Targeted liposomal nanodelivery of E-cadherin as potential therapy for acute myeloid leukaemia
2 Department of Micro-Nano Mechanical Science and Engineering, Nagoya University, Nagoya, Japan, Email: ali.sameh@d.mbox.nagoya-u.ac.jp
Received: 26-Mar-2021 Accepted Date: Apr 09, 2021; Published: 15-Apr-2021
Citation: Sameh A Mohammed, Targeted liposomal nanodelivery of E-cadherin as potential therapy for acute myeloid leukaemia. J Nanosci Nanomed. 2021;5(3):1-2.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Advanced drug delivery system has opened novel approaches to develop the therapeutic effects of potentially-efficient molecules. Specifically, Lipid Nanoparticles (LNs) have recruited as promising Nano carriers in cancer therapy. LNs exhibit remarkable advantages such as reduced toxicity, high bioavailability of drugs, incorporation versatility of lipophilic and hydrophilic drugs, and large-scale production feasibility. Furthermore, LNs overcome numerous physiological barriers that prohibit drug delivery to tumor location and are also able to curb mechanisms of multidrug resistance, unique characteristics of cancer cells. E-cadherin (CDH1) is an epithelial cadherin, belongs to the calcium-dependent adhesion molecule superfamily, and is implicated in the interactions of hematopoietic progenitors and bone marrow stromal cells. Adhesion capacity to bone marrow stroma was impaired for leukemia cells, suggesting that a breakdown of adhesive mechanisms governed by an adhesion molecule may exist in the leukemic microenvironment. Our Nano formulation mainly consists of liposomal nanoparticles to deliver CDH1 for targeting leukemic cells. LNs can deliver CDH1 by different mechanisms, such as passive mechanisms that take advantage of the tumor microenvironment. After the restoration of E-cadherin in leukemia cells, E-cadherin-specific adhesion was enhanced.
Keywords
Lipid Nanoparticles; E-cadherin; Advanced drug delivery system; Acute myeloid leukaemia; E-cadherin-specific adhesion
Introduction
It is well known that lipid-based nanoparticles are less toxic and biocompatible compared to inorganic or polymeric nanoparticles [1]. In particular, lipid nanoparticles (LNs) have emerged as an effective and promising alternative. They are colloidal particles of submicron size, with a diameter between 50 and 1000 nm. LNs can be produced by different methods described exhaustively in the bibliography, such as high shear homogenization and ultrasound, high-pressure homogenization, hot homogenization, cold homogenization, solvent emulsification/evaporation, and micro emulsion [2,3]. Among them, the micro emulsion method stands out for being an easy method that does not need very sophisticated equipment or highenergy input and avoids the use of organic solvents. All these advantages make the production of LNs at large scale technically and economically feasible [4]. Nonetheless, the correct composition is essential for the formation of micro emulsions (thermodynamically stable and transparent mixtures), and therefore the optimization of the mixture is required. LNs can improve drug effect while overcoming resistance mechanisms. The nanometre size of these systems, together with the possibility of chemical and structural modifications, makes them suitable to get through several biological barriers and to deliver drugs at the sites of interest with minimal toxicity [5]. Aside from the already mentioned advantages, the use of LNs in antitumor treatments could also allow oral administration of drugs and improve the exposure time of cancer cells to medicines in comparison to the most frequent administration methods [6]. This would imply the use of simpler and more convenient therapies for patients. Considering the impact of cancer worldwide and the need of more efficient therapies, LNs are demonstrated as particularly promising drug delivery systems for the improvement of cancer chemotherapeutic treatments. Hence, the aims of this study are to gather relevant and current information on the application of LNs as drug delivery systems in antitumor treatments, to determine the main barriers in cancer treatments and the importance of the mechanisms used by LNs to improve drug delivery, and to discuss the advances on the application of SLN and remaining challenges in this field [7,8].
E-cadherin is one of the most important molecules in cellular adhesion in epithelial tissues. It is localized on the surfaces of epithelial cells in regions of cell-cell contact known as adherens junctions (AJ) [9]. As a member of large family of genes coding for calcium-dependent cell adhesion molecules (CAMs), the cadherin glycoproteins are expressed by a variety of tissues, mediating adhesion through homotypic binding. Classical cadherins –Eand N-cadherins being the best characterized – play important roles in the formation of tissues during gastrulation, neurulation and organogenesis [10]. E-cadherin has probably been studied in most detail. It is essential for the formation and maintenance of epithelia, was first identified in chicken, and was originally called LCAM [11,12]. Besides its role in normal cells, this highly conserved gene can play a major role in malignant cell transformation, and especially in tumour development and progression. The suppression of E-cadherin expression is regarded as one of the main molecular events responsible for dysfunction in cellular architecture, and loss of tissue integrity can lead to local invasion. Thus, loss of E-cadherin function as a tumour suppressor protein correlates with increased invasiveness and metastasis of tumours [13,14], resulting in it being referred to as the “suppressor of invasion” gene. Our Nano formulation mainly consists of liposomal nanoparticles to deliver CDH1 for targeting leukemic cells. LNs can deliver CDH1 by different mechanisms, such as passive mechanisms that take advantage of the tumour microenvironment. After restoration of E-cadherin in leukaemia cells, E-cadherin-specific adhesion was enhanced.
