Tadalafil: A new oral therapy for erectile dysfunction
- *Corresponding Author:
- Dr Gerald Brock
St Joseph's Health Centre, 268 Grosvenor Street, Urology Department, London, Ontario N6A 4V2.
Telephone: 519-646-6042
fax: 519-646-6037
E-mail: gebrock@sympatico.ca
Keywords
Efficacy; Erectile dysfunction; Pharmacology; Tadalafil
The oral inhibitors of phosphodiesterase type 5 (PDE5) appear to be the treatment of choice for the management of erectile dysfunction (ED). The combination of a high degree of efficacy (regardless of the underlying etiology of ED), a strong safety record and convenience has made these oral agents the first-line therapy, as recommended by the current Canadian Urological Association guidelines [1]. The first agent in this class to become available in Canada was sildenafil, which has been very widely studied and prescribed. More recently, tadalafil (Cialis, Lilly ICOS, USA, initially known as IC351) has been approved for use in Canada and the United States, while another member of the class, vardenafil (Levitra, Bayer Healthcare/GlaxoSmithKline, USA), is undergoing regulatory review in Canada at the time of writing. This report will focus on tadalafil, providing a comprehensive review of pharmacological and clinical information summarized from recently published studies (including four Canadian trials) and current abstracts.
Pharmacology of Tadalafil
Sildenafil, vardenafil and tadalafil all work by targeting PDE5, but have distinct chemical structures and pharmacological properties.
The time to maximum serum concentration for tadalafil, after oral administration of a single dose, is 2 h [2], compared with that of sildenafil (1 h) [3] or vardenafil (0.7 to 0.9 h) [4]. Tadalafil has been shown to have an onset of action as early as 16 min, which can be related to the minimal inhibitory concentration of this agent required to have a clinical effect.
The most significant pharmacological characteristic differentiating the three agents is the serum half-life: 4 h for sildenafil [3], 4 to 5 h for vardenafil [4] and 17.5 h for tadalafil [2].
As with other PDE5 inhibitors, tadalafil should not be administered in combination with organic nitrates. In clinical investigations, the hemodynamic interaction between tadalafil and sublingual nitroglycerin lasted 24 h but was not seen at 48 h and beyond [5].
Another pharmacological feature of tadalafil is that its absorption and pharmacodynamic properties are not affected by food [6,7]. In contrast, the other PDE5 inhibitors’ absorption can be blunted by fatty food, producing a delay in absorption and reduced peak serum concentration [3].
Outcome Measures
Many trials of new drugs for ED use standardized, validated questionnaires as the major outcome measures. The most commonly used is the International Index of Erectile Function (IIEF), a questionnaire that is available in at least 57 different languages and has been validated in different cultures [8]. The self-administered IIEF consists of 15 questions, six of which make up the erectile function domain [9]. These six questions are shown in Table 1. Each is scored from 1 to 5, so that a maximum score is 30; normal erectile function is defined as a score of 26 or greater [10].
| Question* | Response options |
|---|---|
| Q1: How often were you able to get an erection during sexual activity? | 0 = No sexual activity |
| Q2: When you had erections with sexual stimulation, how often were | 1 = Almost never/never |
| your erections hard enough for penetration? | 2 = A few times (much less than half the time) |
| 3 = Sometimes (about half the time) | |
| 4 = Most times (much more than half the time) | |
| 5 = Almost always/always | |
| Q3: When you attempted sexual intercourse, how often were you able | 0 = Did not attempt intercourse |
| to penetrate (enter) your partner? | 1 = Almost never/never |
| Q4: During sexual intercourse, how often were you able to maintain your | 2 = A few times (much less than half the time) |
| erection after you had penetrated (entered) your partner? | 3 = Sometimes (about half the time) |
| 4 = Most times (much more than half the time) | |
| 5 = Almost always/always | |
| Q5: During sexual intercourse, how difficult was it to maintain your | 0 = Did not attempt intercourse |
| erection to completion of intercourse? | 1 = Extremely difficult |
| 2 = Very difficult | |
| 3 = Difficult | |
| 4 = Slightly difficult | |
| 5 = Not difficult | |
| Q15: How do you rate your confidence that you could get and keep | 1 = Very low |
| an erection? | 2 = Low |
| 3 = Moderate 4 = High | |
| 5 = Very high |
Table 1: Distribution of Nugent scores of vaginal smears for 146 subjects, obtained by one technologist comparing transport and fixation methods, with results for all three methods.
Another frequently used scale is the Sexual Encounter Profile (SEP), a patient diary used to record sexual experiences. In this scale, the most commonly analyzed questions are question 2 (SEP-Q2), “Were you able to insert your penis into your partner’s vagina?” and question 3 (SEP-Q3), “Did your erection last long enough for you to have successful intercourse?” [11]
A summary question widely used in clinical studies is a global assessment question (GAQ), which uses wording such as “Has the treatment you have been taking improved your erections?” The GAQ provides good insight into the subjective clinical response to a therapeutic agent and provides the clinician with an indirect estimate of patient satisfaction.
