The effect of cigarette smoke exposure on the conformational stability and biological activity of a model protein lysozyme
2 Department of Pharmacy Sciences, Creighton University, Nebraska, USA, Email: ssingh@creighton.edu
Received: 11-Oct-2017 Accepted Date: Nov 14, 2017; Published: 18-Nov-2017
Citation: Samuels Jr AR, Singh S. The effect of cigarette smoke exposure on the conformational stability and biological activity of a model protein lysozyme. J Pharmacol Med Chem. 2017;1(1):25-28.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Cigarette smoking has severe health issue resulting in tremendous economic loss. Each year more than 480,000 people in the United States die due to chronic use of cigarettes. The total economic cost of smoking is more than $300 billion a year. Chronic smoking has been associated with many diseases some of which are attributed to conformation changes in proteins. Therefore, this study has investigated the effect of smoking on the conformational changes of a model protein lysozyme and alteration in its biological activity. The cigarette smoke was passed through citrate phosphate (CP) buffer (pH 4.4) which resulted into the formation of cigarette smoke extract (CSE) which is reported to simulate the presence of various chemicals found in such smoke. The CSE (10% to 50% v/v) was added to lysozyme solution (0.06% w/v) samples were taken specific time points. The samples were subjected to Fourier Transform Infrared (FTIR) and ultraviolet (UV) spectroscopic analysis for evaluation of changes in conformational integrity and biological enzyme activity of lysozyme, respectively. The results obtained indicated that CSE causes conformational stability perturbation in lysozyme which is associated with a corresponding decrease in its biological activity. However, this study did not include the effect of water insoluble components of cigarette smoke and the effect of CSE on long term exposure beyond the 72 hrs. Therefore, any future study should include the effect of long-term exposure of water soluble as well as insoluble components on conformation integrity and biological activity of proteins.
Keywords
Cigarette smoke exposure; Conformational stability; Lysozyme
Each year more than 480,000 people in the USA die due to chronic use of cigarettes in addition to 41,000 deaths resulting from secondhand smoke exposure [1,2]. The total economic cost of smoking is more than $300 billion a year which includes about $170 billion in direct medical care for adults [3] and more than $156 billion in lost productivity due to premature death [2]. There are hundreds of chemicals in a cigarette which are proven to be toxic. Smoking cigarette exposes body to harmful chemicals which cause damage to major organs of the body. Smoking is one of the major causes of heart disease, aneurysms, bronchitis, emphysema, and stroke [1,4]. Toxic chemicals from cigarette smoke reduce fertility in men. Consumption of cigarette by pregnant women can lead to miscarriage, premature birth and stillbirth [5]. The Centers for Disease Control and Prevention estimated that adult male smokers lost an average of 13.2 years of life and female smokers lost 14.5 years of life because of smoking [2]. Chronic smoking has been proven to be responsible for various kinds of cancers like lung, esophagus, oral cavity (mouth, tongue, lips) and acute myeloid leukemia, chronic obstructive pulmonary disease (COPD) [6]. Cigarette smoke extract (CSE) contains more than 4000 chemicals, of which more than 50 are carcinogenic [7]. Examples of toxic chemicals found in cigarette smoke include benzene, ammonia, tar, nicotine, formaldehyde, carbon monoxide, arsenic etc. Benzene is a carcinogenic colorless liquid found in cigarette smoke and has been linked with leukemia [8,9]. Nicotine is one of the most addictive chemicals known and is the reason behind addiction for cigarette. Carbon monoxide causes a reduction in the oxygen carrying capacity of the red blood cells. Most diseases reported due to cigarette smoke are believed to be associated with perturbation of structural and conformational integrity of some specific protein [10-12]. Cigarette smoke extract regulates the protein expression in the airway epithelium [13]. Aqueous cigarette smoke extract contains chemicals and free radicals which can oxidize and cause damage to the human plasma proteins and microsomal proteins [12,14]. Degradation of the proteins is believed to be caused due to the oxidation of proteins or proteolytic degradation of oxidized protein by the microsomes. Many diseases are caused due incorrect folding of proteins [11]. Alzheimer’s