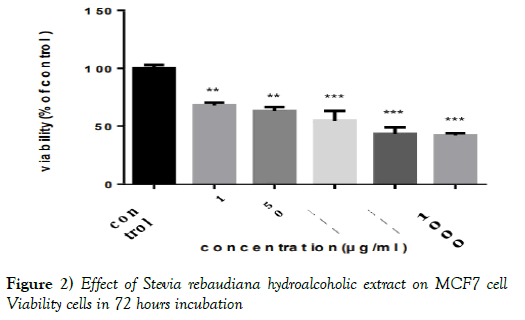
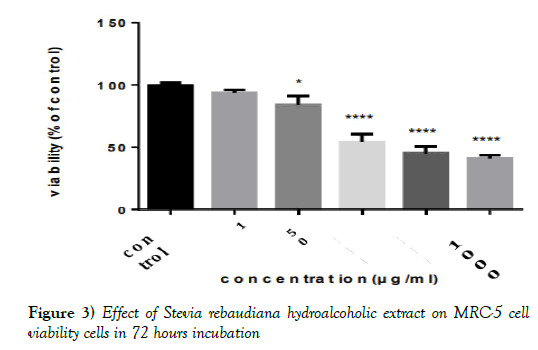
Using the adsorption in the MCF7 and MRC-5 cell lines and the completion of MTT test, the percentage of cell survival (viability %) after exposure to methanolic-aqueous extract of Stevia rebaudiana was determined. It is obvious that the dose of 10 μg/ml doesn’t have toxic effect in both cell lines. (The absence of a significant difference with the DMSO control group).
However, at higher doses there was a significant difference between the group affected by Stevia extract and the control group (P< 0.01) (Figures 2 and 3 & Tables 2 and 3).
Figure 2) Effect of Stevia rebaudiana hydroalcoholic extract on MCF7 cell Viability cells in 72 hours incubation
Figure 3) Effect of Stevia rebaudiana hydroalcoholic extract on MRC-5 cell viability cells in 72 hours incubation
| HBsAg | 0R | CI 95% | p-value | ||
|---|---|---|---|---|---|
| Variables | Positive | Negative | |||
| Previous hepatic pathology | n (%) | 08 (61.5) | |||
| Present | 05 (38.5) | 134 (88.2) | 4,6 | [1.37-15.77] | 0.01 |
| Absent | 18 (11.8) | 1 | |||
| Vaccination against HBV | n (%) | ||||
| Vaccinated | 01 (33.3) | 02 (66.7) | 1 | 0.32 | |
| Unvacinated | 22 (13.5) | 141 (86.5) | 3.2 | [0.27-36.58] | |
| Anterior serology of HBV | n (%) | ||||
| Positive | 09 (81.8) | 02 (18.2) | 0.01 | ||
| Negative | 01 (02.9) | 34 (97.1) | |||
| Unknown | 13 (10.8) | 107 (89.2) | |||
| HIV | n (%) | ||||
| Positive | 08 (28.6) | 20 (71.4) | 0.2 | [0.07-0.65] | 0.01 |
| Negative | 09 (08.6) | 96 (91.4) | 1 | ||
| Unknown | 06 (18.2) | 27 (81.8) | 0.5 | [0.15-1.77] | |
Table 2: Medical history and carriage of HBsAg
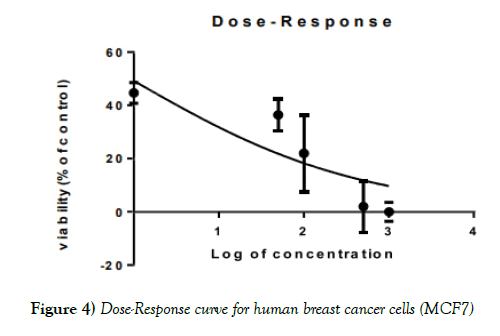
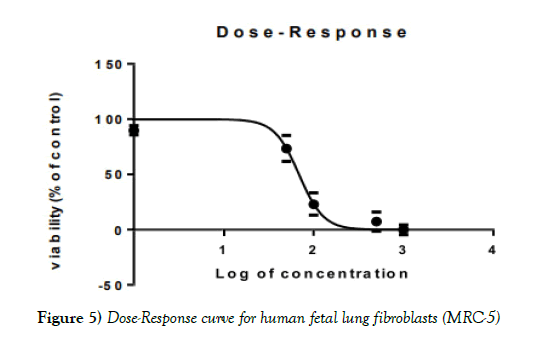
The MTT test with increasing doses of extract showed a decrease in viability in both normal and cancer cells, MCF7 and MRC- 5, respectively (Figures 4-6).