Efficacy
Integrated analysis
The most important data supporting the efficacy of tadalafil for ED come from an integrated analysis of five randomized, doubleblind, placebo-controlled, multicentre trials, four of which enrolled patients from Canada [8]. The trials shared common elements of design, including inclusion and exclusion criteria, which facilitated pooling of their results for analysis. Conducted in a total of 74 centres, the trials enrolled 1112 patients with ED of varying etiologies and severities (41% mild, 23% moderate, 36% severe). The mean age of the patients was 59 years; 30% had hypertension and 21% were diabetic [8].
Doses of tadalafil in the studies ranged from 2.5 mg to 20 mg, with patients instructed to take a dose before sexual intercourse (8). One of the major strengths of tadalafil is that this agent can be taken without regard to food [7]. Because pharmacokinetic and pharmacodynamic data show no interaction with food, dosing in these studies was not restricted with regard to food intake. All of the studies were analyzed over a 12-week period [8]. The major outcome measures were change from baseline in the IIEF erectile function domain scores and the SEP questions 2 and 3.
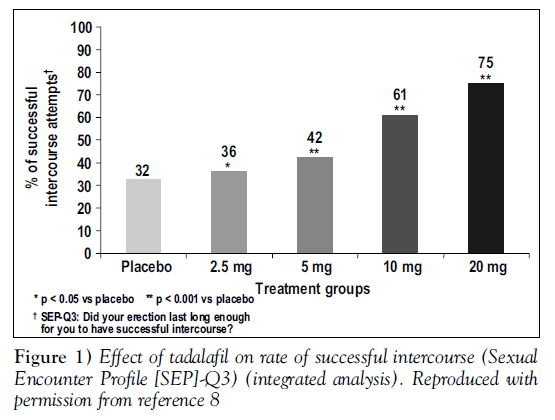
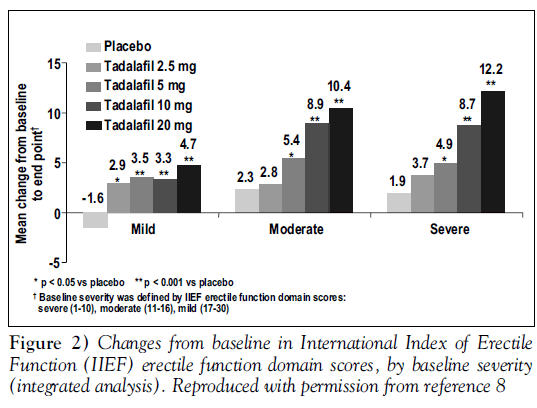
At all doses, tadalafil gave statistically significant improvements from baseline in erectile function compared with placebo. Figure 1 shows responses to SEP question 3, “Did your erection last long enough for you to have successful intercourse” Figure 2 displays changes from baseline in the total IIEF erectile function domain scores, analyzed by baseline severity of ED; tadalafil was effective regardless of baseline severity [8].
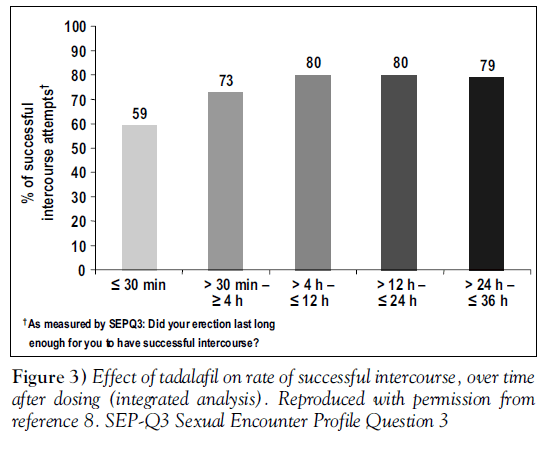
Men were asked to record the times of dosing and intercourse. Rates of successful intercourse (SEP-Q3) at different time intervals, for men taking 20 mg tadalafil, are shown in Figure 3 [8].
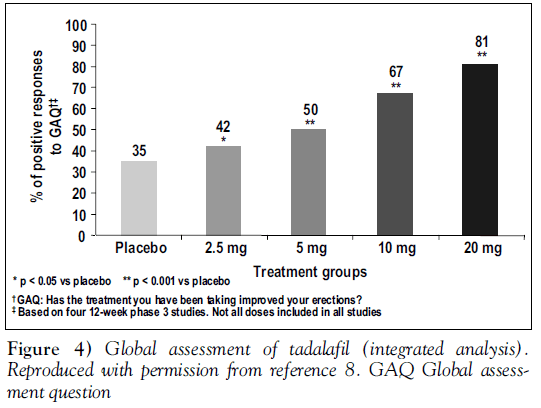
Other outcome measures showed similar improvements with tadalafil. One of the most clinically relevant was the proportion of men who achieved normal erectile function (an IIEF erectile function domain score of 26 or better). Scores increased in a dose-related manner from 11% with placebo to 21% with tadalafil 2.5 mg. For men taking tadalafil 20 mg, 59% achieved a score of 26 points or greater, placing them within the ‘normal’ range [8]. As well, the most commonly quoted measure of ED treatment, the GAQ, showed that 81% of men taking tadalafil 20 mg reported overall improvement in their erections (Figure 4) [8].