disease is a prime example of incorrectly folded proteins being brought together [15]. Damage to the alpha 1-Protease inhibitor has been linked to the development of emphysema. Alpha 1 antitrypsin deficient patient develop emphysema 10 years before in regular smokers compared to non- smokers [16,17]. Disruption of the p53 protein leads to about 50% to 55% of cancers in humans. Other forms of diseases caused due to the incorrect folding of the protein include Transmissible spongiform encephalopathies (TSEs), mad cow disease (bovine spongiform encephalopathy; BSE) and Creutzfeld Jakob disease (CJD) [11]. These are a kind of amyloidosis in which the brain degenerates to a structure similar a porous sponge. The protein responsible for these conditions is prion protein, which is found normally in the nerve cells. Cigarette smoke forms reactive oxidant species which causes damage to the lung and is one of the prime reasons for development of Chronic Obstructive Pulmonary Disease (COPD) [18]. It has been reported that smoking interferes with the protein folding in the endoplasmic reticulum. An unfolded protein response is observed in regular smokers. The unfolded protein interferes with the antioxidant response, cell cycle, inflammation, apoptosis and protein synthesis [18]. A chronic increase in the defective protein structure and a dysregulation of the endoplasmic reticulum plays a pathogenic role in diabetes, cardiovascular diseases, Alzheimer’s disease, Parkinson’s syndrome and cancer [10,11,15,18,19]. Lysozyme is the most investigated protein of which biochemical and biophysical characteristics are well reported in literature. It is a129 amino acid residues enzyme. It catalyzes hydrolysis of 1,4-beta-linkages between N-acetylmuramic acid and N-acetyl-D-glucosamine residues in peptidoglycan present in the bacterial cell walls. Therefore, it is also named as 1,4-N-acetylmuramidase, L-7001, N,O-diacetylmuramidase, PR1-lysozyme, globulin G1, globulin G, lysozyme g, mucopeptide N-acetylmuramoylhydrolase, mucopeptide glucohydrolase and muramidase. Gram-positive bacteria are more susceptible to hydrolysis by lysozyme since its wall contains a high amount of peptidoglycan. However, gram-negative bacteria are less prone to hydrolysis since they have a lower amount of peptidoglycan in the cell wall [20]. M. Luteus is a gram-positive bacterium. Hence it was used as a substrate for quantifying biological activity of lysozyme. Enzyme activity of lysozyme gets affected in the presence of strong oxidizing agents. It loses its antibacterial activity due to a change in the structure and conformation [21,22]. Therefore, in this study we investigated the influence of exposure of CSE on the conformation stability and biological activity of lysozyme which could be related with pathogenesis of many diseases.
Materials and Methods
Materials
Cigarette smoke was prepared by burning five Kentucky University research grade cigarettes. Lysozyme (EC 3.2.1.17) from chicken egg white and Micrococcus lysodeikticus (Micrococcus luteus) were purchased from Sigma Chemical Company, St. Louis, MO. All other chemicals bought were of analytical reagent grade and used as obtained.
Preparation of the cigarette smoke extract
The generated smoke was passed through 10 ml of citrate phosphate (CP) buffer (pH 4.4) which resulted into the formation of cigarette smoke extract (CSE). CSE was added to lysozyme solution (0.06% w/v) in the concentration range of 9% through 50% v/v. Samples were taken from the mixture of lysozyme mixed with CSE at 4, 24, 48, and 72 hours’ time points and subjected to Fourier Transform Infrared (FTIR) and ultraviolet (UV) spectroscopic analysis for conformational changes in secondary structure of lysozyme and its biological enzyme activity, respectively.
Secondary structure analysis using Fourier Transform Infrared (FTIR) spectroscopy
Forty microliters of the lysozyme, a model protein, sample was placed in the sample cell of the IR Prestige FTIR (Shimadzu, Kyoto, Japan). FTIR spectra were obtained in the frequency range 4000-1000 cm-1 in absorbance mode. The parameters chosen for running the scans were resolution of 4 cm-1, average of 15 scans was taken and no apodization option was selected. Forty microliters of CP buffer was used to obtain the background spectra which was subtracted automatically from each sample spectrum. The CSE and lysozyme solution were mixed in various ratios (CSE: lysozyme:: 1-10:10) to produce 10 samples containing CSE in the concentration range of 9% to 50% v/v. We used second derivative spectra for calculation of peaks in the region of 1600-1700 cm-1 wavenumber.
