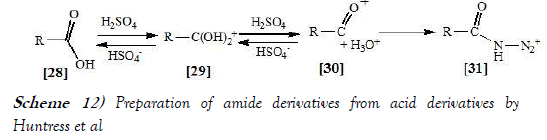Synthesis and biological importance of amide analogues
Received: 26-Mar-2018 Accepted Date: Apr 12, 2018; Published: 26-Mar-2018
Citation: Rajput P, Sharma A. Synthesis and biological importance of amide analogues. J Pharmacol Med Chem 2018;2(1):22-31.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The present research article deals with the amide analogues prepared by available very well-known name reactions. The author have studied the name reactions like Beckmann rearrangement, Schmidt reaction, Passerine reaction, Willgerodt–Kindler reaction and UGI reaction, which involves preparation of amide linage containing compounds. The main purpose of article is to provide information on the development of novel amide derivatives to the scientific community. Doing so, it focuses on mechanisms of action and adverse events, and suggests measures to be implemented in the clinical practice according to bioethical principles.
Keywords
Novel amide derivatives; Amide analogues; Amide formation
The Amide bond formation reactions are among the most important transformations in organic chemistry and biochemistry because of the widespread occurrence of amides in pharmaceuticals, natural products and biologically active compounds. The amide group is widely present in the drugs, intermediates, pharmaceuticals, and natural products. It is also available in large number of industrial materials including polymers, detergents and lubricants [1]. The common method for the preparation of amides involve the reaction of activated carboxylic acid derivatives, such as chlorides, anhydrides or esters, with amines or, alternatively, the direct union of the carboxylic acids with amines assisted by stoichiometric amounts of coupling reagents, such as carbodiimides or 1H-benzotriazole derivatives [1,2]. However, these classical approaches are low in atom efficiency and generate large amounts of waste products, making their environmental profile unfavorable. Therefore, ACS Green Chemistry Institute and members of leading pharmaceutical corporations worldwide, identified ‘‘amide formation avoiding poor atom economy reagents’’ as one of the top challenges in organic chemistry [3]. New efficient and sustainable synthetic routes to access this important class of compounds are therefore needed [4].
In the search of more atom-economical and cost-effective protocols, metal-catalyzed transformations have emerged in the last few years as attractive alternatives, offering the possibility to develop previously unavailable routes starting from substrates other than carboxylic acids and their derivatives [5]. Thus, with the help of transition metals, a plethora of functional groups, such as nitriles, aldehydes, ketones, oximes, primary alcohols or amines, can be now conveniently employed as starting materials for the construction of the amide bond. There are three types of amides available in Chemistry: (i) an organic amide which is also referred as carboxamide, (ii) a sulfonamide, and (iii) a phosphoramide. Amides are usually regarded as derivatives of carboxylic acids in which the hydroxyl group has been replaced by an amine or ammonia.
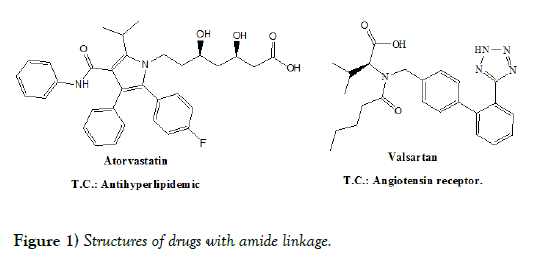
There are many drugs available in the market which contains amide linkage in the nucleus and possesses various therapeutic activities. Few drugs with their structures (Figure 1) are mentioned below:
Literature Review
Beckmann rearrangement
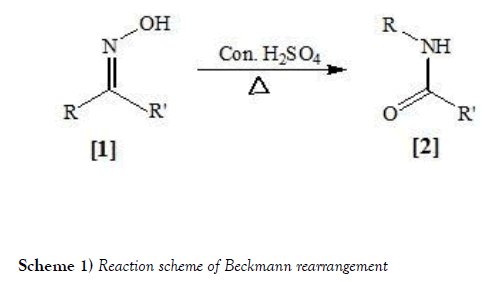
The Beckmann rearrangement developed by German chemist Ernst Otto Beckmann since 1853–1923. It is an acid-catalyzed rearrangement of an oxime to an amide [6-8]. Cyclic oximes yield lactams. Since the discovery of this Beckmann rearrangement (BR) in 1896, successive investigations have been largely carried out and applied in many ways. It is a skeletal rearrangement, which accomplishes both, the cleavage of C-C bond and formation of C-N bond. It has become a very useful andefficient method for incorporation of nitrogen atom in both cyclic andacyclic systems and also for the synthesis of various alkaloids. Severalcatalysts have been used for this rearrangement like zeolites, metalcatalysts, FeCl3/AgSbF6 etc [9-12]. Though BR is a very well-knownand old reaction, rearrange cyclic oximes fused with heterocyclic ringsystems. The reaction scheme of Beckmann rearrangement ismentioned below as Scheme-1:
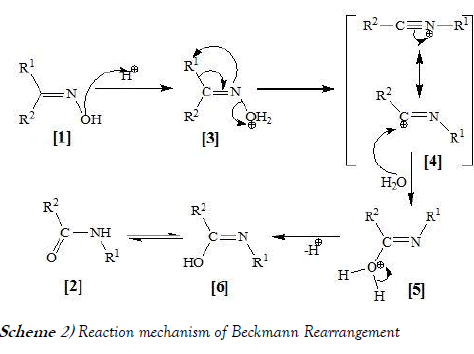
Beckmann rearrangement reaction includes preparation of amide based compound from oxime derivatives using acid catalyst i.e., Con. H2SO4. The same reaction mechanism of Beckmann rearrangement is mentioned below as Scheme-2:
The reaction mechanism of the Beckmann rearrangement is in general believed to consist of an alkyl migration with expulsion of the hydroxyl group to form a nitrilium ion followed by hydrolysis.