Additionally, placebo-controlled clinical trials included in the integrated analysis showed that tadalafil provided statistically significant improvement in partner satisfaction [7].
Efficacy in men with diabetes
The integrated analysis included men with diabetes, who made up 21% of the study population. Results showed significant improvements from baseline in all outcome measures in men with diabetes [8].
More specific information is available, however, from a recently published trial of 216 men with diabetes [11]. This randomized, double-blind, placebo-controlled trial was conducted at 18 sites in Spain. The average duration of diabetes was 11.7 years, 90.7% of men had type 2 diabetes, with only 18.5% of the study population well-controlled (Hemoglobin A1c less than 0.07). Hypertension was diagnosed in 39% of the men, and hyper-cholesterolemia in 19%. Once enrolled, men were randomly assigned to treatment with placebo or tadalafil 10 mg or 20 mg taken as needed up to once daily. As in the integrated analysis, timing of doses with respect to food was not restricted [11].
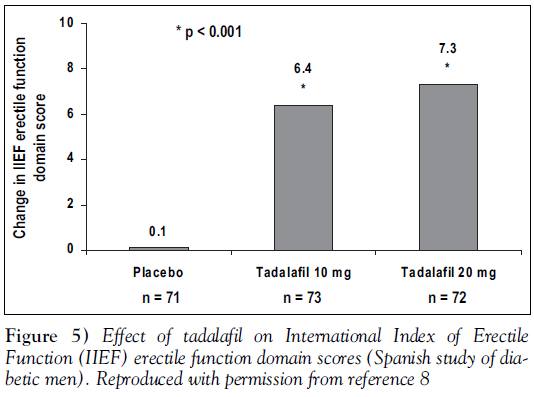
Treatment with tadalafil significantly improved erectile function in these diabetic men. Figure 5 shows the change from baseline in IIEF erectile function domain scores. Men taking placebo showed a 4.1% decline over the study in positive responses to SEP-Q2 (attaining an erection), while those taking tadalafil 10 mg showed a 22.2% improvement, and those taking 20 mg showed a 22.6% improvement (both comparisons with placebo, P<0.001) [11].
In response to the GAQ question, 25% of men taking placebo reported that the treatment improved their erections, compared with 56% of those taking 10 mg of tadalafil and 64% of those taking 20 mg (both comparisons with placebo P<0.001) [11].
Period of responsiveness
The period of effectiveness of tadalafil was assessed in a trial of 348 men with various severities of ED [12]. Men in this doubleblind, placebo-controlled trial were instructed to attempt intercourse on four occasions: twice at roughly 24 h after a dose of either placebo or tadalafil 20 mg, then on two other occasions roughly 36 h after either placebo or tadalafil.
At both 24 h and 36 h after the medication, men who took tadalafil were much more likely to be successful in intercourse attempts (SEP-Q3) than men who received placebo (P<0.001) (Figure 6) [12]. Other secondary measures of erectile function and overall satisfaction showed similar improvements with tadalafil [12].
These results confirm the findings from the integrated analysis (Figure 3) [8], showing a prolonged period of responsiveness after the use of tadalafil.
Patient Preference
Ultimately, once all three PDE5 inhibitors are approved for use in Canada, patient preference as characterized by efficacy, safety, cost, ease of use and as-yet-unknown factors will determine the ED agent of choice. Preference studies are difficult to develop and frequently have significant inherent biases built into their protocols. In spite of these limitations, insight into what patients prefer is a valid concept and deserves to be explored to assist the clinician in counselling patients concerning which erectogenic agent should be tried.
A randomized, double-blind, two-period crossover study [13] examined patient preference for initiating tadalafil or sildenafil treatment among 215 men with ED who either were sildenafil-naïve (85%) or had had a previous inadequate trial of sildenafil (15%). Of 190 evaluable patients, 66.3% preferred to initiate treatment with tadalafil and 33.7% with sildenafil (P<0.001). This study evaluated the 50 mg dose of sildenafil in comparison with the higher 20 mg dose of tadalafil. Data evaluating the two comparable higher doses (sildenafil 100 mg and tadalafil 20 mg) are not available.
Patient preference for tadalafil or sildenafil was also examined in a multicentre, open-label study of 155 current users of sildenafil treatment for ED who were then treated with tadalafil [14]. Though the results are subject to limitations due to the design of the study, including a 35% cap on numbers of men allowed into the study using 100 mg sildenafil, the singlecrossover design, use of tadalafil at the end of the study and the brief period of exposure to sildenafil, patients exhibited a significant preference for tadalafil over sildenafil [14].