Biological activity of lysozyme
Biological activity of the samples was determined by an enzyme activity assay using Micrococcus lysodeikticus (M. luteus) as substrate using UV visible spectrophotometer (UV 1700, Shimadzu). The method used for evaluating biological activity of lysozyme is previously reported [23,24]. Briefly, 100 mL sample of the bacteria M. luteus (0.015% w/v) in phosphate buffer (pH 4.4) was prepared. Ten samples of CSE and lysozyme mixture, containing increasing concentrations of CSE, were prepared. Sample one was prepared by adding 50 μL of CSE to 500 μL of lysozyme solution (1:10) which were further diluted to obtain 9 other samples containing CSE: lysozyme in the range of 1:10-10:10. Thirty μL of each of the 10 samples of CSE/lysozyme mixture were withdrawn at 4, 24, 48, and 72 hrs and added to 2 mL of microbial suspension. Phosphate buffer was used as the reference for the study. This mixture was observed for change in absorbance by operating the spectrophotometer in rate measurement mode using the parameters: wavelength=450.0 nm, measure Time=180 seconds with (δ)=0.2, lag/rate=5 seconds/175 seconds, and temperature=25° C. A rate of change of 0.001 in absorbance at 450 nm (A450 nm) was used to define 1 unit of biologically active lysozyme and was calculated by using following formula [23]
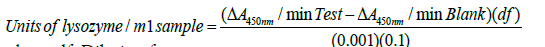
where, df=Dilution factor
0.001=Change in absorbance at A as per the unit definition
0.1=Volume (in ml) of sample/standard used.
Data analysis
Statistical comparisons were made using student’s t-test and analysis of variance (ANOVA). The level of significance was used as p<0.05.
Results and Discussion
Lysozyme was selected as a model protein in this study because it is reported as one of the serum biomarkers related to tobacco elicited injuries of the pulmonary microvasculature as indicated by isolated decrease of diffusion capacity of the lung (DCL) [25-29]. There is an inverse relationship between serum lysozyme concentration and DCL which might suggest a causal relationship between these two factors in early phase of tobacco smoke-related diseases. Furthermore, lysozyme is reported to correctly identify abnormal decrease of DCL in smokers with normal lung function which may suggest it to mirror monocyte/macrophage (and/ or neutrophil) mediated disease process presumably related to pulmonary microvasculature system [30,31]. Moreover, lysozyme is also reported to have acute role in inflammation response associated with pulmonary emphysema.
Lysozyme (1,4-β-N-acetylmuramidase) is a bacteriolytic enzyme that was discovered by Alexander Fleming, the pioneer of penicillin, and exists widely in nature, including in humans and birds. Lysozyme is an enzyme with lytic action that is found in human secretions such as respiratory tract secretions. It is present in high levels in the respiratory epithelium and neutrophil granules and has antibacterial activity against gram-negative and grampositive pathogens [32,33] A study was conducted in pigs [34] to determine the effect of lysozyme and antibiotics on growth performance and immune response during an indirect immune challenge. It was found that lysozyme is a suitable alternative to antibiotics in swine nursery diets, and lysozyme ameliorates the effects of a chronic indirect immune challenge.