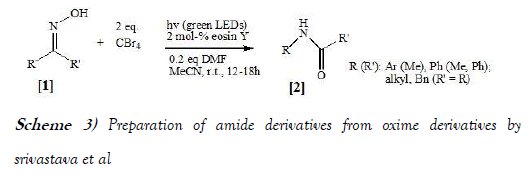
Srivastava et al. [13] have synthesized amide compounds from aldoxide through performing an efficient Beckmann rearrangement at room temperature which involves an eosin Y catalyzed, visible-light-mediated in-situ formation of the Vilsmeier-Haack reagent from CBr4 and a catalytic amount of DMF. This operationally simple method for the activation of ketoximes avoids the need for any corrosive, water-sensitive reagents and elevated temperatures. The reaction scheme of Beckmann Rearrangement performed by Srivastava et al. is mentioned below as Scheme-3:
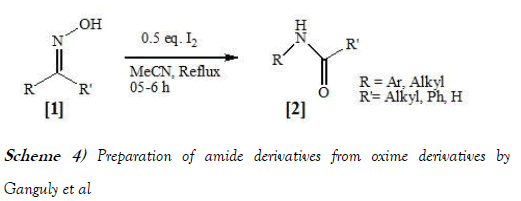
Ganguly et al. [14] have synthesized amides from ketoximes using Mild Neutral Conditions through performing an efficient Iodine-Mediated Beckmann Rearrangement. The reaction scheme of Beckmann Rearrangement performed by Ganguli et al. is mentioned below as Scheme-4:
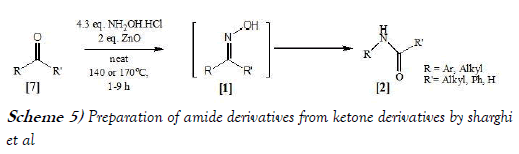
Sharghi et al. [15] have synthesized amides from Ketones and Aldehydes using Zinc Oxide via performing Solvent-Free and One-Step Beckmann rearrangement. The reaction scheme of Beckmann Rearrangement performed by Sharghi et al. is mentioned below as Scheme-5:
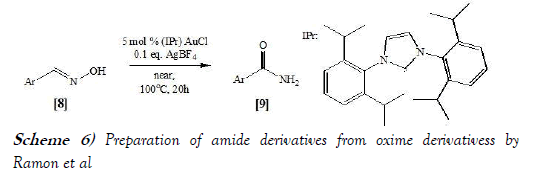
Ramon et al. [16] have synthesized Amides from Aldoximes using Au/Ag-Cocatalyzed through performing Beckmann-Rearrangement under Solvent- and Acid-Free Conditions. The reaction scheme of Beckmann Rearrangement performed by Ramon et al. is mentioned below as Scheme-6:
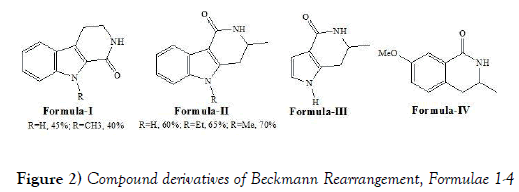
Kusurkar et al. [17] have synthesized derivatives of biologically active scaffolds by performing Beckmann rearrangement successfully. The synthesis of two β-carbolinone, three new γ-carbolinone, new pyrrolopyridinone and tetrahydroisoquinolinone derivatives were achieved in good to moderate yields. Kusurkar et al. have synthesized following compounds i.e., Compound of formulae (Figure 2) I-IV by performing Beckmann Rearrangement:
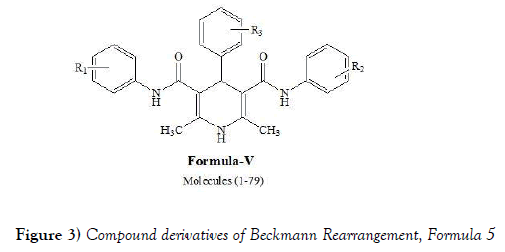
Shah et al. [18] have synthesized 97 various symmetrical, unsymmetrical, and N-substituted 1,4-dihydropyridines. The synthesized molecules were tested for their activity against M. tuberculosis H37Rv strain with rifampin as the standard drug. The percentage inhibition was found in the range 3–93%. In an effort to understand the relationship between structure and activity, 3D-QSAR studies were also carried out on a subset that is representative of the molecules synthesized. For the generation of the QSAR models, a training set of 35 diverse molecules representing the synthesized molecules was utilized. The molecules were aligned using the atom-fit technique like CoMFA and CoMSIA models. The QSAR models revealed the importance of the conformational flexibility of the substituents in antitubercular activity. Shah et al. have synthesized following compounds generically mentioned as compound of formula-V (Figure 3) by performing Beckmann Rearrangement:
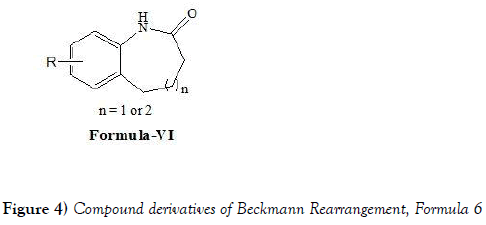
Spring et al. [19] have developed a convenient, efficient, and general method for the synthesis of benzo-fused seven- and eight-membered ring lactams by the Beckmann rearrangement of cyclic oximes. The process is technically simple, proceeds under relatively mild conditions with complete regioselectivity, and forms biologically interesting products that are generally difficult to synthesize by other methods. The biological activities of the synthesized compounds were also evaluated. In addition, several structurally novel N-arylated derivatives were prepared, demonstrating the capability to carry out structural elaboration around the lactam core. Overall, the work reported by Spring et al. represents a convenient approach to benzo-fused eight-membered ring lactams, a biologically interesting chemotype that is underrepresented in current small-molecule collections. The enrichment of screening libraries with compounds of this sort will allow the sampling of previously untapped regions of chemical space, which may thus facilitate the discovery of novel biologically active small-molecule agents. As such, author anticipated that this methodology will prove especially valuable in the diversity-oriented synthesis of structurally diverse small-molecule collections. Spring et al. have synthesized following compounds as compound of formula-VI (Figure 4) by performing Beckmann Rearrangement:
Schmidt Reaction
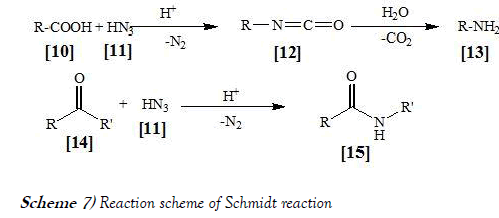