Safety
In all studies published to date, tadalafil has been generally well tolerated, with side effects comparable to those seen with other PDE5 inhibitors. The most common side effects have been dyspepsia and headache. Table 2 shows the rate of side effects reported in the integrated analysis of 1112 men [8]. The side effects tended to occur shortly after initial doses of tadalafil and generally became less frequent and less severe with repeated dosing. The duration of side effects was similar between tadalafil and placebo. No life-threatening or cumulative side effects were seen, and most side effects became less noticeable with ongoing use of tadalafil. Figure 7 illustrates this attenuation of side effects with continued use [15,16].
| Tadalafil (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Safety variable | Placebo | All tadalafil | 2.5 | 5 | 10 | 20 | ||
| Patients (n) | 308 | 804 | 74 | 151 | 321 | 258 | ||
| Overall, n (%) | ||||||||
| Subjects with at least one treatment- | 159 (52) | 479 (60) | 38 (51) | 68 (45) | 185 (58) | 188 (73) | ||
| emergent adverse event | ||||||||
| Discontinuations from adverse events | 4 (1.3) | 17 (2.1) | 3 (4.1) | 1 (0.7) | 5 (1.6) | 8 (3.1) | ||
| Most common treatment- emergent adverse events, n (%) |
||||||||
| Headache | 19 (6) | 114 (14) | 5 (7) | 17 (11) | 37 (12) | 55 (21) | ||
| Dyspepsia | 7 (2) | 81 (10) | 1 (1) | 7 (5) | 28 (9) | 45 (17) | ||
| Back pain | 15 (5) | 50 (6) | 3 (4) | 5 (3) | 20 (6) | 22 (9) | ||
| Rhinitis (nasal congestion) | 12 (4) | 40 (5) | 4 (5) | 6 (4) | 18 (6) | 12 (5) | ||
| Myalgia | 6 (2) | 38 (5) | 2 (3) | 2 (1) | 16 (5) | 18 (7) | ||
| Vasodilatation (flushing) | 6 (2) | 30 (4) | 1 (1) | 4 (3) | 11 (3) | 14 (5) | ||
Adapted from reference 8
Table 2: Summary of most commonly reported adverse events and discontinuations from treatment.
The rates of adverse events in the Spanish study of diabetic men were comparable, with dyspepsia in 11% of men taking tadalafil, headache in 9%, back pain in 3.4%, myalgia in 4.8% and flushing in 3.4%. Of these, only dyspepsia was reported at a rate statistically significantly different from that seen with placebo (P=0.005) [11].
A review of placebo-controlled phase 3 studies found that tadalafil was not associated with an increase in cardiovascular adverse events or an increase in the incidence of potentially clinically significant changes in blood pressure in patients taking antihypertensive agents when compared with those patients not taking antihypertensive agents [17].
Conclusion
Clinical trial evidence currently available on tadalafil has shown it to be an effective and well-tolerated agent for the oral treatment of ED. Instructions that tadalafil may be taken without regard to food make the therapy simple to administer. Tadalafil’s extended period of responsiveness, with studies demonstrating improved erectile function out to 36 h, may offer clinical benefits to patients. Tadalafil should provide an important new therapeutic option for physicians treating ED and for their patients.
Acknowledgements
Writing and editorial support for this article were provided by Alan Yoshioka, PhD (AY’s Edit) through a grant from Eli Lilly Canada Inc, which also provided an honorarium for the author.
References
- Erectile dysfunction practice guidelines. Can J Urol 2002;9:1583-7.
- Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract 2002;56:300-4.
- Krane R, Brock G, Eardley I, et al. Oral non-endocrine treatment. In: Jardin A, Wagner G, Khoury S, Giuliano F, Padma-Nathan H, Rosen R, eds. Erectile Dysfunction. Plymouth UK: Health Publication Ltd 2000:241-78.
- Pryor J. Vardenafil: Update on clinical experience. Int J Impot Res 2002;14(Suppl 1):S65-9.
- Kloner RA, Hutter AM, Emmick JT, Mitchll MI, Denne J, Jackson G. Time course of the interaction between tadalafil and nitrates. J Am Coll Cardiol 2003;42:1855-60.
- Patterson B, Bedding A, Jewell H, Payne C, Mitchell M. The effect of intrinsic and extrinsic factors on the pharmacokinetic properties of tadalafil (IC351). 4th Congress of the European Society for Sexual and Impotence Research. Rome, Italy Sep 30-Oct 3, 2001.
- Cialis Product Monograph, September 17, 2003.
- Brock GB, McMahon CG, Chen KK, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: Results of integrated analyses. J Urol 2002;168:1332-6.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30.
- Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999;54:346-1.
- Saenz de Tejada I, Anglin G, Knight JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care 2002;25:2159-64.
- Porst H, Rosen RC, Padma-Nathan H, Varanese L, Anglin G, Giuliano F. Tadalafil allows men with erectile dysfunction to have successful intercourse up to 36 hours postdose. Presented at the 97th Annual Meeting of the American Urological Association; 2002 May 25-30; Orlando, USA.
- Govier F, Potempa A-J, Kaufman J, Denne J, Kovalenko P, Ahuja S. A multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20 mg or sildenafil citrate 50 mg during initiation of treatment for erectile dysfunction. Clin Ther 2003;25:2709-3.
- Ströberg P, Murphy A, Costigan T. Switching patients with erectile dysfunction from sildenafil citrate to tadalafil: Results of a multicenter open-label study investigating patient preference. Clin Ther 2003;25:2724-7.