Evaluation of conformational changes in lysozyme by FTIR
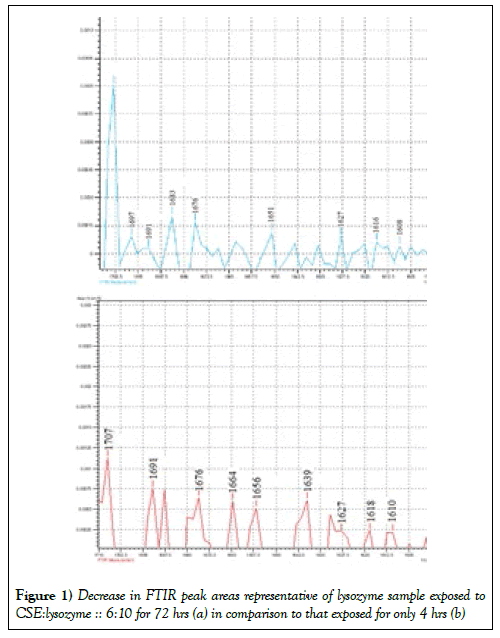
IR spectrophotometer was used to investigate the conformational changes in the lysozyme sample mixed with CSE. We focused on region 1600-1700 cm-1 because different secondary protein structures absorb IR in this region [25]. We observed significant (p<0.05) decrease in peak heights and areas for the lysozyme sample exposed to CSE for 72 hrs (Figure 1a) in comparison to those of exposed only for 4 hrs (Figure 1b). Similar trends were found for all the samples exposed for other time points.
Table 1 shows the splitting of β sheets with increase in exposure time in all samples. We observed bathochromic shift (Table 2) corresponding to α helix and β turns in all the samples at most of the time points. The different secondary structures like β sheet, random coils, α helix, and β turns absorb in the range 1640-1620, 1650-1640, 1660-1650, and 1695-1660 cm-1, respectively [17,25,26]. We observed some random coils in 24 and 48 h samples which disappeared in 72 h samples containing 25% v/v or more CSE. FTIR data suggests that the exposure of cigarette smoke to protein lysozyme lead to change in the structural conformation. The β sheet structure is found in the 1620-1640 cm-1 region [17]. Splitting of peak in this region shows breaking of some bonds. Due to the change in structure of lysozyme there is an expected reduction in enzymatic action which was observed by carrying out the enzyme assay technique.
| Samples | Exposure Time (hr) | Location (cm-1) |
|---|---|---|
| Control (lysozyme) | 4 | 1624 ± 1 |
| 24 | 1624 ± 1 | |
| 48 | 1624 ± 1 | |
| 72 | 1624 ± 1 | |
| CSE:lysozyme (1:10) | 4 | 1639 ± 2 |
| 24 | 1630 ± 2 | |
| 1637 ± 1 | ||
| 48 | 1630 ± 1 | |
| 1636 ± 1 | ||
| 72 | 1628 ± 3 | |
| 1636 ± 1 | ||
| CSE:lysozyme (1:1) | 4 | 1635 ± 1 |
| 1639 ± 1 | ||
| 24 | 1631 ± 2 | |
| 1639 ± 2 | ||
| 48 | 1631 ± 1 | |
| 1639 ± 1 | ||
| 72 | 1631 ± 1 | |
| 1620 ± 3 |
Table 1: Splitting of β sheet secondary structure of lysozyme exposed to CSE
| Sample | Exposure Time (hr) | α helix Location (cm-1) | β turn Location (cm-1) | Rando coil location (cm-1) |
|---|---|---|---|---|
| Control (lysozyme) | Apr-72 | 1658 ± 1 | 1671 ± 1 | 1693 ± 1 |
| CSE: lysozyme (1:10) | 4 | 1656 ± 1 | 1666 ± 1 | 1687 ± 3 |
| 24 | 1653 ± 2 | 1664 ± 1 | 1686 ± 1 | |
| 48 | 1653 ± 1 | 1662 ± 2 | 1684 ± 1 | |
| 72 | 1653 ± 2 | 1662 ± 2 | 1684 ± 2 | |
| CSE: lysozyme (1:1) | 4 | 1653 ± 1 | 1676 ± 1 | 1683 ± 2 |
| 24 | 1649 ± 3 | 1666 ± 1 | 1676 ± 1 | |
| 48 | 1649 ± 2 | 1666 ± 1 | 1683 ± 1 | |
| 72 | No peak | 1664 ± 2 | 1683 ± 2 |
Table 2: The limitations of ROTEM.
The reduction in peak height and area could be due to loss in secondary character of protein. Longer exposure to CSE led to the corresponding greater reduction in secondary character of lysozyme.