Figure 4) Dose-Response curve for human breast cancer cells (MCF7)
IC50 for the methanolic-aqueous extract of Stevia rebaudiana for human breast cancer cells (MCF7), 98.82 ± 2.52 μg/ml for human fetal lung fibroblasts (MRC- 5), 68.88± 0.30 and for cisplatin is 15.96 ± 0.26 μg/ml. HIV infection was significantly associated with carriage of HBsAg (p=0.01); (Table 1). None of the usual risk factors for HBV transmission were significantly associated with the carriage of HBsAg in our study (Table 3).
| Variables | HBs Ag | OR | CI 95% | p-value | ||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Multiple sex partners | n (%) | |||||
| Present | 03 (11.6) | 23 (88.4) | 0.8 | [0.22-2.98] | 0.76 | |
| Absent | 20 (14.3) | 120 (85.7) | 1 | |||
| Hepatitis B in the entourage | n (%) | |||||
| Positive | 02 (09.6) | 19 (90.4) | 0.8 | [0.06-10] | 0.85 | |
| Negative | 01 (12.5) | 07 (87.5) | 1 | |||
| Unknown | 20 (14.6) | 117 (85.4) | 1.2 | [0.13-10.25] | ||
| Manicure by common material | n(%) | |||||
| Present | 11 (14.1) | 67 (85.9) | 1.1 | [0.43-2.55] | 0.90 | |
| Absent | 12 (13.6) | 76 (86.4) | 1 | |||
| Tattoo | n (%) | |||||
| Tattoed | 07 (29.2) | 17 (70.8) | 1,6 | [0.59-4.14] | 0.36 | |
| No tattoed | 31 (21.9) | 111 (78.1) | 1 | |||
| Scarifications | n (%) | |||||
| Scarified | 17 (14.7) | 99 (85.3) | 1.3 | [0.46-3.44] | 0.63 | |
| Not scarified | 06 (12) | 44 (88) | 1 | |||
| Surgery | n (%) | |||||
| Operated | 00 (00) | 03 (100) | 0.56 | |||
| Not operated | 23 (14.1) | 140 (85.9) | ||||
| Piercings | n (%) | |||||
| Yes | 04 (21.1) | 15 (78.9) | 2 | [0.26-5.04] | 0.28 | |
| No | 19 (12.9) | 128 (87.1) | 1 | |||
| Blood transfusion | n (%) | |||||
| Transfused | 00 (00) | 11 (100) | 0.34 | |||
| Not transfused | 02 (01.3) | 153 (98.7) | ||||
| Drug IV | n (%) | |||||
| Present | 02 (20) | 08 (80) | 1.8 | [0.35-9.44] | 0.46 | |
| Absent | 21 (13.5) | 135 (86.5) | 1 | |||
Table 3: Risk Factors and Portage of Hbs Ag
Discussion
The global cancer report shows a high sudden increase in the rate of cancer. Cancer is the second largest disease which has a sizable contribution in the total number of deaths [43]. Cancer rates can rise to 50% of new cases by 2020 [44]. Nowadays, compounds with natural origin due to the frequency, less side effects and drug interactions get the most attention for synthesis of new drugs [45,46]. The effects of various compounds such as cisplatin, methotrexate and methanol- aqueous extract of Stevia rebaudiana (which were investigated in this study) on cell lines in a controlled environment make it possible to identify mechanism and their biological effects on different intracellular factors. Toxic compounds, that are currently measurable by cellbased toxicology methods using toxicity testing methods are one of the best candidates for the synthesis of anticancer drugs in chemotherapy of cancers [45]. All of these can lead to improved therapeutic ways [46]. Cisplatin is an alkylating agent that acts on S phase of the cell cycle.