- Porst H, Giuliano F, Meuleman E, Saoud J, Ferguson K, Whitaker S. Daily IC351 treatment of ED. Int J Impot Res 2000;12(Suppl 3):S76.
- Porst H. IC351 (tadalafil, Cialis): Update on clinical experience. Int J Impot Res 2002;14(Suppl 1):S57-4.
- Emmick JT, Stuewe SR, Mitchell M. Overview of the cardiovascular effects of tadalafil. Eur Heart J Supplements 2002;4(Suppl H):H32-47.
- *Corresponding Author:
- Dr Gerald Brock
St Joseph's Health Centre, 268 Grosvenor Street, Urology Department, London, Ontario N6A 4V2.
Telephone: 519-646-6042
fax: 519-646-6037
E-mail: gebrock@sympatico.ca
Abstract
Oral phosphodiesterase type 5 (PDE5) inhibitor therapy is recommended in guidelines as first-line therapy for erectile dysfunction because of its convenience, high efficacy and low rates of side effects. Tadalafil (Cialis, Lilly ICOS, USA), recently approved in Canada and the United States as an oral therapy for erectile dysfunction, has a half-life of 17.5 h, which offers men a longer period of effectiveness than other PDE5 inhibitors. Five randomized, double-blind, placebocontrolled, multicentre trials (four with patients in Canada) studied tadalafil at fixed doses of 2.5, 5, 10, or 20 mg. In an integrated analysis, tadalafil gave statistically significant improvements in all efficacy outcomes compared with placebo. A global assessment question found 81% of men taking tadalafil 20 mg reported overall improvement in their erections. In a separate study, tadalafil significantly improved erectile function 36 h after dosing. Tadalafil has been generally well-tolerated, with side effects comparable to those seen with other PDE5 inhibitors.
-Keywords
Efficacy; Erectile dysfunction; Pharmacology; Tadalafil
The oral inhibitors of phosphodiesterase type 5 (PDE5) appear to be the treatment of choice for the management of erectile dysfunction (ED). The combination of a high degree of efficacy (regardless of the underlying etiology of ED), a strong safety record and convenience has made these oral agents the first-line therapy, as recommended by the current Canadian Urological Association guidelines [1]. The first agent in this class to become available in Canada was sildenafil, which has been very widely studied and prescribed. More recently, tadalafil (Cialis, Lilly ICOS, USA, initially known as IC351) has been approved for use in Canada and the United States, while another member of the class, vardenafil (Levitra, Bayer Healthcare/GlaxoSmithKline, USA), is undergoing regulatory review in Canada at the time of writing. This report will focus on tadalafil, providing a comprehensive review of pharmacological and clinical information summarized from recently published studies (including four Canadian trials) and current abstracts.
Pharmacology of Tadalafil
Sildenafil, vardenafil and tadalafil all work by targeting PDE5, but have distinct chemical structures and pharmacological properties.
The time to maximum serum concentration for tadalafil, after oral administration of a single dose, is 2 h [2], compared with that of sildenafil (1 h) [3] or vardenafil (0.7 to 0.9 h) [4]. Tadalafil has been shown to have an onset of action as early as 16 min, which can be related to the minimal inhibitory concentration of this agent required to have a clinical effect.
The most significant pharmacological characteristic differentiating the three agents is the serum half-life: 4 h for sildenafil [3], 4 to 5 h for vardenafil [4] and 17.5 h for tadalafil [2].
As with other PDE5 inhibitors, tadalafil should not be administered in combination with organic nitrates. In clinical investigations, the hemodynamic interaction between tadalafil and sublingual nitroglycerin lasted 24 h but was not seen at 48 h and beyond [5].
Another pharmacological feature of tadalafil is that its absorption and pharmacodynamic properties are not affected by food [6,7]. In contrast, the other PDE5 inhibitors’ absorption can be blunted by fatty food, producing a delay in absorption and reduced peak serum concentration [3].
Outcome Measures
Many trials of new drugs for ED use standardized, validated questionnaires as the major outcome measures. The most commonly used is the International Index of Erectile Function (IIEF), a questionnaire that is available in at least 57 different languages and has been validated in different cultures [8]. The self-administered IIEF consists of 15 questions, six of which make up the erectile function domain [9]. These six questions are shown in Table 1. Each is scored from 1 to 5, so that a maximum score is 30; normal erectile function is defined as a score of 26 or greater [10].
