The acute effects of therapeutic concentrations of simvastatin lactone on mitochondrial respiratory capacity in human skeletal muscle: the LIFESTAT study
2 Department of Geriatrics, Bispebjerg University Hospital, Copenhagen, Denmark, Email: fdela@sund.ku.dk
Received: 10-Sep-2017 Accepted Date: Sep 12, 2017; Published: 25-Sep-2017
Citation: Dohlmann TL, Helge JW, Dela F, et al.. The acute effects of therapeutic concentrations of simvastatin lactone on mitochondrial respiratory capacity in human skeletal muscle: the LIFESTAT study Clin Pharmacol Toxicol Res September-2017;1(1):4-12.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Objectives: Simvastatin is a potent cholesterol lowering drug that may carry myalgia as a side effect. Whilst the underlying mechanism is unclear, statin therapy has been associated with impaired mitochondrial function. Two studies have differentiated between toxicity of simvastatin in its active acid and inactive lactone form, and found the inactive lactone form to be more toxic to myoblasts in vitro. Thus, the aim of this study was to investigate if therapeutic concentrations of simvastatin lactone in vitro impair mitochondrial respiration in skeletal muscle tissue, and if a dose dependent relationship exists.
Methods: Muscle biopsies from 9 healthy adults were incubated with simvastatin corresponding to the maximal previously reported peak plasma concentration (Cmax), and in a 10 fold higher concentration to mimic intramuscular statin accumulation (0.1 μM and 1 μM, respectively). We measured mitochondrial maximal complex I and I+II linked respiration, which was succeeded by a stepwise simvastatin titration protocol to investigate a possible dose-dependent relationship.
Results: We found no effect on mitochondrial respiration using therapeutic concentrations of simvastatin (0.1 and 1 μM), but supratherapeutic concentrations yielded a dose-dependent inhibition of mitochondrial respiration.
Conclusion: These results implicate a possible toxic effect of simvastatin lactone on mitochondrial respiration, which may play a role in the development of statin induced myalgia, but only if the intramuscular accumulation of simvastatin lactone is high in myalgic statin users . This study emphasizes the need for investigating the degree of skeletal muscle statin accumulation in statin users experiencing myalgia.
Keywords
Mitochondria, Simvastatin lactone, Myalgia
Introduction
Statins are a class of drugs that lower blood cholesterol levels by inhibition of HMG-CoA reductase which thereby reduces the endogenous synthesis of cholesterol and other end-products of the essential mevalonate pathway. They are one of the most used drugs in the world, but have been associated with development of muscular side effects (myopathies), including muscle ache and pain (myalgia) [1-3] and in rare cases rhabdomyolysis [4].
The mechanism behind statin induced myalgia is unclear, but prior studies have associated statin induced myalgia with impaired mitochondrial function [5-7], altered calcium homeostasis [8] and apoptosis [9,10]. Statins exist in various chemical structures differing in solubility and pharmacokinetic properties [11].
Simvastatin is the most frequently prescribed statin [12] and is highly lipophilic. It is consumed orally in its inactive lactone form, and after ingestion it is hydrolysed to its active, but less lipophilic, compound simvastatin B-hydroxy acid, by carboxyesterases in the intestinal mucosa, liver and plasma [13,14].
Drug transporters have been shown to facilitate influx (organic aniontransporting polypeptides (OATP2B1) [13,15,16] and efflux (multidrug resistance-associated protein (MRP) 1,4,5 [16]) of mainly simvastatin acid in skeletal muscle tissue. But due to their lipophilic structure, both the acid and lactone forms of simvastatin are capable of entering various tissues including skeletal muscle by passive diffusion.
Thus, simvastatin acid and lactone have the potential to accumulate in skeletal muscle, and cause myotoxic effects. However, the intramuscular simvastatin concentration in patients in simvastatin therapy has never been investigated and is thus unknown.
Previous studies have used supratherapeutic simvastatin concentrations to simulate intramuscular statin accumulation, and found impaired mitochondrial complex I linked respiration in human myotubes [17] and rat skeletal muscle [18] in vitro. However, the simvastatin concentrations used were up to 1000 times higher than the reported human plasma concentrations, and it is unclear if the simvastatin used was in its acid or lactone form. A few studies have differentiated between simvastatin in its lactone and acid form and the former has been found more toxic than the latter in in vitro in rat [19] and human myoblasts [20].
To date, there is a lack of studies on toxicology of simvastatin lactone in humans, and the pharmacological property of simvastatin lactone is generally poorly understood. To our knowledge, a toxic effect of simvastatin on mitochondrial respiratory capacity in human skeletal muscle has never been investigated before. Therefore, we aimed to investigate if therapeutic concentrations of simvastatin lactone impair mitochondrial respiration in skeletal muscle tissue obtained from healthy adults.