Determination of biological activity of lysozyme
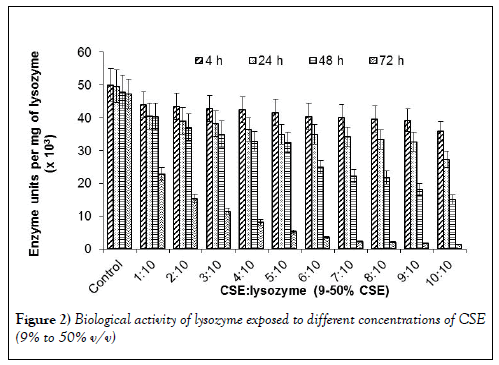
The IR data were supported by a corresponding decrease in activity of lysozyme in the samples with increasing ratios of CSE and exposure times (Figure 2).
The lysozyme activity was monitored at different time points on addition of increasing concentrations of CSE. The ratio of CSE added to lysozyme was increased from 1:10 to 1:1. The biological activity of lysozyme was found to decrease with increasing concentrations of CSE. As the concentration of CSE was increased the biological activity of lysozyme decreased by approximately 10 folds when kept together for 72 hrs duration. The decrease in lysozyme activity is attributed to a change in conformation due to chemicals present in the CSE. There are about 4000 chemicals in the cigarette smoke which can affect the conformation of lysozyme which in turn affected its enzymatic action on the cell wall of the bacteria. CSE contains chemicals like carbon monoxide, nicotine, nitrous oxide and other free radicals. These chemical cause the protein to go under oxidative stress. Methionine (Met) amino acid from the lysozyme undergoes oxidation in presence of CSE and gets converted to Met sulfoxide.
Trypsin, histidine and tyrosine residue get oxidized and render the protein inactive [19,27,28]. We used a water soluble extract of smoke which did not include the water insoluble components of cigarette smoke. Those components could also affect the stability and conformation of lysozyme. Further research is required to study the effect of water insoluble components on proteins. Also, our study was carried out to study effect of smoke extract on lysozyme for a period of 72 hrs. Further research could be aimed to study the effect of cigarette components on protein conformation for a longer duration. Looking at the results obtained from this study we can predict that longer exposure to smoke extract will affect the protein in a negative way thereby reducing its activity against the microbial attack. Results show that the cigarette smoke extract will cause changes in the structure and conformation of proteins thereby rendering them inactive. Slight changes in the protein structure or unfolding of protein are prime reasons for development of diseases like Alzheimer’s disease, Parkinson’s disease, cardiovascular diseases and also cancer. Although, this study did not account for the various barriers which the cigarette smoke has to face before coming in contact with lysozyme a future study using animal models could be carried out to test the amount of cigarette smoke chemicals reaching the body fluids.
Conclusion
It is concluded that CSE causes conformational stability perturbation in lysozyme which is associated with a corresponding decrease in its biological activity. In this study lysozyme has been used as a model protein. Our study showed that CSE causes conformational changes in lysozyme which might be expected in other protein also such as alpha synuclein [35,36] which is involved in the pathogenesis of Alzheimer and Parkinson’s disease. Therefore, any future study should involve alpha synuclein also.
Acknowledgements
We are thankful to Health Science Multicultural and Community Affairs (HS-MACA) Summer Research Program, CreightonUniversity for financial support to Alvin and this study.
REFERENCES
- Alberg AJ. Cigarette smoking: Health effects and control strategies. Drugs Today 2008;44:895-904.
- https://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm
- Xu X, Bishop EE, Kennedy SM, et al. Annual healthcare spending attributable to cigarette smoking: An update. Am J Prev Med. 2014;48:326-33.
- Boyle JO, Gumus ZH, Kacker A, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res. 2010;3:266-78.
- Coelho C, Julio C, Silva G, et al. 2009. Tobacco and male infertility: A retrospective study in infertile couples. Acta Med Port. 2009;22:753-8.
- Beasley MB. Smoking-related Small airway disease-A review and update. Adv Anat Pathol. 2010;17:270-6.
- Richter PA, Bishop EE, Wang J, et al. Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Int J Environ Res Public Health. 2009;6:1930-46.