The Schmidt reaction is an organic reaction in which an azide reacts with a carbonyl group to give an amine or amide, with expulsion of nitrogen. It is named after Karl Friedrich Schmidt (1887–1971), who first reported it in 1924 by successfully converting benzophnone and hydrazoic acid to benzanilide (20). Surprisingly, the intramolecular reaction was not reported until 1991 but has become important in the synthesis of natural product. The reaction scheme of Schmidt reaction is mentioned below as Scheme-7:
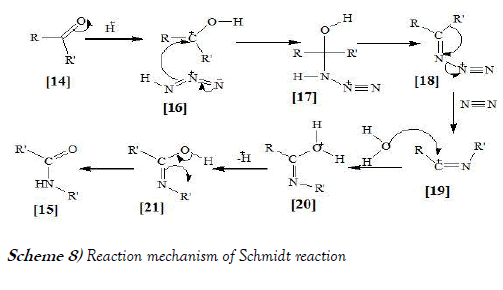
In the reaction mechanism for the ketone Schmidt reaction, the carbonyl group is activated by protonation for nucleophilic addition by the azide, forming intermediate compound, which loses water in an elimination reaction to temporary imine, over which one of the alkyl groups migrates from carbon to nitrogen with loss of nitrogen. Attack by water and proton loss converts intermediate compounds, which is a tautomer of the final amide [21]. The said mechanism is mentioned below as Scheme-8:
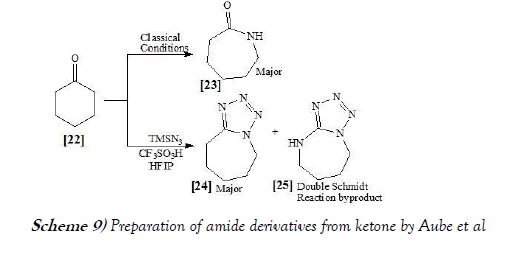
Aube et al. [22] have performed Schmidt reaction via carrying out two variations in which ketone electrophiles in hexafluoroisopropanol (HFIP) solvent has been studied. When TMSN3 is reacted with ketones in the presence of triflic acid (TfOH) promoter, tetrazoles are obtained as the major products. This observation is in contrast to established methods, which usually lead to amides or lactams arising from formal NH insertion as the major products. The full product profiles of several examples of this reaction are also reported and found to include mechanistically interesting products (e.g., double ring expansion). Application of TfOH promoter in HFIP was also found to promote the reaction of a hydroxyalkyl azide with a ketone, which affords lactams following nucleophilic opening of initially formed iminium ether more efficiently than previously reported methods. The reaction scheme of Schmidt reaction performed by Aube et al. is mentioned below as Scheme-9:
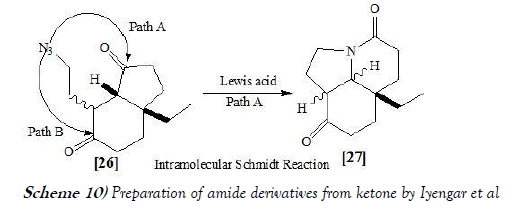
Iyengar et al. [23] have disclosed a total synthesis of (+)-aspidospermidine, which featuring an intramolecular Schmidt reaction as the key step. The effects of stereochemistry and protecting group status on the regio- and chemoselectivity of this reaction were examined. Further motivation was provided by the opportunity to examine a challenging intramolecular Schmidt reaction, in which selectivity for insertion into only one of two regioisomeric ketones is required for the preparation of a key tricyclic lactam. The reaction scheme of Schmidt reaction performed by Iyengar et al. is mentioned below as Scheme-10:
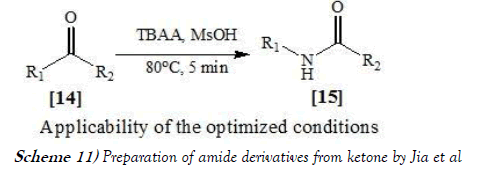
Jia et al. [24] have discloses the Schmidt reaction, which includes the treatment with hydroazoic acid in the presence of a strong acid, converts ketones to amides directly and has been broadly applied in organic synthesis. In this communication, the Schmidt reaction of ketones was carried out in DME solution in a continuous-flow micro reactor and gave the amide products. The enclosed small-volume feature of the micro reactor made this reaction safe, fast, and cost effective. The reaction scheme of Schmidt reaction performed by Jia et al. is mentioned below as Scheme-11:
Huntress et al. [25] have discloses Schmidt reaction which include preparation of amide from primay, seconday and tertiary acid. The organic acid is shown reacting with hydroazoic acid only in the form of a positive ion, since changes in the system which result in decreased ionization of the organic acid, also result in incomplete reaction. In reaction which yield both carbon monoxide and carbon dioxide, complex ionization of the organic acids is assumed, the dihydroxy-carbonium ions [29]reacting to form carbon dioxide and primary amines or cleavageproducts, the oxocarbonium ions [30] reacting to form carbon monoxideand cleavage products (but no primary amine). This interpretation ischosen to explain the following observations: as reaction conditions arechanged to decrease the yield of carbon monoxide, the yield of primaryamine is increased. The proportion of carbon monoxide formed isreduced by factors (dilution and accumulation of reaction products)which would reverse the mobile equilibrium shown. The proportion ofprimary amine to cleavage products was not observed to vary on dilutionof the reaction medium, if no carbon monoxide was evolved. Thealternative assumption is that only one ion [29 or 30] can react in threedifferent ways, only one of which is sensitive to dilution, appears a lesssatisfactory explanation.