Methods
The muscle samples for the present study were generated from a subgroup of a larger ongoing study: the LIFESTAT study [12]. The study followed the Helsinki declaration and was approved by the local - ethics committee (protocol number: H-2-2013164), and all subjects signed a written consent form before enrolling in the study.
Muscle sampling
Skeletal muscle biopsies were obtained from the vastus lateralis of 9 healthy untrained adults (Age 59 ± 2 years, body mass index: 30 ± 1 kg•m-2 ) using the Bergström needle modified for suction under local anesthetic (Lidocaine 5%). Approximately 15 mg muscle tissue was immediately transferred to the relaxing buffer “Biops” (2.8 mM Ca2K2EGTA, 7.2 mM K2EGTA, 5.8 mM ATP, 6.6 mM MgCl2, 20 mM taurine, 15 mM Na2phosphocreatine, 20 mM imidazole, 0.5 mM dithiotreitol, 50 mM MES, pH 7.1) and kept on ice for approximately two hours until teased and permeabilised.
Permeabilisation and incubation procedure
Under magnification, the muscle biopsy was carefully teased with sharp forceps to separate the muscle fibers and remove connective tissue. The bundle of teased muscle fibers was then divided into 3 portions, which were permeabilised with saponine (5 μg/ml) in BIOPS [21] on ice for 30 minutes. Two parts were added simvastatin lactone dissolved in dimethyl sulfoxide (DMSO) (0.1 μM simvastatin lactone (Sim 0.1 μM) and 1 μM (Sim 1 μM )) for simultaneous statin incubation and permeabilisation, while the third part served as a control.
Following permeabilisation, the muscle bundles were washed twice for ten minutes in the mitochondrial respiration medium “MiR05” (0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM sucrose, 0.1% (w/v) bovine serum albumin; pH 7.1) [21]. The “Sim 0.1 μM” and “Sim 1 μM” muscle fiber bundles were washed in MiR05 with simvastatin 0.1 μM and 1 μM, respectively, for simultaneous statin incubation. The total simvastatin lactone incubation time was 50 minutes.
Measurements of mitochondrial respiration
Mitochondrial respiration was measured with the respiration medium MiR05 with simvastatin lactone concentrations similar to the permeabilisation procedure. The respiration measurements were done in duplicate with hyperoxygenation (450–200 μM) using high resolution respirometry (Oxygraph-2k, Oroboros Instruments, Innsbruck, Austria) as described elsewhere [21]. Reoxygenation was performed if oxygen concentration declined below 200 μM. The protocol investigated maximal complex I linked respiration with malate (2 mM), glutamate (10 mM), pyruvate (5 mM), and ADP (5 mM).
This was followed by Cytochrome C (10 μM ) to ensure integrity of the outer mitochondrial membrane. 10 mM succinate was added to yield maximal complex I+II linked respiration, followed by rotenone (0.5 μM) to inhibit complex I and isolate complex II linked respiration. Finally, muscle that initially was incubated with Sim 1 μM was finished by a stepwise simvastatin lactone titration into the respiration chamber, after the rotenone titration (complex II), with the purpose of increasing simvastatin lactone doses to concentrations of: 8.5 μM, 13.5 μM, 18.5 μM, and 23.5 μM to mimic intramuscular accumulation.
Chemicals
Pure simvastatin in its lactone form was kindly donated by Teva Pharmaceutical Works Private Limited Company, Hungary, and dissolved in DMSO to a stock solution of 0.5 mM. The solution was prepared fresh on each experimental day.
Statistics
Data was analyzed by two-way ANOVA for repeated measures and Holm- Sidak was used as post hoc test. The effect of simvastatin titrations on mitochondrial respiration was analyzed by one way ANOVA for repeated measures. Statistical analyses were performed using SigmaPlot 13.0 (Systat Software, San Jose, CA, USA). Data is presented as mean ± standard error of the mean (SE). P<0.05 was considered significant.