Therefore, it is a cytotoxic drug that causes cell death [47]. Also, methotrexate is a structural analogue of folic acid and hence interferes with the synthesis of nucleic acid [4]. Figures 1, 2 and 3 in the findings shows that although there is a significant difference between the amount of IC50 of the extract of the Stevia rebaudiana plant compared to the cisplatin anticancer drug, it can be argued that the extract of this plant has an significant inhibitory effect on the growth of human breast cancer cell line (MCF7) and human fetal lung fibroblasts (MRC-5). It is intelligible that sensitivity of cancer cells to Stevia extract was significantly higher than that of human fetal lung fibroblasts according to the MTT test results. This difference can be due to dysfunction of cellular organisms that cause cancer with higher proliferation and increased cellular intake [48].
The reason for significant difference between IC50 and Cisplatin with Stevia rebaudiana extract on cell cells is that cisplatin is a pure chemical compound, but the Stevia extract is a mixture of many compounds, including a variety of glycosides, namely dulcoside A, rebaudiosides A_E, steviolbioside, and stevioside [22] with stevioside being the major constituent (3_8% by weight of the dried leaves) [49]. The main constituents were glycosides such as stevioside, steviol and rebaudioside A and B. Stevioside is a natural sweetener extracted from leave of Stevia [50]. In addition, S. rebaudiana Bertoni contains stigmasterol, b-sitosterol, and campesterol [51]. It also contains steviol, a product formed by enzymatic hydroxylation within the plant [52]. Steviol can exhibit some toxic and mutagenic activity effect in some tests [33,53]. Labdane sclareol, compound present in leaf extract of Stevia has anti-tumorous and cytotoxic properties [54].
Stevioside, the Stevia leaf aglycones, steviol, isosteviol, and their metabolites have been reported to inhibit tumor promotion by blocking Epstein-Barr virus early antigen (EBV-EA) induction [55] as well as by reducing tumor formation in the two-stage mouse skin carcinogenesis model following sequential exposure to 7,12-dimethylbenz [a]anthracene (DMBA) and 12- O- tetradecanoylphorbol-13-acetate (TPA) [56-58]. The hydrolysis product of stevioside, isosteviol, potently inhibits DNA replication and human cancer cell growth in vitro (with LD50 values of 84 to 167 μMol) [59]. Effect of stevioside against tumor was examined and stevioside slowed the tumor promoting agent (TPA) induced tumor promotion in a skin carcinogenesis in mice [60].
The study of toxic and carcinogenic effects of stevioside showed that this substance had no effect on progression of pre-neoplastic or neoplastic lesions in the urinary bladder [60]. The toxicological effects of low concentrations of stevioside on apoptosis induced by serum deprivation using the PC12 cell system was examined by using DNA electrophoresis and TUNEL signal assays and on the basis of data it was found that stevioside increased apoptosis caused by serum deprivation and this enhancement was enhanced by increased expression of Bax and of cytochrome c released into the cytosol which make a sign that stevioside affects regulation of the normal apoptotic condition [60,61]. Ascorbic acid, b-carotene, chromium, cobalt, magnesium, iron, potassium, phosphorous, riboflavin, thiamin, tin and zinc are other constituents exist in Stevia [62]. Since differential cytotoxicity is also a useful feature for potential antitumor agents, the cytotoxicity activity of the Stevia rebaudiana leaf extract was evaluated in both cancer and normal cell lines by the MTT assay. In previous studies the sensitivity of lymphocytes isolated from peripheral blood has been reported into chemicals more than the lymphocytes of the blood as whole blood. This is due to presence of some protective factors in the blood and also other targets rather than lymphocytes are known for the chemical. Since all of these targets and protective factors are in the human body’s peripheral blood, it was decided in this study to use whole blood cultures for further similarity of test conditions with the human body [61].