Ion [30] is shown decomposing to form carbon monoxide and a carbonium ion only after reaction with hydroazoic acid and not directly as does triphenylacetic acid since no gas evolution occurred with these acids until sodium azide was added to the reaction mixture. The assumption that in complex [31] electron-withdrawal from the alkyl group proceeds in preference to the cleavage of the nitrogen-nitrogen bond does not appear unlikely in view of the tendency for Carbonium ion formation when carbon dioxide is evolved. The reaction scheme of Schmidt reaction performed by Huntress et al. is mentioned below as Scheme-12:
Willgerodt–Kindler reaction
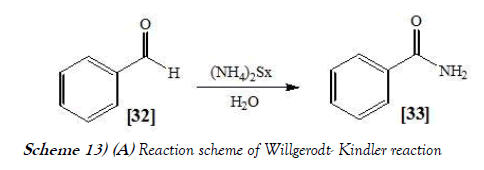
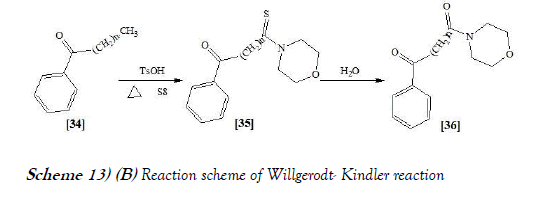
The Willgerodt rearrangement or Willgerodt reaction is an organic reaction which converting an aryl alkyl ketone to the corresponding amide by reaction with ammonium polysulfide, named after Conrad Willgerodt. The related Willgerodt-Kinder reaction takes place with elemental sulfur and an amine like morpholine. The reaction is name after Karl Kindler. The Willsgerodt Reaction allows the synthesis of amides from aryl ketones under the influence of a secondary amine and a thiating agent. The reaction mainly relates to the preparation of thioamides, which can be converted into carboxamide by treatment with water (26). The reaction scheme of Willgerodt–Kindler reaction is mentioned below as Scheme-13a & 13b:
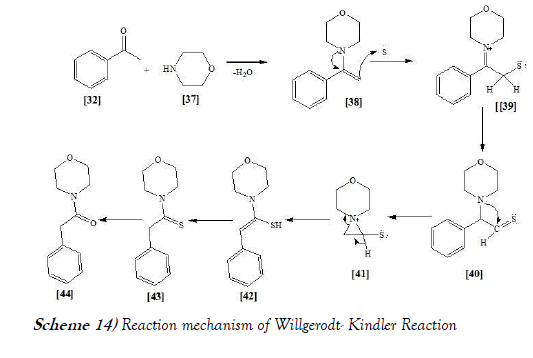
The reaction mechanism of Willgerodt-Kindler reaction is mentioned below as Scheme-14:
The reaction mechanism of Willgerodt-Kindler reaction involves the formation of an enamine which undergoes thiation, and the carbonyl group migrates to the end of the chain via a cascade of thio-substituted iminium-aziridinium rearrangements [27].
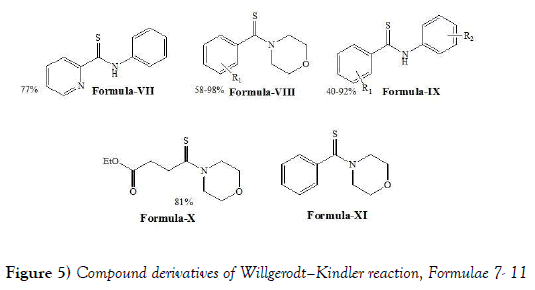
Bolm et al. [28] have discloses that Willgerodt-Kindler reaction is an efficient method for the preparation of (thio) amides from a range of substrates and additionally allows the terminal functionalisation of arylalkyl ketones. Probably due to concerns regarding both the yield and the complex reaction mixtures the Willgerodt–Kindler reaction has, to date, been relatively under utilised in organic synthesis. Bolm et al. have mainly discloses the use of microwave techniques for the preparation of thio-amide using Willgerodt—Kindler reaction and proved that microwave techniques is application in organic syntheses and be no longer plagued by the poor reputation it has held in the past. Moreover, thioamides have been employed as valuable intermediates in the preparation of S- and N-heterocycles. To this end, the Willgerodt–Kindler reaction has also shown potential for the preparation of numerous sulfur-containing heterocycles, but this is yet to be fully exploited. Investigations into the preparation of many new sulfur- and/or nitrogen containing heterocyclic compounds under Willgerodt–Kindler reaction conditions are warranted. Although much progress has been made in the development of the Willgerodt–Kindler reaction there is still much work to be done. To allow this reaction process to find wider utility in organic synthesis, the challenge now stands for synthetic chemists to identify conditions that allow the selective functionalisation of certain moieties under Willgerodt–Kindler conditions. In addition, the further development of this reaction process to allow the efficient preparation of thioamides from arylalkyl ketones with much longer alkyl chains would be of significant synthetic utility and as such warrants investigation. The Bolm et al. have synthesized following compounds i.e., compound of formulae VII-XI (Figure 5) by performing Willgerodt–Kindler reaction:
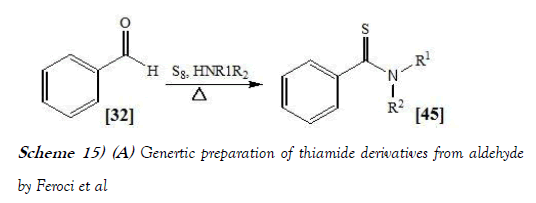
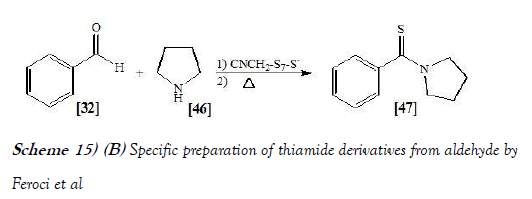
Feroci et al. [29] have discloses the synthesis of thiobenzamides from benzaldehydes, elemental sulfur and cyclic secondary amines was carried out by Willgerodt-Kindler reaction following three different protocols, avoiding the harsh conditions usually reported in the literature. Protocol A was solventless, protocol B was in acetonitrile, while protocol C was electrochemical in acetonitrile (using electrogenerated acetonitrile anion).