Materials and Methods
L-α-phosphatidylcholine, cholesterol are supplied from Sigma Chemical Company (St Louis, MO). E-cadherin was purchasing from Sigma-Aldrich. All other chemicals or solvents were of the analytical or high-performance liquid chromatography (HPLC) grade available.
Physicochemical characterization of particle size and zeta potential
The measurement of mean particle size and poly disparity of samples was performed by Malvern Nano ZS90 Zetasizer (Malvern Instruments). The measurements were carried out at a fixed angle of 90° opposite the incident light source. Small amounts (2 mL) of each sample were added to the quartz cell of the photon correlation spectroscope. Zetasizer 2000 (Malvern Instruments, UK) was used to measure zeta potential.
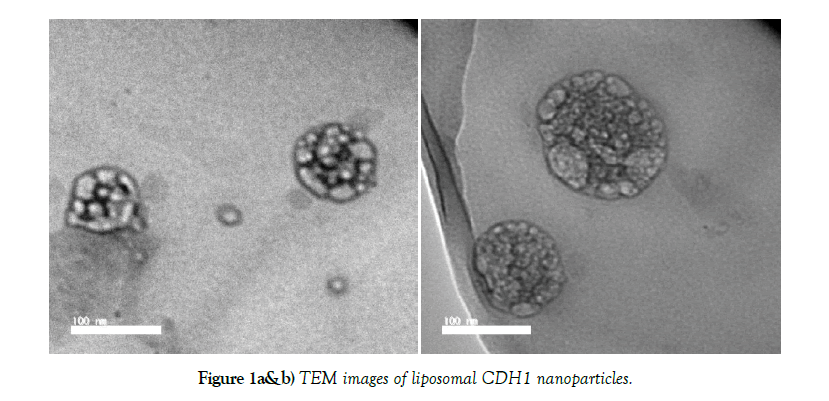
Transmission electron microscope (TEM) imaging
Transmission electron microscopy (TEM) were also analysed by a JEOL 1200 EX transmission electron microscope.
Results and Discussion
Physicochemical characteristics of liposomal CDH1 nanoparticles are demonstrated in (Table 1). Microscopic imaging of liposomal CDH1 nanoparticles showed possessing uniform spherical shape (Figure 1a and b). Polydispersity Index (PDI) values of liposomal CDH1 nanoparticles was (≤1) that indicating narrow size dispersion. Zeta potential results indicate adequate dispersion stability of nanoparticles. Accordingly, liposomal CDH1 nanoparticles are promising candidate as.
Table 1: Size and zeta potential characterizations of liposomal CDH1 nanoparticles.
| Group | Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| liposomal CDH1 nanoparticles | 115.2 ± 7.5 | 0.312 ± 0.004 | -43 ± 1.78 |
Conflict of Interest
None
REFERENCES
- Patra JK, Das G, Fraceto LF et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71.
- Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: a modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71(4):349-358.
- Mishra V, Bansal KK, Verma A et al. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics. 2018;10(4):191.
- Tartaro G, Mateos H, Schirone D et al. Microemulsion Microstructure(s): A Tutorial Review. Nanomaterials (Basel). 2020;10(9):1657.
- Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol Rev. 2016;68(3):701-787.
- Bayón-Cordero L, Alkorta I, Arana L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials (Basel). 2019;9(3):474.
- Zhao CY, Cheng R, Yang Z, et al. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules. 2018;23(4):826.
- Senapati S, Mahanta AK, Kumar S et al. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7.
- Maître JL, Heisenberg CP. Three functions of cadherins in cell adhesion. Curr Biol. 2013;23(14):R626-R633.
- Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z et al. Role of Cadherins in Cancer-A Review. Int J Mol Sci. 2020;21(20):7624.
- Bryan RT. Cell adhesion and urothelial bladder cancer: the role of cadherin switching and related phenomena. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140042.
- Ortega FE, Rengarajan M, Chavez N et al. Adhesion to the host cell surface is sufficient to mediate Listeria monocytogenes entry into epithelial cells. Mol Biol Cell. 2017;28(22):2945-2957.
- Bruner HC, Derksen PWB. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb Perspect Biol. 2018;10(3):a029330.
- Carafa V, Altucci L, Nebbioso A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front Pharmacol. 2019;10:38.