Conclusion
The use of cell culture methods provides a valuable insight of the effects of various drugs on normal and cancerous cells. Gene toxicity is a very important factor in the safety of a substance because tumors can be caused by mutagenic substances [60]. Despite the Stevia extract’s IC50 in cancer cell line was higher than the cisplatin’s IC50, its value is considerably low because the lower IC50 the more power of sample in killing cell or inhibiting their growth [48]. Accordingly, we can suggest that the hydro-alcoholic extract of Stevia rebaudiana can be used as a complementary therapy in cancer.
Acknowledgement
This study was supported by a grant from the vice chancellor of research of Mazandaran University of Medical Sciences, Sari, Iran.
Declaration Of Interest
The authors declare no conflicts of interest.
REFERENCES
- Stewart BW, Kleihues P. World Cancer Report: IARC press Lyon. 2003.
- Tiwari AK. Imbalance in antioxidant defence and human diseases: Multiple approach of natural antioxidants therapy. Current science. 2001;1179-1187.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751-760.
- Ortiz Z, Shea B, Suarez-Almazor M, et al. The efficacy of folic acid and folinic acid in reducing methotrexate gastrointestinal toxicity in rheumatoid arthritis. A metaanalysis of randomized controlled trials. 1998.
- Keshava C, Keshava N, Whong W-Z, et al. Inhibition of methotrexate-induced chromosomal damage by folinic acid in V79 cells. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 1998;397:221-228.
- Choudhury RC, Palo AK. Modulatory effects of caffeine on methotrexate-induced cytogenotoxicity in mouse bone marrow. Environmental Toxicology and Pharmacology. 2004;15:79-85.
- Kasahara Y, Nakai Y, Miura D, et al. Mechanism of induction of micronuclei and chromosome aberrations in mouse bone marrow by multiple treatments of methotrexate. Mutation Research/Genetic Toxicology. 1992;280:117-128.
- Choudhury RC, Ghosh SK, Palo AK. Cytogenetic toxicity of methotrexate in mouse bone marrow. Environmental Toxicology and Pharmacology. 2000;8:191-196.
- Brumen V, Horvat D. Work environment influence on cytostatics‐induced genotoxicity in oncologic nurses. American Journal of Industrial Medicine. 1996;30:67-71.
- Ramos-Remus C, Dorazco-Barragan G, Aceves-Avila F, et al. Genotoxicity assessment using micronuclei assay in rheumatoid arthritis patients. Clinical and experimental rheumatology. 2002;20:208-212.
- Behrens BC, Hamilton TC, Masuda H, et al. Characterization of a cis-diammine dichloroplatinum (II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer research. 1987;47:414-418.
- Bedford P, Walker MC, Sharma HL, et al. Factors influencing the sensitivity of two human bladder carcinoma cell lines to cis-diamminedichloro-platinum (II). Chemico-Biological Interactions. 1987;61:1-15.
- Pratheeshkumar P, Kuttan G. Ameliorative action of Vernonia cinerea L. on cyclophosphamide-induced immunosuppression and oxidative stress in mice. Inflammopharmacology. 2010;18:197-207.
- De Stefani E, Deneo‐Pellegrini H, Boffetta P, et al. Dietary patterns and risk of cancer: a factor analysis in Uruguay. International journal of cancer. 2009;124:1391-1397.
- Lin J, Kamat A, Gu J, et al. Dietary intake of vegetables and fruits and the modification effects of GSTM1 and NAT2 genotypes on bladder cancer risk. Cancer Epidemiology and Prevention Biomarkers. 2009;18:2090-2097.
- Kim MK, Kim K, Han JY, et al. Modulation of inflammatory signaling pathways by phytochemicals in ovarian cancer. Genes & nutrition. 2011;6:109-115.
- Pan MH, Lai CS, Wu JC, et al. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Molecular Nutrition & Food Research. 2011;55:32-45.
- Zhai DD, Zhong JJ. Simultaneous analysis of three bioactive compounds in Artemisia annua hairy root cultures by reversed‐phase high‐performance liquid chromatography–diode array detector. Phytochemical Analysis. 2010;21:524-530.
- Goenadi D. Water tension and fertilization of Stevia rebaudiana Bertoni on Oxic Tropudalf (English abstr.). Menara Perkebunan. 1983;51:85-90.