For all protocols the temperature was 6°C to 80°C. In all cases, a stoichiometric amount of reagents was used, with waste lessening with respect to literature syntheses. Good to high yields were obtained by chemical or electrochemical methods, neat or in acetonitrile. Advantages and limits of these sustainable syntheses are highlighted. The reaction scheme of Willgerodt–Kindler reaction performed by Feroci et al. is mentioned below generically as Scheme-15a & specifically as Scheme-15b:
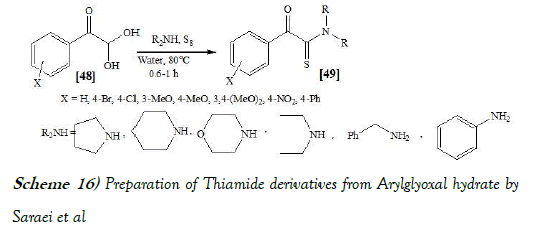
Saraei et al. [30] have discloses a simple and efficient method for the synthesis of α-ketothioamides via the Willgerodt–Kindler reaction is developed. Reactions were carried out between arylglyoxal hydrates, amines and elemental sulfur in water at 80◦C to afford corresponding α-ketothioamides in good to high yields in a short reaction time. The reaction scheme of Willgerodt–Kindler reaction performed by Saraei et al. is mentioned below as Scheme-16:
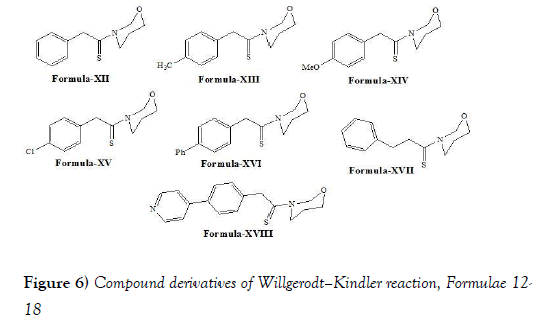
Moghaddam et al. [31] have discloses that Aldehydes and aryl alkyl ketones were efficiently transformed to thioamides with the same number of carbon atoms via Willgerodt-Kindler reaction under microwave irradiation in solvent-free conditions and hydrolysis of same thiomides into carboxylic acids. Moghaddam et al. have synthesized following compounds i.e., compound of formulae XII-XVIII (Figure 6) via performing Willgerodt-Kindler reaction:
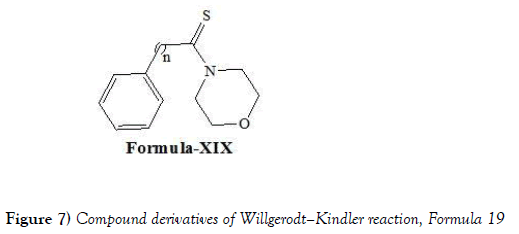
Darabi et al. [32] have discloses the use of Willgerodt-Kindler reaction for reaction of several aryl alkyl ketones with sulfur and morpholine under solvent-free conditions was performed in a domestic microwave oven. Good results including good yield and purity were obtained in a very short reaction time (between 3.5- minutes). Darabi et al. have synthesized following compounds i.e., compound of formula XIX (Figure 7) via performing Willgerodt-Kindler reaction:
Passerini Reaction
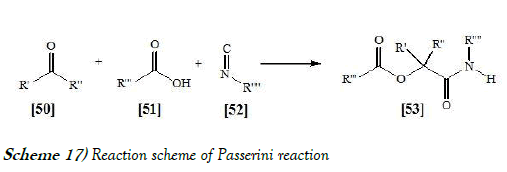
This three-component reaction between a carboxylic acid, a carbonyl compound such as a ketone or aldehyde, and an isocyanide, offers direct access to α-hydroxy carboxamides [33]. The reaction scheme of Passerini Reaction is mentioned below as Scheme-17:
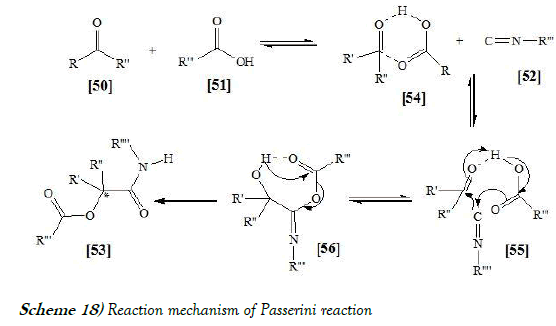
The Passerini reaction proceeds rapidly if the reaction is performed in aprotic solvents at room temperature. High yields are obtained with high concentrations of the starting materials in the reaction mixture. From these findings, it is assumed that the Passerini Reaction does not follow an ionic pathway. Hydrogen bonding is believed to play a crucial role in the formation of the presumed cyclic transition state for this reaction [34]. The reaction mechanism of Passerini reaction is mentioned below as Scheme-18:
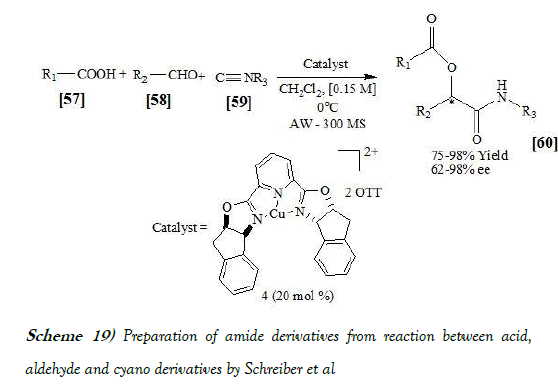
Schreiber et al. [35] have synthesized amide analogues by performing catalytic asymmetric Passerini reaction using tridentate indan (pybox) Cu(II) Lewis acid complex 4 with substrates capable of bidentate coordination. The reaction occurs via ligand-accelerated catalysis. Schreiber et al. have reacted different aliphatic and aromatic aldehydes, acids and isocyanides to form required amide analogues. In all cases, they were able to obtain the desired product in fair to excellent yields and enantioselectivities. The reaction scheme of Passerini reaction performed by Schreiber et al. is mentioned below as Scheme-19:
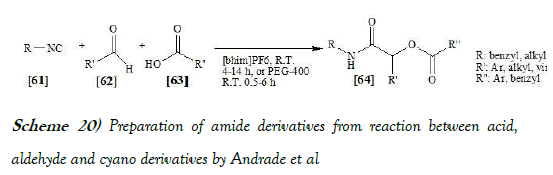
Andrade et al. [36] have α-Acyloxy carboxamides were easily assessed in one step by the multicomponent reaction between carboxylic acids, aldehydes and C-isocyanides (Passerini reaction) using ionic liquids or polyethyleneglycol (PEG 400) as green reaction media. The reaction scheme of Passerini reaction performed by Andrade et al. is mentioned below as Scheme-20:
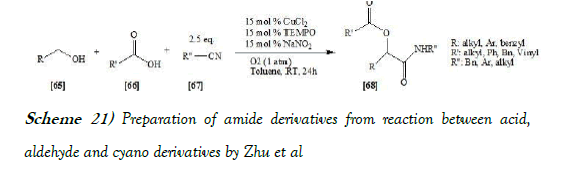
Zhu et al. [37] have discloses the alcohols instead of aldehydes were used in the Passerini three-component reaction under catalytic aerobic conditions. Mixing alcohols, isocyanides, and carboxylic acids in toluene in the presence of a catalytic amount of cupric chloride, NaNO2, and TEMPO afforded, under an oxygen atmosphere, the P-3CR adducts in good yields. The reaction scheme of Passerini reaction performed by Zhu et al. is mentioned below as Scheme-21:
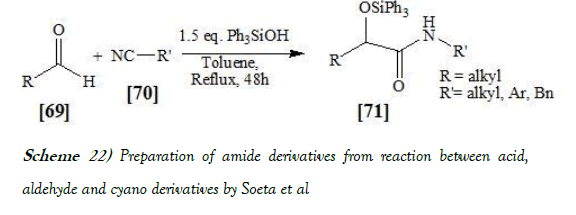
Soeta et al. [38] have synthesized a new method for a highly effective addition of isocyanides to aldehydes proceeded smoothly in the presence of a silanol to give the corresponding α-siloxyamides in high yields. A wide range of aldehydes and isocyanides are applicable in this reaction. The reaction scheme of Passerini reaction performed by Soeta et al. is mentioned below as Scheme-22:
It is to be noted that Soeta et al. have developed a direct O-silylative Passerini reaction consisting of aldehydes, isocyanides, and silanols. The said reaction is the first example of the isocyanide-based multicomponent reaction using a silanol instead of a carboxylic acid component, giving the corresponding R-siloxyamides in high yields. A wide range of aldehydes and isocyanides are applicable to this reaction.