Results
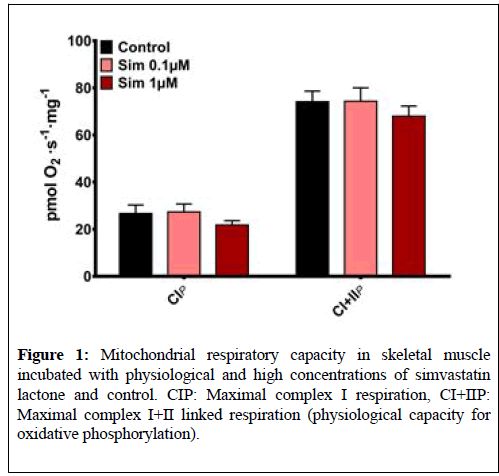
There were no differences between maximal mitochondrial complex I or I +II linked respiration between the untreated muscle tissue (Control) and muscle tissue incubated with simvastatin lactone (Sim 0.1 μM and Sim 1 μM) (Figure 1). Cytochrome C addition yielded no significant increase in respiration rate in either protocol (data not shown).
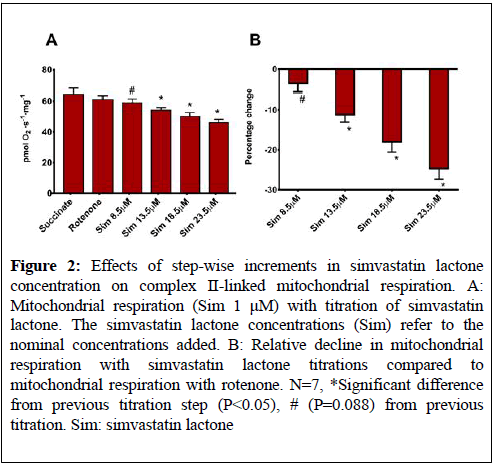
The step-wise increase in simvastatin lactone concentration in the Sim 1 μM protocol yielded a tendency (P=0.088) towards a decline (-4% ± 2%) in mitochondrial respiration when the nominal simvastatin lactone concentration was 8.5 μM (Figure 2).
Figure 2: Effects of step-wise increments in simvastatin lactone concentration on complex II-linked mitochondrial respiration. A: Mitochondrial respiration (Sim 1 μM) with titration of simvastatin lactone. The simvastatin lactone concentrations (Sim) refer to the nominal concentrations added. B: Relative decline in mitochondrial respiration with simvastatin lactone titrations compared to mitochondrial respiration with rotenone. N=7, *Significant difference from previous titration step (P<0.05), # (P=0.088) from previous titration. Sim: simvastatin lactone
When the simvastatin lactone concentration was further increased to 13.5 μM, 18.5 μM, and 23.5 μM, oxygen flux declined in a dose-dependent manner (-11% ± 2%, -18% ± 3%, and -25% ± 3%, respectiely, (P<0.05), (Figure 2).
Discussion
The novel finding of this study is that therapeutic concentrations of simvastatin lactone have no acute effects on mitochondrial respiration, whereas supratherapeutic concentrations induce a dose-dependent impairment.
Several studies have investigated the prevalence of statin induced myalgia with results that range from <1% to >60% in athletes [22,23]. Yet, the underlying mechanism(s) remain unknown. Different theories have been put forward to explain these statin induced side-effects. One theory is that reduced cholesterol synthesis results in reduced cholesterol content in the skeletal muscle membranes, rendering them unstable [24]. Another theory is based on reduced synthesis of other end-products of the mevalonate pathway, including coenzyme Q10 (CoQ10) [25].
Reduced CoQ10 in the mitochondrial electron transfer system may impair the capacity for oxidative phosphorylation and compromise the energetic state in the skeletal muscle, which could explain the myalgia and exercise intolerance reported with statin treatment [26].
In fact, studies have reported impaired mitochondrial respiratory capacity with statin treatment [7,27], but the current literature on whether statin therapy reduces CoQ10 levels in skeletal muscle are inconclusive [28-31]. Although reduced muscle CoQ10 and impaired mitochondrial respiratory capacity is a plausible explanation for development of statin induced myalgia, it is unlikely the sole underlying mechanism. Since the ratelimiting enzyme in the mevalonate pathway is inhibited by the active simvastatin acid and not the inactive lactone, the mechanism behind the acute toxicity seen with high concentrations of simvastatin lactone in vitro must be unrelated to the mevalonate pathway. However, if toxicity is only observed in vitro with supratherapeutic concentrations, the underlying mechanism may not be clinically relevant.
In spite of persistent research in statin pharmacokinetics, the intramuscular simvastatin concentration has never been investigated in humans. One study administered high doses of simvastatin (in its lactone form) to rats to induce myopathy, and found the intramuscular concentration of simvastatin acid to be one-third of the serum levels [32]. Another study has argued, without measuring, that the intramuscular concentration of simvastatin in vivo is 2-5 μM in humans [17], which would be a result of bioaccumulation, as it is ~1000 fold higher than the average human plasma concentration [11,33].