| Question* | Response options |
|---|---|
| Q1: How often were you able to get an erection during sexual activity? | 0 = No sexual activity |
| Q2: When you had erections with sexual stimulation, how often were | 1 = Almost never/never |
| your erections hard enough for penetration? | 2 = A few times (much less than half the time) |
| 3 = Sometimes (about half the time) | |
| 4 = Most times (much more than half the time) | |
| 5 = Almost always/always | |
| Q3: When you attempted sexual intercourse, how often were you able | 0 = Did not attempt intercourse |
| to penetrate (enter) your partner? | 1 = Almost never/never |
| Q4: During sexual intercourse, how often were you able to maintain your | 2 = A few times (much less than half the time) |
| erection after you had penetrated (entered) your partner? | 3 = Sometimes (about half the time) |
| 4 = Most times (much more than half the time) | |
| 5 = Almost always/always | |
| Q5: During sexual intercourse, how difficult was it to maintain your | 0 = Did not attempt intercourse |
| erection to completion of intercourse? | 1 = Extremely difficult |
| 2 = Very difficult | |
| 3 = Difficult | |
| 4 = Slightly difficult | |
| 5 = Not difficult | |
| Q15: How do you rate your confidence that you could get and keep | 1 = Very low |
| an erection? | 2 = Low |
| 3 = Moderate 4 = High | |
| 5 = Very high |
Table 1: Distribution of Nugent scores of vaginal smears for 146 subjects, obtained by one technologist comparing transport and fixation methods, with results for all three methods.
Another frequently used scale is the Sexual Encounter Profile (SEP), a patient diary used to record sexual experiences. In this scale, the most commonly analyzed questions are question 2 (SEP-Q2), “Were you able to insert your penis into your partner’s vagina?” and question 3 (SEP-Q3), “Did your erection last long enough for you to have successful intercourse?” [11]
A summary question widely used in clinical studies is a global assessment question (GAQ), which uses wording such as “Has the treatment you have been taking improved your erections?” The GAQ provides good insight into the subjective clinical response to a therapeutic agent and provides the clinician with an indirect estimate of patient satisfaction.
Efficacy
Integrated analysis
The most important data supporting the efficacy of tadalafil for ED come from an integrated analysis of five randomized, doubleblind, placebo-controlled, multicentre trials, four of which enrolled patients from Canada [8]. The trials shared common elements of design, including inclusion and exclusion criteria, which facilitated pooling of their results for analysis. Conducted in a total of 74 centres, the trials enrolled 1112 patients with ED of varying etiologies and severities (41% mild, 23% moderate, 36% severe). The mean age of the patients was 59 years; 30% had hypertension and 21% were diabetic [8].
Doses of tadalafil in the studies ranged from 2.5 mg to 20 mg, with patients instructed to take a dose before sexual intercourse (8). One of the major strengths of tadalafil is that this agent can be taken without regard to food [7]. Because pharmacokinetic and pharmacodynamic data show no interaction with food, dosing in these studies was not restricted with regard to food intake. All of the studies were analyzed over a 12-week period [8]. The major outcome measures were change from baseline in the IIEF erectile function domain scores and the SEP questions 2 and 3.
At all doses, tadalafil gave statistically significant improvements from baseline in erectile function compared with placebo. Figure 1 shows responses to SEP question 3, “Did your erection last long enough for you to have successful intercourse” Figure 2 displays changes from baseline in the total IIEF erectile function domain scores, analyzed by baseline severity of ED; tadalafil was effective regardless of baseline severity [8].
Men were asked to record the times of dosing and intercourse. Rates of successful intercourse (SEP-Q3) at different time intervals, for men taking 20 mg tadalafil, are shown in Figure 3 [8].
Other outcome measures showed similar improvements with tadalafil. One of the most clinically relevant was the proportion of men who achieved normal erectile function (an IIEF erectile function domain score of 26 or better). Scores increased in a dose-related manner from 11% with placebo to 21% with tadalafil 2.5 mg. For men taking tadalafil 20 mg, 59% achieved a score of 26 points or greater, placing them within the ‘normal’ range [8]. As well, the most commonly quoted measure of ED treatment, the GAQ, showed that 81% of men taking tadalafil 20 mg reported overall improvement in their erections (Figure 4) [8].
Additionally, placebo-controlled clinical trials included in the integrated analysis showed that tadalafil provided statistically significant improvement in partner satisfaction [7].
Efficacy in men with diabetes
The integrated analysis included men with diabetes, who made up 21% of the study population. Results showed significant improvements from baseline in all outcome measures in men with diabetes [8].
More specific information is available, however, from a recently published trial of 216 men with diabetes [11]. This randomized, double-blind, placebo-controlled trial was conducted at 18 sites in Spain. The average duration of diabetes was 11.7 years, 90.7% of men had type 2 diabetes, with only 18.5% of the study population well-controlled (Hemoglobin A1c less than 0.07). Hypertension was diagnosed in 39% of the men, and hyper-cholesterolemia in 19%. Once enrolled, men were randomly assigned to treatment with placebo or tadalafil 10 mg or 20 mg taken as needed up to once daily. As in the integrated analysis, timing of doses with respect to food was not restricted [11].
Treatment with tadalafil significantly improved erectile function in these diabetic men. Figure 5 shows the change from baseline in IIEF erectile function domain scores. Men taking placebo showed a 4.1% decline over the study in positive responses to SEP-Q2 (attaining an erection), while those taking tadalafil 10 mg showed a 22.2% improvement, and those taking 20 mg showed a 22.6% improvement (both comparisons with placebo, P<0.001) [11].