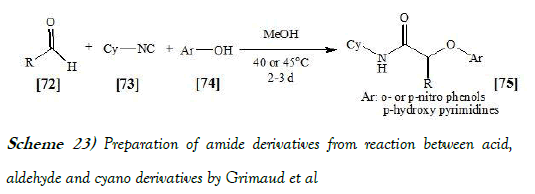
Grimaud et al. have [39] discloses a three-component addition of isocyanides to phenol derivatives and aldehydes in methanol forms O-arylated compounds in a new Passerini-type reaction. The key step is an irreversible Smiles rearrangement of intermediate phenoxyimidate adducts. The reaction scheme of Passerini reaction performed by Grimaud et al. is mentioned below as Scheme-23:
UGI reaction
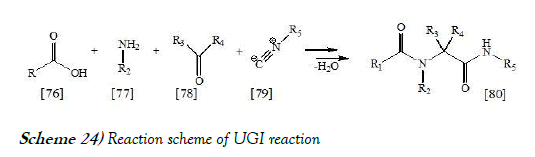
reactionThe UGI reaction is a multi-component reaction in organic chemistry involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide [40]. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959. The reaction scheme of UGI reaction is mentioned below as Scheme-24:
The Ugi reaction is exothermic and usually complete within minutes of adding the isocyanide. High concentration (0.5M - 2.0M) of reactants gives the highest yields. Polar, aprotic solvents, like DMF, work well. However, methanol and ethanol have also been used successfully. This uncatalyzed reaction has an inherent high atom economy as only a molecule of water is lost and chemical yield in general are high. Recent research has shown that the Ugi reaction is accelerated in water.
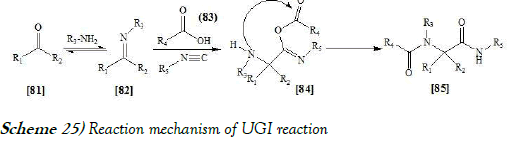
In the reaction mechanism of Ugi reaction, the initial reaction is the formation of an imine [82] from the amine and the ketone. Subsequent reaction of the imine with the isocyanide and the carboxylic acid gives intermediate [84], which rearranges via an acyl transfer into the bis-amide [85]. The exact mechanism of the trimolecular reaction to form intermediate [84] is not known [41]. The reaction mechanism of UGI reaction is mentioned below as Scheme-25:
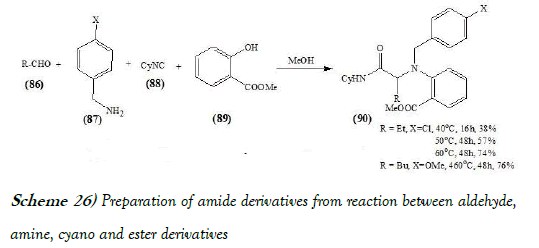
Grimaud et al. [40] have discloses a novel and very efficient four-component reaction from readily available substrates. The creation of four bonds that should be reasonably resistant to hydrolysis makes this process particularly attractive for the design of pharmaceutical and agrochemical libraries. Moreover, a straightforward elaboration of these adducts gives easy access to various heterocyclic structures. Further studies are under way to examine more thoroughly the scope and limitations of this MCR system. The reaction scheme of UGI reaction performed by Grimaud et al. is mentioned below as Scheme-26:
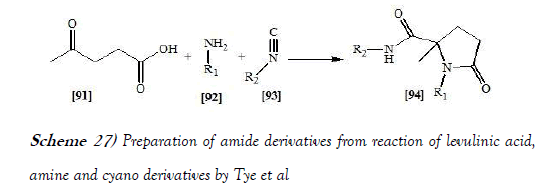
Tye et al. [42] have discloses a utility of a Design of Experiments (DoE) approach for the rapid and efficient optimisation of a microwave assisted UGI reaction of levulinic acid is demonstrated. DoE methods have also been applied to the assessment of the reaction scope for a range of amine and isonitrile substrates. The optimal procedure developed using this approach has enabled the preparation of lactam derivatives in moderate to excellent yields (17–90%) in a reaction time of only 30 min compared to the conventional methodology which required up to 48 h. The reaction scheme of UGI reaction performed by Tye et al. is mentioned below:
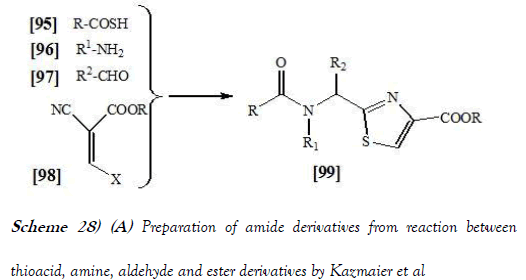
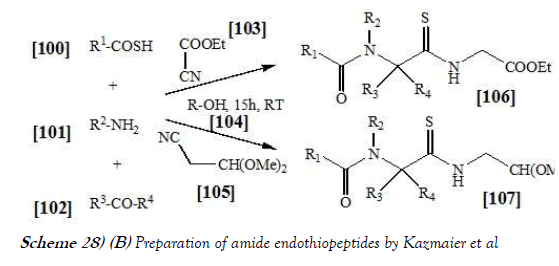
Kazmaier et al. [43] have discloses an Endothiopeptides can easily be obtained via Ugi reaction using thio acids as acid components. If isonitriles with an acetal group are applied, the endothiopeptides can directly be converted into thiazoles using TMSCl–NaI under microwave irradiation. In conclusion Kazmaier et al. have shown that the application of thio acids in Ugi reactions gives rise to endothiopeptides in one step with the option of combinatorial synthesis. If suitable isonitriles are used, these endothiopeptides can be converted into alpeptidic thiazoles also in one step under microwave irradiation. The reaction scheme of UGI reaction performed by Kazmaier et al. is mentioned below as Scheme-28a & 28b:
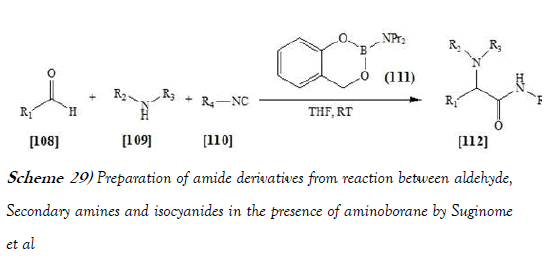