- Cocco P, Fadda D, Melis M, et al. Occupational exposure to solvents and risk of lymphoma subtypes: results from the Epilymph case-control study. Occup Environ Med. 2010; 67:341-7.
- Xing C, Marchetti F, Li G, et al. Benzene exposure near the U.S. Permissible limit is associated with sperm aneuploidy. Environ Health Perspect. 2010;118:833-9.
- Kitamura S. Effects of cigarette smoking on metabolic events in the lung. Environ Health Perspect. 1987;72:283-96.
- Li H, Guo M. Protein degradation in Parkinson disease revisited: It's complex. J Clin Invest. 2009;119:442-5.
- Panda K, Chattopadhyay R, Chattopadhyay D, et al. Cigarette smoke-induced protein oxidation and proteolysis is exclusively caused by its tar phase: prevention by vitamin C. Toxicol Lett. 2001;123:21-32.
- Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979-2004.
- Carnevali S, Petruzzelli S, Longoni B, et al. Cigarette smoke extract induces oxidative stress and apoptosis in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;284:L955-63.
- Jorgensen E, Stinson A, Shan L, et al. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. 2008;8:229.
- Pryor WA, Dooley MM, Church DF. Mechanisms of cigarette smoke toxicity: the inactivation of human alpha-1-proteinase inhibitor by nitric oxide/isoprene mixtures in air. Chem Biol Interact. 1985;54:171-83.
- Pelton JT, McLean LR. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 2000;277:167-76.
- Kelsen SG, Duan X, Ji R, et al. Cigarette smoke induces an unfolded protein response in the human lung: A proteomic approach. Am J Respir Cell Mol Biol. 2008;38:541-50.
- Hawkins CL, Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: Role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol. 2005;18:1600-10.
- Callewaert L, Michiels CW. Lysozymes in the animal kingdom. J Biosci. 2010;35:127-60.
- Jori G, Galiazzo G, Marzotto A, et al. Selective and reversible photo-oxidation of the methionyl residues in lysozyme. J Biol Chem. 1968;243:4272-8.
- Singh S, Singh J. Effect of polyols on the conformational stability and biological activity of a model protein lysozyme. AAPS Pharm Sci Tech. 2003;4:E42.
- Shugar D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952;8:302-9.
- Chhabra S, Sachdeva V, Singh S. Influence of end groups on in vitro release and biological activity of lysozyme from a phase-sensitive smart polymer-based in situ gel forming controlled release drug delivery system. Int J Pharm. 2007;342:72-7.
- Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39:549-59.
- Sethuraman A, Belfort G. Protein structural perturbation and aggregation on homogeneous surfaces. Biophys J. 2005;88:1322-33.
- Pattison DI, Davies MJ. Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry. 2004;43:4799-809.
- Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci. 2004;3:17-25.
- Schmekel B, Blomstrand P, Venge P. Serum lysozyme- a surrogate marker of pulmonary microvascular injury in smokers? Clin Physiol Funct Imaging. 2013;33:307-12.
- Amin K, Jansson A, Lofdahl C, et al. Relationship between inflammatory cells and structural changes in the lungs of asymptomatic and never smokers: A biopsy study. Thorax. 2003;58:135-42.
- Shields PG, Berman M, Brasky TM, et al. A review of pulmonary toxicity of electronic cigarettes in the context of smoking: A focus on inflammation. Cancer Epidemiol Biomarkers Prev. 2017;26:1175-91.
- Dajani R, Zhang Y, Taft PJ, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol. 2005;32:548-52.
- Ellison RT, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88:1080-91.
- Oliver WT, Wells JE, Maxwell CV. Lysozyme as an alternative to antibiotics improves performance in nursery pigs during an indirect immune challenge. J Anim Sci. 2014;92:4927-34.
- Malek N, Swallow D, Grosset KA, et al. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson's disease- a systematic review. Acta Neurol Scand. 2014;130:59-72.
- Goedert M. Alzheimer's and Parkinson's diseases: The prion concept in relation to assembled Aß, tau, and a-synuclein. Science. 2015;7:349(6248):1255555.