In response to the GAQ question, 25% of men taking placebo reported that the treatment improved their erections, compared with 56% of those taking 10 mg of tadalafil and 64% of those taking 20 mg (both comparisons with placebo P<0.001) [11].
Period of responsiveness
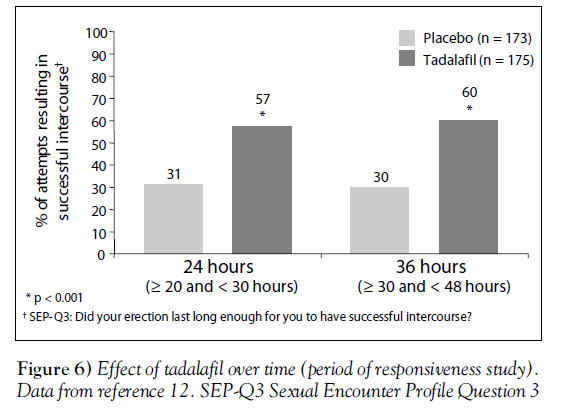
The period of effectiveness of tadalafil was assessed in a trial of 348 men with various severities of ED [12]. Men in this doubleblind, placebo-controlled trial were instructed to attempt intercourse on four occasions: twice at roughly 24 h after a dose of either placebo or tadalafil 20 mg, then on two other occasions roughly 36 h after either placebo or tadalafil.
At both 24 h and 36 h after the medication, men who took tadalafil were much more likely to be successful in intercourse attempts (SEP-Q3) than men who received placebo (P<0.001) (Figure 6) [12]. Other secondary measures of erectile function and overall satisfaction showed similar improvements with tadalafil [12].
These results confirm the findings from the integrated analysis (Figure 3) [8], showing a prolonged period of responsiveness after the use of tadalafil.
Patient Preference
Ultimately, once all three PDE5 inhibitors are approved for use in Canada, patient preference as characterized by efficacy, safety, cost, ease of use and as-yet-unknown factors will determine the ED agent of choice. Preference studies are difficult to develop and frequently have significant inherent biases built into their protocols. In spite of these limitations, insight into what patients prefer is a valid concept and deserves to be explored to assist the clinician in counselling patients concerning which erectogenic agent should be tried.
A randomized, double-blind, two-period crossover study [13] examined patient preference for initiating tadalafil or sildenafil treatment among 215 men with ED who either were sildenafil-naïve (85%) or had had a previous inadequate trial of sildenafil (15%). Of 190 evaluable patients, 66.3% preferred to initiate treatment with tadalafil and 33.7% with sildenafil (P<0.001). This study evaluated the 50 mg dose of sildenafil in comparison with the higher 20 mg dose of tadalafil. Data evaluating the two comparable higher doses (sildenafil 100 mg and tadalafil 20 mg) are not available.
Patient preference for tadalafil or sildenafil was also examined in a multicentre, open-label study of 155 current users of sildenafil treatment for ED who were then treated with tadalafil [14]. Though the results are subject to limitations due to the design of the study, including a 35% cap on numbers of men allowed into the study using 100 mg sildenafil, the singlecrossover design, use of tadalafil at the end of the study and the brief period of exposure to sildenafil, patients exhibited a significant preference for tadalafil over sildenafil [14].
Safety
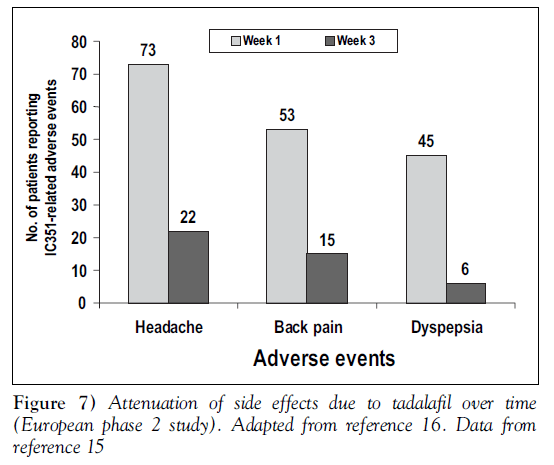
In all studies published to date, tadalafil has been generally well tolerated, with side effects comparable to those seen with other PDE5 inhibitors. The most common side effects have been dyspepsia and headache. Table 2 shows the rate of side effects reported in the integrated analysis of 1112 men [8]. The side effects tended to occur shortly after initial doses of tadalafil and generally became less frequent and less severe with repeated dosing. The duration of side effects was similar between tadalafil and placebo. No life-threatening or cumulative side effects were seen, and most side effects became less noticeable with ongoing use of tadalafil. Figure 7 illustrates this attenuation of side effects with continued use [15,16].