Suginome et al. [44] discloses a variety of secondary amines have become utilized in the Ugi reaction by using aminoborane as an iminium ion generator. Aldehydes, secondary amines, and isocyanides are coupled in the presence of aminoborane at room temperature, giving the corresponding r-amino amides in good yields. The nonacidic reaction conditions are beneficial for unique chemoselectivity, where the aldimine functionality is left intact in the present Ugi-type reaction. The reaction scheme of UGI reaction performed by Suginome et al. is mentioned below as Scheme-29:
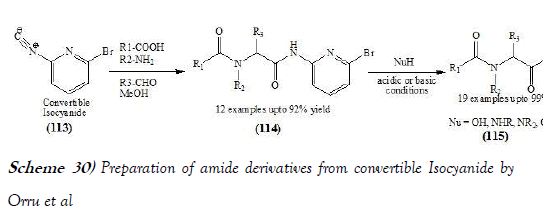
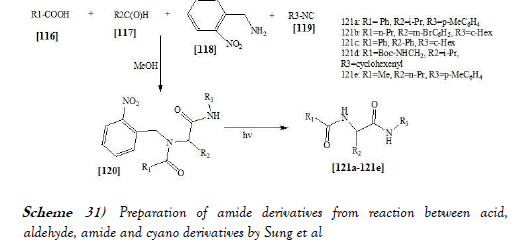
Orru et al. [45] have discloses a development of 2-isocyanopyridines as novel convertible isocyanides for multicomponent chemistry. Comparison of representatives of this class revealed 2-bromo-6-isocyanopyridine as the optimal reagent in terms of stability and synthetic efficiency. It combines sufficient nucleophilicity with good leaving group capacity of the resulting amide moiety under both basic and acidic conditions. To demonstrate the practical utility of this reagent, an efficient two-step synthesisof the potent opioid carfentanil is presented. The reaction scheme of UGI reaction performed by Orru et al. is mentioned below as Scheme-30: Sung et al. [46] have discloses a modified U-4CR reaction, which has been done by using commercially available 2-nitrobenzylamine as an ammonia equivalent and it involves a multi-component reaction, followed by photochemical cleavage of the 2-nitrobenzyl group, which can be done in one pot with good yields. Thereaction scheme of UGI reaction performed by Sung et al. is mentionedbelow as Scheme-31:
Discussion and Conclusion
The present review article includes an overview of well-known name reactions like Beckmann rearrangement, Schmidt reaction, Passerine reaction, Willgerodt–Kindler reaction and UGI reaction, which contains amide group formation in nucleus of synthesized organic compounds.
Number of organic and a natural product which contains peptide linkage are possessing interesting biological activities. For this reason, the development of new peptide linkage containing compounds by using name reactions has become a most fascinating field of research for many organic chemists with various backgrounds. It is believed that this review presents a systematic overview of use of well-known name reactions, which contains peptide linkage serves as an excellent guideline for the organic synthesis of bioactive molecules bearing peptide linkages.
Acknowledgements
The authors are thankful to Department of Chemistry, Noida International University, Noida (UP) for providing research and library facilities.
REFERENCES
- Greenberg A. The amide linkage: Structural significance in chemistry, biochemistry, and materials science. John Wiley & Sons; 2000.
- Carlsen L, Dopp D, Dopp H, et al. Houben-Weyl Methods in Organic Chemistry.
- Constable DJ, Dunn PJ, Hayler JD,et al. Key green chemistry research areas—a perspective from pharmaceutical manufacturers. Green Chem. 2007;9(5):411-20.
- Carey JS, Laffan D, Thomson C, et al. . Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 2006;4(12):2337-47.
- Roy S, Gribble GW. Metal-catalyzed amidation. Tetrahedron. 2012;68:9867-9923.
- Beckmann E. Isonitroso compounds. Ber. Dtsch. Chem Ges. 1886;19:988-93.
- Donaruma LG, Heldt WZ. The beckmann rearrangement. Org React 1960;11:2-36.
- Gawley RE. The Beckmann Reactions: Rearrangements, elimination–additions, fragmentations, and rearrangement–cyclizations. Org react. 1988.
- Opanasenko M, Shamzhy M, Lamač M, Čejka J. The effect of substrate size in the Beckmann rearrangement: MOFs vs. zeolites. Catal. Today. 2013;204:94-100.
- Thomas B, Sugunan S. Rare-earth (Ce3+, La3+, Sm3+, and Re3+) exchanged Na-Y zeolites and K-10 clay as solid acid catalysts for the synthesis of benzoxazole via Beckmann rearrangement of salicylaldoxime. Microporous Mesoporous Mater. 2006;96(1-3):55-64.
- Priya SV, Mabel JH, Palanichamy M, et al. Catalytic activity of MAPO-36 and ion-exchanged MAPO-36 in Beckmann rearrangement. Stud Surf Sci Catal 2008;Vol. 174, pp. 1147-1150).
- Jefferies LR, Weber SR, Cook SP. Iron-catalyzed C–N bond formation via the Beckmann rearrangement. Synlett. 2015;26(03):331-4.
- Srivastava VP, Yadav AK, Yadav LD. The Beckmann Rearrangement Executed by Visible-Light-Driven Generation of Vilsmeier–Haack Reagent. Synlett. 2014;25(05):665-70.