In the present study we report an acute impairment of mitochondrial respiration only when increasing the simvastatin concentration to 13.5 μM, which mimics an extreme 2500-7000 fold accumulation, and thus we consider it supratherapeutic. When simvastatin lactone doses were increased even further from 13.5 μM to 18.5 μM and 23.5 μM, mitochondrial respiration declined in a dose-dependent manner. While it is well established, that HMG-CoA reductase is inhibited by statins in a competitive dose-dependent manner [34,35], dose-dependency in the relationship between statin therapy and development of myopathies is less apparent [36]. The results of the present study may well be an indication that a dose-dependent impairment only occurs when the intracellular simvastatin lactone concentration rises above a certain threshold.
Therefore individual susceptibility and differences in the capacity for absorption, transport and elimination of statins may play a role in explaining why some develop myopathies. Simvastatin is generally well absorbed from the intestine [37], but individual differences in the abundance of Cytochrome P450 3A4 (CYP3A4) that metabolizes simvastatin [38] has previously been reported [39,40]. A low abundance of CYP3A4 in the liver and/or skeletal muscle would result in an elevated bioavailability and presumably an elevated intramuscular statin concentration, which would increase the potential for developing myopathies.
Genetic factors may be responsible for individual differences in the abundance of CYP3A4 and the transport capacity of MCT4, OATP2B1 and MRP1,4,5, and could be the reason some experience accumulation of statins and myopathies. We speculate that patients experiencing muscular side-effects are genetically disposed to statin accumulation in the muscle.
Conclusion
Unless simvastatin lactone accumulates more than 1000 fold within the skeletal muscle, the muscular side effects with simvastatin treatment cannot be explained by an impaired mitochondrial respiratory capacity. However, the dose-dependent relationship between simvastatin lactone and mitochondrial respiratory capacity only found at supratherapeutical doses, cannot allow us to exclude a role of the simvastin lactone metabolite in the pathogenesis of statin induced myalgia.
Thus, in order to more closely elucidate the role of simvastin´s metabolites in the development of muscular side effects of statin therapy, further studies addressing the dynamics of intramuscular simvastatin concentrations and its individual susceptibility are warranted.
Acknowledgements
Anja Birk Kuhlman and Thomas Hoffmann Morville are thanked for collecting muscle biopsies, and Maria Dahl is thanked for assisting with preparation for the analysis of mitochondrial respiration.
Funding
This work was supported by the University of Copenhagen 2016 Center of Excellence grant to the LIFESTAT–Living with statins project, and by the Nordea Foundation.
Conflict of Interest
All authors have read and approved the final version of this manuscript. None of the authors have conflicts of interest to disclose.
REFERENCES
- Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation 2013;127(1):96-103.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403-14.
- Cohen JD, Brinton EA, Ito MK, et al. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6(3):208-15.
- Schech S, Graham D, Staffa J, et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16(3):352-8.
- Sirvent P, Fabre O, Bordenave S, et al. Muscle mitochondrial metabolism and calcium signaling impairment in patients treated with statins. Toxicol Appl Pharmacol. 2012;259(2):263-8.
- Galtier F, Mura T, Raynaud de Mauverger E, et al. Effect of a high dose of simvastatin on muscle mitochondrial metabolism and calcium signaling in healthy volunteers. Toxicol Appl Pharmacol. 2012;263(3):281-6.
- Larsen S, Stride N, Hey-Mogensen M, et al. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J Am Coll Cardiol. 2013;61(1):44-53.
- Tesfamariam B, Frohlich BH, Gregg RE. Differential effects of pravastatin, simvastatin, and atorvastatin on Ca2+ release and vascular reactivity. J Cardiovasc Pharmacol. 1999;34(1):95-101.
- Tsubaki M, Fujiwara D, Takeda T, et al. The sensitivity of head and neck carcinoma cells to statins is related to the expression of their Ras expression status, and statin-induced apoptosis is mediated via suppression of the Ras/ERK and Ras/mTOR pathways. Clin Exp Pharmacol Physiol. 2017;44(2):222-34.
- Kang S, Kim K, Noh JY, et al. Simvastatin induces the apoptosis of normal vascular smooth muscle through the disruption of actin integrity via the impairment of RhoA/Rac-1 activity. Thromb Haemost. 2016;116(3):496-505.
- Gazzerro P, Proto MC, Gangemi G, et al. Pharmacological actions of statins: a critical appraisal in the management of cancer. Pharmacol Rev. 2012;64(1):102-46.
- Christensen CL, Wulff Helge J, Krasnik A, et al. LIFESTAT - Living with statins: An interdisciplinary project on the use of statins as a cholesterol-lowering treatment and for cardiovascular risk reduction. Scand J Public Health. 2016;44(5):534-9.