| Tadalafil (mg) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Safety variable | Placebo | All tadalafil | 2.5 | 5 | 10 | 20 | ||
| Patients (n) | 308 | 804 | 74 | 151 | 321 | 258 | ||
| Overall, n (%) | ||||||||
| Subjects with at least one treatment- | 159 (52) | 479 (60) | 38 (51) | 68 (45) | 185 (58) | 188 (73) | ||
| emergent adverse event | ||||||||
| Discontinuations from adverse events | 4 (1.3) | 17 (2.1) | 3 (4.1) | 1 (0.7) | 5 (1.6) | 8 (3.1) | ||
| Most common treatment- emergent adverse events, n (%) |
||||||||
| Headache | 19 (6) | 114 (14) | 5 (7) | 17 (11) | 37 (12) | 55 (21) | ||
| Dyspepsia | 7 (2) | 81 (10) | 1 (1) | 7 (5) | 28 (9) | 45 (17) | ||
| Back pain | 15 (5) | 50 (6) | 3 (4) | 5 (3) | 20 (6) | 22 (9) | ||
| Rhinitis (nasal congestion) | 12 (4) | 40 (5) | 4 (5) | 6 (4) | 18 (6) | 12 (5) | ||
| Myalgia | 6 (2) | 38 (5) | 2 (3) | 2 (1) | 16 (5) | 18 (7) | ||
| Vasodilatation (flushing) | 6 (2) | 30 (4) | 1 (1) | 4 (3) | 11 (3) | 14 (5) | ||
Adapted from reference 8
Table 2: Summary of most commonly reported adverse events and discontinuations from treatment.
The rates of adverse events in the Spanish study of diabetic men were comparable, with dyspepsia in 11% of men taking tadalafil, headache in 9%, back pain in 3.4%, myalgia in 4.8% and flushing in 3.4%. Of these, only dyspepsia was reported at a rate statistically significantly different from that seen with placebo (P=0.005) [11].
A review of placebo-controlled phase 3 studies found that tadalafil was not associated with an increase in cardiovascular adverse events or an increase in the incidence of potentially clinically significant changes in blood pressure in patients taking antihypertensive agents when compared with those patients not taking antihypertensive agents [17].
Conclusion
Clinical trial evidence currently available on tadalafil has shown it to be an effective and well-tolerated agent for the oral treatment of ED. Instructions that tadalafil may be taken without regard to food make the therapy simple to administer. Tadalafil’s extended period of responsiveness, with studies demonstrating improved erectile function out to 36 h, may offer clinical benefits to patients. Tadalafil should provide an important new therapeutic option for physicians treating ED and for their patients.
Acknowledgements
Writing and editorial support for this article were provided by Alan Yoshioka, PhD (AY’s Edit) through a grant from Eli Lilly Canada Inc, which also provided an honorarium for the author.
References
- Erectile dysfunction practice guidelines. Can J Urol 2002;9:1583-7.
- Eardley I, Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int J Clin Pract 2002;56:300-4.
- Krane R, Brock G, Eardley I, et al. Oral non-endocrine treatment. In: Jardin A, Wagner G, Khoury S, Giuliano F, Padma-Nathan H, Rosen R, eds. Erectile Dysfunction. Plymouth UK: Health Publication Ltd 2000:241-78.
- Pryor J. Vardenafil: Update on clinical experience. Int J Impot Res 2002;14(Suppl 1):S65-9.
- Kloner RA, Hutter AM, Emmick JT, Mitchll MI, Denne J, Jackson G. Time course of the interaction between tadalafil and nitrates. J Am Coll Cardiol 2003;42:1855-60.
- Patterson B, Bedding A, Jewell H, Payne C, Mitchell M. The effect of intrinsic and extrinsic factors on the pharmacokinetic properties of tadalafil (IC351). 4th Congress of the European Society for Sexual and Impotence Research. Rome, Italy Sep 30-Oct 3, 2001.
- Cialis Product Monograph, September 17, 2003.
- Brock GB, McMahon CG, Chen KK, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: Results of integrated analyses. J Urol 2002;168:1332-6.
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30.
- Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999;54:346-1.
- Saenz de Tejada I, Anglin G, Knight JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care 2002;25:2159-64.
- Porst H, Rosen RC, Padma-Nathan H, Varanese L, Anglin G, Giuliano F. Tadalafil allows men with erectile dysfunction to have successful intercourse up to 36 hours postdose. Presented at the 97th Annual Meeting of the American Urological Association; 2002 May 25-30; Orlando, USA.
- Govier F, Potempa A-J, Kaufman J, Denne J, Kovalenko P, Ahuja S. A multicenter, randomized, double-blind, crossover study of patient preference for tadalafil 20 mg or sildenafil citrate 50 mg during initiation of treatment for erectile dysfunction. Clin Ther 2003;25:2709-3.
- Ströberg P, Murphy A, Costigan T. Switching patients with erectile dysfunction from sildenafil citrate to tadalafil: Results of a multicenter open-label study investigating patient preference. Clin Ther 2003;25:2724-7.
- Porst H, Giuliano F, Meuleman E, Saoud J, Ferguson K, Whitaker S. Daily IC351 treatment of ED. Int J Impot Res 2000;12(Suppl 3):S76.
- Porst H. IC351 (tadalafil, Cialis): Update on clinical experience. Int J Impot Res 2002;14(Suppl 1):S57-4.
- Emmick JT, Stuewe SR, Mitchell M. Overview of the cardiovascular effects of tadalafil. Eur Heart J Supplements 2002;4(Suppl H):H32-47.