- Geuns JM. Safety of Stevia and stevioside. Recent Research Developments in Phytochemistry. 2000;4:75-88.
- Cardello H, Da Silva M, Damasio M. Measurement of the relative sweetness of Stevia extract, aspartame and cyclamate/saccharin blend as compared to sucrose at different concentrations. Plant Foods for Human Nutrition (Formerly Qualitas Plantarum). 1999;54:119-129.
- Kinghorn AD, Soejarto D, Nanayakkara N, et al. A phytochemical screening procedure for sweet ent-kaurene glycosides in the genus Stevia. Journal of Natural Products. 1984;47:439-444.
- Chan P, Tomlinson B, Chen YJ, et al. A double‐blind placebo‐controlled study of the effectiveness and tolerability of oral stevioside in human hypertension. British Journal of Clinical Pharmacology. 2000;50:215-220.
- Lee C-N, Wong K-L, Liu J-C, et al. Inhibitory effect of stevioside on calcium influx to produce antihypertension. Planta Medica. 2001;67:796-799.
- Melis M. Influence of calcium on the blood pressure and renal effects of stevioside. Brazilian journal of medical and biological research= Revista brasileira de pesquisas medicas e biologicas. 1992;25:943-949.
- Suanarunsawat T, Chaiyabutr N. The effect of stevioside on glucose metabolism in rat. Canadian Journal of Physiology and pharmacology. 1997;75:976-982.
- Debnath M. Clonal propagation and antimicrobial activity of an endemic medicinal plant Stevia rebaudiana. Journal of Medicinal Plants Research. 2007;2:45-51.
- Tadhani MB, Subhash R. In vitro antimicrobial activity of Stevia rebaudiana Bertoni leaves. Tropical Journal of Pharmaceutical Research. 2006;5:557-560.
- Tomita T, Sato N, Arai T, et al. Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coli O157: H7 and other food-borne pathogenic bacteria. Microbiology and Immunology. 1997;41:1005-1009.
- Melis M. Chronic administration of aqueous extract of Stevia rebaudiana in rats: renal effects. Journal of Ethnopharmacology. 1995;47:129-134.
- Madan S, Ahmad S, Singh G, et al. Stevia rebaudiana (Bert.) Bertoni-A review. 2010.
- Ismail M, Bagalkotkar G, Iqbal S, et al. Anticancer properties and phenolic contents of sequentially prepared extracts from different parts of selected medicinal plants indigenous to Malaysia. Molecules. 2012;17:5745-5756.
- Matsui M, Matsui K, Kawasaki Y, et al. Evaluation of the genotoxicity of stevioside and steviol using six in vitro and one in vivo mutagenicity assays. Mutagenesis. 1996;11:573-579.
- Salimi M, Majd A, Sepahdar Z, et al. Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharmaceutical biology. 2012;50:1416-1422.
- Kumar A, Partap S, Sharma NK. Phytochemical, ethnobotanical and pharmacological profile of Lagenaria siceraria:- A review. Journal of Pharmacognosy and Phytochemistry. 2012;1.
- Ghosh K, Chandra K, Ojha AK, et al. Structural identification and cytotoxic activity of a polysaccharide from the fruits of Lagenaria siceraria (Lau). Carbohydrate research. 2009;344:693-698.
- Sankari M, Chitra V, Jubilee R, et al. Immunosuppressive activity of aqueous extract of Lagenaria siceraria (Standley) in mice. Der Pharmacia Lettre. 2010;2:291-296.
- Saravi SS, Shokrzadeh M, Shirazi FH. Cytotoxicity of Sambucus ebulus on cancer cell lines and protective effects of vitamins C and E against its cytotoxicity on normal cell lines. African Journal of Biotechnology. 2013;12.
- Fenech M, Morley AA. Measurement of micronuclei in lymphocytes. Mutation Research/Environmental Mutagenesis and Related Subjects. 1985;147:29-36.
- Abdel-Hameed E-SS, Bazaid SA, Hagag HA. Chemical characterization of Rosa damascena Miller var. trigintipetala Dieck essential oil and it’s in vitro genotoxic and cytotoxic properties. Journal of Essential Oil Research. 2016;28:121-129.