- Ganguly NC, Mondal P. Efficient iodine-mediated Beckmann rearrangement of ketoximes to amides under mild neutral conditions. Synthesis. 2010;2010(21):3705-9.
- Sharghi H, Hosseini M. Solvent-free and one-step Beckmann rearrangement of ketones and aldehydes by zinc oxide. Synthesis. 2002;2002(08):1057-60.
- Ramon RS, Bosson J, Díez-González S, et al. Au/Ag-cocatalyzed aldoximes to amides rearrangement under solvent-and acid-free conditions. J Org Chem. 2010;75(4):1197-2022.
- Naik PN, Alkobati NA, Kusurkar RS. Beckmann rearrangement for the synthesis of derivatives of β-and γ-carbolinones, dihydropyrrolopyridinone and tetrahydroisoquinolinone. ARKIVOC: c. 2015;2015.
- Manvar AT, Pissurlenkar RR, Virsodia VR, et al. Synthesis, in vitro antitubercular activity and 3D-QSAR study of 1, 4-dihydropyridines. Mol Divers. 2010;14(2):285-305.
- Kenwright JL, Galloway WR, Wortmann L, et al. Mild and efficient synthesis of benzo-fused seven-and eight-membered ring lactams: A convenient approach to biologically interesting chemotypes. Synth Commun. 2013;43(11):1508-16.
- Aube J, Milligan GL. Intramolecular Schmidt reaction of alkyl azides. J Am Chem Soc. 1991;113(23):8965-6.
- Wolff H. The Schmidt Reaction. Organic Reactions. 1946:307–336.
- Motiwala HF, Charaschanya M, Day VW, et al. Remodeling and enhancing Schmidt reaction pathways in hexafluoroisopropanol. J Org Chem. 2016;81(4):1593-609.
- Iyengar R, Schildknegt K, Aubé J. Regiocontrol in an intramolecular Schmidt reaction: total synthesis of (+)-aspidospermidine. Organic letters. 2000;2(11):1625-7.
- Chen Y, Liu B, Liu X, et al. Schmidt reaction of ketones in DME solution in a continuous-flow microreactor. Org Process Res. Dev. 2014;18(11):1589-92.
- Schuerch C, Huntress EH. The Schmidt reaction. I. Conditions and reaction mechanism with primary, secondary and tertiary aliphatic acids. J Am Chem Soc. 1949;71(6):2233-7.
- Priebbenow DL, Bolm C. Recent advances in the Willgerodt–Kindler reaction. Chem Soc Rev. 2013;42(19):7870-80.
- Carmack M. The willgerodt-Kindler reactions. 7. The mechanisms. J Heterocycl Chem. 1989;26(5):1319-23.
- Priebbenow DL, Bolm C. Recent advances in the Willgerodt–Kindler reaction. Chem Soc Rev. 2013;42(19):7870-80.
- Papa M, Chiarotto I, Feroci M. Willgerodt‐Kindler reaction of benzaldehydes: A comparative study for a sustainable synthesis of secondary thiobenzamides. Chemistry Select. 2017;2(10):3207-10.
- Eftekhari-Sis B, Vahdati-Khajeh S, Amini SM, et al. Willgerodt–Kindler reaction of arylglyoxals with amines and sulfur in aqueous media: A simple and efficient synthesis of α-ketothioamides. J. Sulfur Chem.2013;34(5):464-73.
- Moghaddam FM, Ghaffarzadeh M. Microwave-assisted rapid hydrolysis and preparation of thioamides by Willgerodt-Kindler reaction. Synth Commun.200;31(2):317-21.
- Nooshabadi M, Aghapoor K, Darabi HR, et al. The rapid synthesis of thiomorpholides by Willgerodt-Kindler reaction under microwave heating. Tetrahedron Lett. 1999;40(42):7549-52.
- Reza Kazemizadeh A, Ramazani A. Synthetic applications of Passerini reaction. Curr Org Chem. 2012;16(4):418-50 .
- Ramozzi R, Morokuma K. Revisiting the Passerini reaction mechanism: existence of the nitrilium, organocatalysis of its formation, and solvent effect. J Org Chem. 2015;80(11):5652-7.
- Andreana PR, Liu CC, Schreiber SL. Stereochemical control of the Passerini reaction. Org Lett. 2004;6(23):4231-3.
- Andrade CK, Takada SC, Suarez PA, et al. Revisiting the Passerini reaction under eco-friendly reaction conditions. Synlett. 2006; 2006(10):1539-42.
- Brioche J, Masson G, Zhu J. Passerini three-component reaction of alcohols under catalytic aerobic oxidative conditions. Org Lett. 2010;12(7):1432-5.
- Soeta T, Kojima Y, Ukaji Y, et al. O-Silylative Passerini reaction: A new one-pot synthesis of α-siloxyamides. Org. Lett. 2010;12(19):4341-3.
- El Kaim L, Gizolme M, Grimaud L. O-Arylative passerini reactions. Org lett. 2006;8(22):5021-3.
- El Kaïm L, Grimaud L, Oble J. Phenol Ugi–Smiles Systems: Strategies for the Multicomponent N‐Arylation of Primary Amines with Isocyanides, Aldehydes, and Phenols. Angewandte Chemie. 2005;117(48):8175-8.
- Iacobucci C, Reale S, Gal JF, De Angelis F. Insight into the Mechanisms of the Multicomponent Ugi and Ugi–Smiles Reactions by ESI‐MS (/MS). J Org Chem. 2014;2014(32):7087-90.
- Tye H, Whittaker M. Use of a design of experiments approach for the optimisation of a microwave assisted Ugi reaction. Org. Biomol. Chem. 2004;2(6):813-5.
- Kazmaier U, Ackermann S. A straightforward approach towards thiazoles and endothiopeptides via Ugi reaction. Org. Biomol Chem. 2005;3(17):3184-7.
- Tanaka Y, Hasui T, Suginome M. Acid-free, aminoborane-mediated Ugi-type reaction leading to general utilization of secondary amines. Org lett. 2007;9(22):4407-10.
- Orru VA, Ruijter E, Eelco J. 2-Bromo-6-isocyanopyridine as a Universal Convertible Isocyanide for Multicomponent Chemistry. Org Lett. 2016;18(5);984-987.
- Sung K, Chen FL, Huang PC. A modified U-4CR reaction with 2-nitrobenzylamine as an ammonia equivalent. Synlett. 2006;2006(16):2667-9.