- Taha DA, De Moor CH, Barrett DA, et al. The role of acid-base imbalance in statin-induced myotoxicity. Transl Res 2016;174:140-60.e114.
- Vree TB, Dammers E, Ulc I, et al. Variable Plasma/Liver and Tissue Esterase Hydrolysis of Simvastatin in Healthy Volunteers after a Single Oral Dose. Clin Drug Investig. 2001;21(9):643-52.
- Tamai I, Tsuji A. Transporter-mediated permeation of drugs across the blood-brain barrier. J Pharm Sci. 2000;89(11):1371-88.
- Knauer MJ, Urquhart BL, Meyer zu Schwabedissen HE, et al. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106(2):297-306.
- Kwak HB, Thalacker-Mercer A, Anderson EJ, et al. Simvastatin impairs ADP-stimulated respiration and increases mitochondrial oxidative stress in primary human skeletal myotubes. Free Radic Biol Med 2012;52(1):198-207.
- La Guardia PG, Alberici LC, Ravagnani FG, et al. Protection of rat skeletal muscle fibers by either L-carnitine or coenzyme Q10 against statins toxicity mediated by mitochondrial reactive oxygen generation. Front Physiol. 2013;4:103.
- Nakahara K, Yada T, Kuriyama M, et al. Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun. 1994;202(3):1579-85.
- Skottheim IB, Gedde-Dahl A, Hejazifar S, et al. Statin induced myotoxicity: the lactone forms are more potent than the acid forms in human skeletal muscle cells in vitro. Eur J Pharm Sci. 2008;33(4-5):317-25.
- Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol. 2012;810:25-58.
- Sinzinger H, O'Grady J. Professional athletes suffering from familial hypercholesterolaemia rarely tolerate statin treatment because of muscular problems. Br J Clin Pharmacol. 2004;57(4):525-8.
- Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301-8.
- Mason RP, Walter MF, Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109(21 Suppl 1):II34-41.
- Chariot P, Abadia R, Agnus D, et al. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am J Med. 1993;94(1):109-10.
- Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62(8):709-14.
- Bouitbir J, Charles AL, Echaniz-Laguna A, et al. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a 'mitohormesis' mechanism involving reactive oxygen species and PGC-1. Eur Heart J. 2012;33(11):1397-407.
- Paiva H, Thelen KM, Van Coster R, et al. High-dose statins and skeletal muscle metabolism in humans: a randomized, controlled trial. Clin Pharmacol Ther. 2005;78(1):60-8.
- Laaksonen R, Jokelainen K, Sahi T, et al. Decreases in serum ubiquinone concentrations do not result in reduced levels in muscle tissue during short-term simvastatin treatment in humans. Clin Pharmacol Ther. 1995;57(1):62-6.
- Laaksonen R, Jokelainen K, Laakso J, et al. The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle. Am J Cardiol. 1996;77(10):851-4.
- Lamperti C, Naini AB, Lucchini V, et al. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62(11):1709-12.
- Sidaway J, Wang Y, Marsden AM, et al. Statin-induced myopathy in the rat: relationship between systemic exposure, muscle exposure and myopathy. Xenobiotica. 2009;39(1):90-8.
- Keskitalo JE, Pasanen MK, Neuvonen PJ, et al. Different effects of the ABCG2 c.421C>A SNP on the pharmacokinetics of fluvastatin, pravastatin and simvastatin. Pharmacogenomics. 2009;10(10):1617-24.
- Saito Y, Goto Y, Nakaya N, et al. Dose-dependent hypolipidemic effect of an inhibitor of HMG-CoA reductase, pravastatin (CS-514), in hypercholesterolemic subjects. A double blind test. Atherosclerosis. 1988;72(2-3):205-11.
- Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet. 1997;32(5):403-25.
- Fujita M, Morimoto T, Ikemoto M, et al. Dose-dependency in pleiotropic effects of atorvastatin. Int J Angiol. 2007;16(3):89-91.
- Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet. 1993;24(3):195-202.
- Fujino H, Saito T, Tsunenari Y, et al. Metabolic properties of the acid and lactone forms of HMG-CoA reductase inhibitors. Xenobiotica. 2004;34(11-12):961-71.
- Molina-Ortiz D, Camacho-Carranza R, Gonzalez-Zamora JF, et al. Differential expression of cytochrome P450 enzymes in normal and tumor tissues from childhood rhabdomyosarcoma. PLoS One. 2014;9(4):e93261.
- Bieche I, Narjoz C, Asselah T, et al. Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics. 2007;17(9):731-42.