- Shokrzade Lamoki M, Pakravan N, Sheikholeslamian S. The protective effect of N-acetyl cysteine on glutathione levels and serum cholinesterase in acute poisoning of diazinon, in mice. Journal of Mazandaran University of Medical Sciences. 2013;22:2-11.
- Burt S. Essential oils: Their antibacterial properties and potential applications in foods—a review. International journal of food microbiology. 2004;94:223-253.
- Marimuthu P. Projection of cancer incidence in five cities and cancer mortality in India. Indian Journal of Cancer. 2008;45:4.
- Salimi M, Ardestaniyan M, Mostafapour Kandelous H, et al. Anti‐proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves. Cell Proliferation. 2014;47:172-179.
- Mongelli E, Pampuro S, Coussio J, et al. Cytotoxic and DNA interaction activities of extracts from medicinal plants used in Argentina. Journal of Ethnopharmacology. 2000;71:145-151.
- Deshpande J, Choudhari A, Mishra M, et al. Beneficial effects of Lagenaria siceraria (Mol.) Standley fruit epicarp in animal models. 2008.
- Clerici WJ, Hensley K, DiMartino DL, et al. Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hearing Research. 1996;98:116-124.
- Hussain AI. Characterization and biological activities of essential oils of some species of Lamiaceae. PhD Th esis Faculty of Sciences, University of Agroculture, Faisalbad, Pakistan. 2009.
- Melis MS. Renal excretion of stevioside in rats. Journal of Natural Products. 1992;55(5):688-690.
- Geuns JM. Stevioside. Phytochemistry. 2003;64:913-921.
- D'Agostino M, De Simone F, Pizza C, et al. Sterols in Stevia rebaudiana Bertoni. Bollettino della Societa italiana di biologia sperimentale. 1984;60:2237-2240.
- Kim KK, Sawa Y, Shibata H. Hydroxylation ofent-Kaurenoic Acid to Steviol in stevia rebaudiana Bertoni—Purification and Partial Characterization of the Enzyme. Archives of biochemistry and biophysics. 1996;332:223-230.
- Tateo F, Fugazza M, Fäustle S, et al. Technical and toxicological problems connected with the formulation of low-energy foods. 2. Mutagenic and fertility-modifying activity of extracts and constituents of Stevia rebaudiana Bertoni. Revista della Societa Italiana di Scienza dell'Alimentazione. 1990;19:13-22.
- Kaushik R, Narayanan P, Vasudevan V, et al. Nutrient composition of cultivated Stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. Journal of Food Science and Technology. 2010;4:27-33.
- Akihisa T, Hamasaki Y, Tokuda H, et al. Microbial Transformation of Isosteviol and Inhibitory Effects on Epstein− Barr Virus Activation of the Transformation Products. Journal of Natural Products. 2004;67:407-410.
- Konoshima T, Takasaki M. Cancer-chemopreventive effects of natural sweeteners and related compounds. Pure and Applied Chemistry. 2002;74:1309-1316.
- Yasukawa K, Kitanaka S, Seo S. Inhibitory effect of stevioside on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Biological and Pharmaceutical Bulletin. 2002;25:1488-1490.
- Takasaki M, Konoshima T, Kozuka M, et al. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorganic & Medicinal Chemistry. 2009;17:600-605.
- Mizushina Y, Akihisa T, Ukiya M, et al. Structural analysis of isosteviol and related compounds as DNA polymerase and DNA topoisomerase inhibitors. Life sciences. 2005;77:2127-2140.
- Nakamura Y, Sakiyama S, Takenaga K. Suppression of syntheses of high molecular weight nonmuscle tropomyosins in macrophages. Cytoskeleton. 1995;31:273-282.
- Takahashi K, Sun Y, Yanagiuchi I, et al. Stevioside enhances apoptosis induced by serum deprivation in PC12 cells. Toxicology mechanisms and methods. 2012;22:243-249.
- Sharma N, Kaushal N, Chawla A, et al. Stevia rebaudiana-A review. Agrobios Newsletter. 2006;5:46-48.